Abstract
BRD4, characterized by two acetyl-lysine binding bromodomains and an extra-terminal (ET) domain, is a key chromatin organizer that directs gene activation in chromatin through transcription factor recruitment, enhancer assembly, and pause release of the RNA polymerase II complex for transcription elongation. BRD4 has been recently validated as a new epigenetic drug target for cancer and inflammation. Our current knowledge of the functional differences of the two bromodomains of BRD4, however, is limited, hindered by the lack of selective inhibitors. Here, we report our structure-guided development of diazobenzene-based small molecule inhibitors for the BRD4 bromodomains that have over 90% sequence identity at the acetyl-lysine binding site. Our lead compound MS436, through a set of water-mediated interactions, exhibits low nanomolar affinity (estimated Ki of 30–50 nM) with preference for the first bromodomain over the second. We demonstrated that MS436 effectively inhibits BRD4 activity in NF-κB-directed production of nitric oxide and pro-inflammatory cytokine interleukin-6 in murine macrophages. MS436 represents a new class of bromodomain inhibitors and will facilitate further investigation of the biological functions of the two bromodomains of BRD4 in gene expression.
INTRODUCTION
Human DNA is tightly packaged into chromatin by wrapping around the core histone octamer of nucleosomes. The N- and C-termini of the core histones, protruding out from the nucleosome, are subject to a wide variety of post-translational amino acid modifications including acetylation, methylation, phosphorylation, ubiquitinalytion, and SUMOlytion.1 These chemical modifications of the histones, in combination with DNA modifications, function in a synergistic fashion to regulate gene activation or silencing in chromatin. The ε-N-acetylation of lysine neutralizes positive charges on histones weakening their interactions with negatively charged DNA. Site-specific lysine acetylation also plays an active role in transitioning chromatin into a relaxed state, and directing the recruitment of the gene transcriptional machinery complex for gene activation.
The evolutionarily conserved bromodomain (BrD) serves as the acetyl-lysine binding domain2, 3 to regulate gene activation in chromatin. The 61 human bromodomains embedded in 46 proteins4 are divided into eight subfamilies with distinctive features based on sequence similarities.5 One major group is BET (bromodomain and extra-terminal domain) proteins comprised of BRD2, BRD3, BRD4, and BRDT, each of which contains two tandem bromodomains (BrD1 and BrD2). All bromodomains share a conserved left-handed helix-bundle that is made out of four α helices, named αZ, αA, αB, and αC, respectively. The inter-helical loop regions known as the ZA and BC loops form the acetyl-lysine binding pocket, located at one end of the helix bundle. The amino acid residues in the acetyl-lysine binding pocket are highly conserved with over 90% sequence identity between the two bromodomains in each BET proteins. Of these is a highly conserved Asn residue that is essential for lysine-acetylated histone recognition by forming a hydrogen bond to the acetyl amide group of the acetylated lysine.
BRD4, arguably the most extensively characterized BET protein has implicated functions in a wide array of human disorders including cancer,6–8 obesity,9 kidney disease,10 lung fibrosis,11 and other inflammatory diseases.12 There is growing evidence that the two bromodomains of BRD4 have different biological functions.10, 12, 13 Despite several potent BET inhibitors reported in recent studies,6, 7, 10, 14–17 there is still no small molecule inhibitor shown to be capable of differentiating between the two bromodomains within any individual BET protein. Developing such a selective inhibitor is a challenging task because of the extremely high sequence identity of these bromodomains, particularly at their acetyl-lysine binding pockets.
In this study, we report the structure-guided development of a new class of potent and selective diazobenzene-based small-molecule inhibitors for the BET bromodomains. Our ligand design started with a diazobenzene compound MS120, originally discovered as an inhibitor for the CBP BrD.18 Guided by our new structural insights into recognition of the diazobenzenes by both CBP and BRD4 BrDs, we designed specific chemical modifications to acquire lead selectivity towards BRD4 BrDs and conducted extensive structure-activity relationship studies. Our lead compound MS436 exhibits potent affinity of an estimated Ki = 30–50 nM for the BRD4 BrD1 and a 10-fold selectivity over the BrD2, which is achieved through a unique set of water-mediated intermolecular interactions. We further demonstrated cellular efficacy of our lead diazobenzene inhibitors in blocking BRD4 transcriptional activity in NF-κB-directed production of nitric oxide and pro-inflammatory cytokine interleukin-6 in murine macrophages. We expect that this new class of bromodomain inhibitors will facilitate further mechanistic investigation of the biological functions of the two bromodomains of BRD4 in gene activation in human biology and disease.
RESULTS AND DISCUSSION
Structure-guided Design of Diazobenzenes as BET Bromodomain Inhibitors
We have previously reported MS120 (compound 1, see Figure 1), (E)-5-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-2,4-dimethylbenzenesulfonic acid, as a small molecule inhibitor for the bromodomain of HAT co-activator CBP18. This compound shows modest activity as assessed in a fluorescent polarization assay toward BRD4 BrD1. The NMR solution structure of the CBP BrD/MS120 complex (PDB: 2L84) reveals that MS120 binds at the acetyl-lysine binding pocket with the phenolic hydroxyl group forming a hydrogen bond with the amide nitrogen on the side chain of Asn1168 of the BrD. This Asn residue is critically important in acetyl-lysine binding and highly conserved in the bromodomain family.3 The overall molecule fits in the narrow groove constituted by the inter-helical ZA and BC loops, in which both the sulfonate and the amino groups are locked by a pair of electrostatic interactions with the guanidinium group of Arg1173 in the BC loop, a unique residue in the CBP BrD (Figure 1A). Our structure-based sequence analysis suggested chemical modifications to engineer this diazobenzene scaffold compound to be selective inhibitors for the BET bromodomains. For example, additional π–π interactions may be established between the ligand and Trp81 in the BRD4 BrD1, which corresponds to Leu1109 of CBP. The acetyl-lysine binding pocket of the BRD4 BrD1 is larger in volume than that of the CBP BrD, thus allowing further modifications to build target selectivity.5
Figure 1. Structure-guided development of diazobenzene-based BrD inhibitors.
(A) The 3D solution structure of the BrD inhibitor MS120 (yellow) bound to the CBP BrD (PDB: 2L84). The key amino acid residues in the acetyl-lysine binding site are shown in sticks. (B) Structure-activity relationship table illustrating binding affinity of a select number of diazobenzene-based BrD inhibitors to the two BrDs of BRD4. CLogP values were calculated using ChemBioDraw Ultra 12.0
Chemical Synthesis of Diazobenezene-Based Bromodomain Inhibitors
Based on the structure-guided design, we first extended the diazobenzene by adding a ring to benzenesulfonate (Figure 1B). In this case, we constructed the extended molecule based on compound 2, a MS120 analog, which itself showed a 2–3-fold improvement in binding affinity to the BRD4 BrDs over MS120 (1). The synthetic schemes and procedures are described in detail in Experimental Procedure. Binding affinity of these newly synthesized compounds for various bromodomain proteins was assessed using a fluorescence anisotropy binding assay as described previously10. Specifically, compound 3, an analog of 2 inherited the sulfonyl group at meta position and extended the system by coupling to 2-aminopyridine. Compound 3 retained the affinity of 2, indicating extension at meta position is tolerated. Interestingly, as the sulfonamide was repositioned to the para position, the affinity for the BRD4 BrD1 was improved by over 6-fold (MS435, compound 4 vs. 3). However, when we attempted trifluoromethyl-benzylamine as an alternative ring system with an extra carbon in the linker (5 in Figure 1B; compounds 35 and 36 in Supplemental Table 1), the inhibitory capacity deteriorated drastically. Therefore, we adopted the optimized 2-aminopyridine as a model system as we continued our lead optimization campaign.
Structure Activity Relationship of the C Ring
Encouraged by the initial results, we commenced an investigation of the structure-activity relationship (SAR) on the C ring toward the BRD4 BrD1 (Table 1). Hydrogen on R5 is required; substitution of H with a methyl group resulted in a 9-fold loss in activity (6 vs. MS435). Substitution at this position possibly causes a steric collision with Pro82 (see below). While substitutions at R6 and R8 are generally not tolerated (7 and 8 vs. MS435), substitutions at R7 are well tolerated. In particular, electron-withdrawing groups at R7 such as carboxyl group in compound 9 displayed activities about 3-fold better affinity than MS435. Chlorine substitution at R7 (compound 10) was tolerated; however, when bromine was introduced instead (11), the inhibitory activity toward BRD4 BrD1 declined by 10 fold. The nitrogen on the C ring is preferred, and corresponding carbon analogs displayed lower affinity, for example 11 or 12 vs. 13, 14, or 15; 16 vs. 17. Notably, we observed that the nitrogen in the C ring resulted in a 13-fold improvement in binding affinity to the BRD4 BrD1, whereas a 3-fold increase for the BRD4 BrD2, thus yielding an overall 4-fold binding selectivity between these two BrDs (12 vs. 13). Non-substituted 2-aminopyridine was one of the best pieces in the C ring. Considering its simplicity, stability, and potential impact on the selectivity between the BrD1 and the BrD2 of BRD4, we decided to choose 2-aminopyridine as a conserved moiety as we continued to optimize A and B rings.
Table 1.
Structure-Activity Relationship of C Ring Analogs of Diazobenzenes
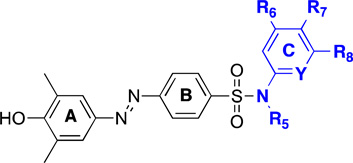 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound ID |
R5 | R6 | R7 | R8 | Y | BRD4 BrD1 |
BRD4 BrD2 |
CLogPa |
| Ki µM | Ki µM | |||||||
| 6 | Me | H | H | H | N | 7.89 | 7.12 | 4.91 |
| 7 | H | H | H | CF3 | C | 6.13 | 7.77 | 6.54 |
| 8 | H | CI | H | H | N | 0.90 | 5.92 | 5.40 |
| 9 | H | H | COOH | H | N | 0.32 | 1.38 | 4.37 |
| 10 | H | H | CI | H | N | 0.73 | 5.84 | 5.40 |
| 11 | H | H | Br | H | N | 6.54 | 38.7 | 5.67 |
| 12 | H | H | OMe | H | N | 0.50 | 2.23 | 4.66 |
| 13 | H | H | OMe | H | C | 6.48 | 6.59 | 5.50 |
| 14 | H | H | CF3 | H | C | 10.7 | 7.14 | 6.54 |
| 15 | H | H | H | C | 9.60 | 12.2 | 4.37 | |
| 16 | H | H | F | H | N | 1.30 | 3.44 | 4.45 |
| 17 | H | H | F | H | C | 1.84 | 8.87 | 5.75 |
CLogP values were calculated using ChemBioDraw Ultra 12.0; n.b.: no binding
Structure Activity Relationship of A and B Rings
Substituted B ring, such as methoxyl group, did not lead to tighter binding (compound 18 vs. MS435) (Table 2). Analysis of the crystal structure of MS435 bound to the BRD4 BrD1 (see Figure 2c) revealed that the hydroxyl group on the phenol ring was hydrogen bonded to the conserved Asn140. Hence, we primarily concentrated on the optimization of the A ring, and built a focused library of over 30 diazobenzene compounds.
Table 2.
Structure-Activity Relationship of A or B Ring Analogs of Diazobenzenes
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound ID |
R1 | R2 | R3 | R4 | X | BRD4 BrD1 |
BRD4 BrD2 |
CLogP |
| Ki µM | Ki µM | |||||||
| 18 | Me | H | Me | OMe | c | 3.13 | 7.77 | 3.82 |
| 19 | CI | H | CI | H | c | 1.27 | 4.89 | 4.27 |
| 20 | Br | H | Me | H | c | 2.79 | 4.48 | 4.50 |
| 21 | CI | H | Me | H | c | 1.40 | 4.81 | 4.30 |
| 22 | Br | H | H | H | c | 4.67 | 4.69 | 4.05 |
| 23 | CI | H | H | H | c | 1.30 | 6.16 | 3.85 |
| 24 | Me | H | H | H | c | 6.43 | 8.20 | 3.81 |
| 25 | Br | H | Br | H | c | 0.52 | 4.29 | 4.67 |
| 26 | CF3 | H | H | H | c | 9.45 | 6.83 | 4.35 |
|
27 (MS436) |
H | NH2 | Me | H | c | <0.085 | 0.34 | 2.65 |
|
28 (MS267) |
Me | NH2 | Me | H | c | 0.15 | 0.44 | 3.05 |
|
29 (MS363) |
CI | NH2 | Me | H | c | 0.17 | 0.64 | 3.17 |
| 30 | H | NH2 | OMe | H | c | 1.28 | 5.39 | 1.96 |
| 31 | Me | H | Me | H | N | 2.31 | 24.60 | 4.44 |
| 32 | Me | Me | Me | H | N | 6.02 | 9.10 | 4.92 |
| 33 | Me | H | OH | H | C | 2.39 | 3.27 | 3.29 |
| 34 | H | H | OH | H | C | 4.59 | 3.91 | 2.84 |
Figure 2. Molecular basis of the BRD4 BrD1 recognition of the lead diazobenzene-based BrD inhibitors.
(A) The 3D crystal structure of MS436 (yellow) bound to BRD4-BrD1, depicted in a ribbon diagram. Key amino acid residues at the acetyl-lysine binding site are shown in sticks. The ligand MS436 is color-coded by atom type. (B) Electrostatic surface representation of the BRD4-BrD1 bound to MS436. (C) Superimposition of MS436, MS435 and MS267 when bound to the BRD4-BrD1. Only MS436 bound protein structure is shown. Side chains of the key residues involved in ligand binding are depicted in sticks and color-coded by atom type. The bound water molecules are shown as spheres in magenta. (D), (E), and (F) Schematic diagrams highlighting key interactions in the BRD4 BrD1 recognition of MS436, MS435 and MS267, respectively. Water molecules are shown in magenta spheres, and hydrogen bonds are drawn in dashed line. The figure was generated by using the program LIGPLOT32
We first examined the importance of the hydroxyl group on the A ring. When it was replaced with a methoxy (37) or an acetamide group (38) (Supplemental Table 1), the resulting compounds completely lost binding activity, confirming our structural analysis. Detailed study further revealed that bulky groups such as tert-butyl and isopropyl at R1 and R3 are not tolerated. When one bulky group was introduced (39 and 40 in Supplemental Table 1), the affinity decreased about 10 fold as compared to MS435; when two bulky groups were introduced simultaneously (41 and 42), the compounds almost completely lost their activities (Supplemental Table 1). The limited space in the acetyl-lysine binding pocket simply cannot accommodate large functional groups. For the same reason, bicyclic (compounds 43–46) systems did not show good activities towards the BRD4 BrD1. Flexible substitutions such as aminomethyl and hydroxymethyl were not tolerated neither (compounds 47–49 in Supplemental Table 1). On the other hand, halides, especially bromides, are generally tolerated such as compounds 19–25 (Table 2). It is worth pointing out that symmetrically bi-substituted phenols appear to outperform their mono-substituted counterparts, suggesting that steric hindrance constrains the rotational freedom between A ring and diazo linker, therefore locking the substituted A ring in a more favorable conformation. Strong electron withdrawing groups such as CF3 (26, Table 2) likely weakens the hydrogen bond between phenolic OH and Asn140, and is therefore not preferred.
Notably, MS436 (compound 27) is the most potent compound of this set of diazobenzene compounds as determined in an in vitro fluorescent anisotropy assay with Ki better than 85 nM for the BRD4 BrD1 (limited by the binding affinity of the assay probe; the estimated Ki is 30–50 nM), and an approximately 10-fold selectivity for the BrD1 over the BrD2 of BRD4 (Table 2). This is the first reported low nanomolar affinity BrD inhibitor with clear selectivity between these two BrDs of BRD4 as far as we know. MS436 is ten times more potent than the model compound MS435 towards the BRD4 BrD1. Interestingly, MS267 (compound 28), a “hybrid” compound incorporating the key features of MS435 and MS436 exhibited an about 2-fold reduction in potency than MS436, although it is still much better than MS435. In addition, placing a Cl at the ortho position with respect to the hydroxyl group in the A ring in another hybrid compound MS363 (compound 29) showed a 2-fold decrease in binding affinity for both BrDs of BRD4. Nevertheless, both MS267 and MS363 maintained the selectivity for the BRD4 BrD1 over the BrD2. Notably, change of the methyl group at R3 in MS436 to methoxy (30) resulted in a 15-fold drop of binding affinity to either BRD4 BrD1 or BrD2. This explains the importance of this methyl group in mimicking the methyl group of acetyl-lysine in a biological ligand. Finally, our endeavors to employ heterocycles in the A ring were unfruitful. Introduction of a nitrogen atom into the A ring aromatic system led to a 3-fold drop in affinity (31 and 32 vs. MS435).
Structural Basis for Ligand Selectivity
We solved three high-resolution crystal structures of BRD4 BrD1 bound to MS436, MS435 or MS267 in order to determine the molecular basis of binding affinity and selectivity of these lead BrD inhibitors (Figure 2A–F). In a similar orientation to that of MS120 in the CBP BrD, these much improved diazobenzene inhibitors bind across the acetyl-lysine binding pocket in the ZA and BC loops in the BRD4 BrD1. The hydroxyl group in the A ring forms a hydrogen bond to the amide of the conserved Asn140, and another water (W1)-mediated hydrogen bond to the phenoxyl group for Tyr97 (Figure 2C–F). In addition, the second nitrogen in the diazo linker is engaged in a water (W8)-mediated hydrogen bond to the backbone carbonyl oxygen of the conserved Pro82 that plays an important structural role in many BrDs3. The core diazobenzene moiety is sandwiched between hydrophobic walls formed by Val87, Leu92, Leu94, and Tyr139 on one side, and Pro82, Phe83 and Ile146 on the other. Further, the pyridine ring (C ring), particularly in MS435 and MS267, forms π-π interactions with the side-chain idol of Trp81. Collectively, these intermolecular interactions explain the diazobenzene scaffold as a preferred platform as BrD inhibitors for BRD4.
Notably, besides five water molecules (W1-W5) commonly observed in BrDs and stably located at the bottom of the acetyl-lysine binding pocket3, we observed several more bound water molecules that are intimately engaged in ligand recognition with these diazobenzene inhibitors, particularly for MS436 (Figure 2C). Specifically, in addition to W8, which facilitates a water-mediated hydrogen bond between one nitrogen atom of the diazo linker and carbonyl oxygen of Pro82 as described above for all three BrD inhibitors, MS436 bound structure reveals three additional water molecules. For example, W6 bridges hydrogen bonding interactions between the amino group of the A ring and carbonyl oxygen of the conserved Asn140; the former is engaged in a hydrogen bond with another surface-exposed bound water, W7. In addition, W9 establishes a set of hydrogen bonding interactions between the sulfonamide oxygen in the B-C ring linker and side-chain amine of Lys91. The latter is one of very few unique residues in the BrD1 of BRD4; the corresponding residue in the BrD2 is Ala384. Notably, when the 2-aminopyridine sulfonamide (C ring) connectivity to the B ring was moved to meta position to the diazo linker, we observed a 10-fold or 3-fold reduction in binding affinity to the BRD4 BrD1 and the BrD2, respectively (compound 51 vs. MS436, see Supplemental Table 1). Finally, W6, W7 and W9 are missing in the MS435 bound structure, whereas only W7 and W9 are present in the MS267 structure.
The protein structures of the BRD4 BrD1 bound to these diazobenzene inhibitors are nearly identical. Superimposition of these structures reveals that MS435 and MS267 are bound in almost identical position and orientation whereas MS436 is upward shifted anchoring on the hydroxyl group of the A ring, which is positioned at nearly same position in the protein with all three ligands (Figure 2C). This shift of MS436 resulted in an increased distance between the pyridine ring (C ring) and Trp81, as compared to that in MS435 or MS267, but yielded additional hydrogen bonding interactions involving W6, W7 and W9 as compared to MS435 (Figure 2D vs. 2E–2F). In addition, W8 and W9 in the MS436 bound structure are re-positioned accordingly to maintain hydrogen bonds between the protein residues and the ligand as described above. Most notably, the key hydrogen bond formed between the hydroxyl group in the A ring and the amide of Asn140 is reduced to 2.8Å in MS436 from 3.0Å in MS435 and MS267. Taken together, these structural insights explain the detailed molecular basis for the potent binding affinity of MS436 superior to MS435 and MS267 as a selective BrD inhibitor for the BrD1 over the BrD2 of BRD4.
Activity of Diazobenzene Compounds against the Bromodomain Panel
We profiled four lead diazobenzene-based BET BrD inhibitors, MS435, MS436, MS267 and MS363 against a panel of bromodomains that represent different subgroups of the human bromodomain family (Table 3). All four inhibitors preferentially bind to the BrDs of BRD4 and BRD3 over the other BrDs tested. Notably, MS436 activity towards CBP BrD is four times better than the initial compound MS120. It is worth mentioning that these diazobenzene compounds have a different bromodomain activity profile from diazepine-based BrD inhibitors such as JQ1,6 MS417,10 and I-BET7. Specifically, MS267 showed binding affinity, Ki of <1.43 µM, <1.70 µM, and 2.26 µM for the BrDs of BRD7, BPTF and BAZ2b, respectively. MS435 showed Ki of <6.56 µM for SMARCA4 BrD. These unique activity profiles make them potentially useful probes for studies of these non-BET BrDs.
Table 3.
Lead BrD Inhibitors against a Panel of BrDs
| BrD Protein | 27 (MS436) Ki µM |
4 (MS435) Ki µM |
28 (MS267) Ki µM |
29 (MS363) Ki µM |
|---|---|---|---|---|
| BRD4-BrD1 | <0.085 | 0.91 | 0.15 | 0.17 |
| BRD4-BrD2 | 0.34 | 4.30 | 0.45 | 0.64 |
| BRD3-BrD1 | 0.10 | 1.64 | 0.29 | 0.24 |
| BRD3-BrD2 | 0.14 | 2.48 | 0.22 | 0.32 |
| CBP | 2.18 | 7.15 | 5.12 | 5.59 |
| PCAF | 5.52 | -- | <3.34 | <3.34 |
| BRD7 | 2.72 | 3.54 | <1.43 | <1.43 |
| BPTF | 6.06 | -- | <1.7 | 1.62 |
| BAZ2b | 3.29 | -- | 2.26 | 3.30 |
| SMARCA4 | 7.97 | <6.56 | <6.56 | <6.56 |
Physiochemical Properties of the Top Diazobenzene Bromodomain Inhibitors
We evaluated the physicochemical properties including CLogP, ligand efficiency (LE) and lipophilic ligand efficiency (LLE)19 of our top four diazobenzene-based bromodomain inhibitors, MS435, MS436, MS267 and MS363 to further understand their potential in cellular study. Of these, MS436 shows the best physiochemical properties (Table 4). As suggested in recent toxicology studies, compounds with CLogP <3 have much lower potential adverse effects in vivo.20, 21 MS436 has CLogP of 2.65, thus belonging to the low-risk category. MS436 also displays higher ligand efficiency than recently reported 3,5-dimethoxylisoxazole-based bromodomain inhibitors,14 striking an excellent balance between lipophilicity and potency. The lipophilic ligand efficiency of MS436 (LLE = 3.42) is also well above the other diazobenzene analogs and in line with the most probable LLE distribution (2–4) as reported in a recent statistical analysis of successful drug molecules.19
Table 4.
Physiochemical Properties of Lead BrD Inhibitors against BRD4-BrD1
| ID | Compound | Ki | p Ki | Ligand Efficiency |
CLogP | Lipophilic Ligand Efficiency |
|---|---|---|---|---|---|---|
| 27 | MS436 | <0.085 | 6.07 | 12.66 | 2.65 | 3.42 |
| 4 | MS435 | 0.91 | 5.04 | 10.5 | 4.26 | 0.78 |
| 28 | MS267 | 0.15 | 5.82 | 11.7 | 3.05 | 2.77 |
| 29 | MS363 | 0.17 | 5.77 | 11.6 | 3.17 | 2.60 |
Note: Ligand efficiency (LE) and lipophilic ligand efficiency (LLE) were calculated using the following equations: LE = ∆Gbinding/number of heavy atoms; LLE = pKi – ClogP, respectively19.
Activities of Diazobenzene Compounds in Murine Macrophage RAW264.7 Cells
These four lead diazobenzene bromodomain inhibitors exhibited little observable cytotoxicity on cell growth and proliferation, as determined in a MTT assay in murine macrophage RAW264.7 cells. As shown by this MTT study, the cell viability was fairly stable with these bromodomain inhibitors at concentrations up to 100 µM (Figure 3A). We next evaluated the cellular efficacy of these bromodomain inhibitors in blocking BRD4 functions in gene transcription. Nitric oxide synthase (NOS) catalyzes a stoichiometric production of nitric oxide (NO) from L-arginine in cells. NO production in macrophages is mediated by inducible nitric oxide synthase (iNOS), which is regulated by the NF-κB pathway.22 As shown in our recent study, NF-κB transcriptional activity for target gene activation is dependent upon its lysine-acetylation-mediated interactions with BRD4,10 which recruits the activated NF-κB to its target gene sites by binding to di-acetylated histone H4, particularly H4K5ac/K8ac. Indeed, we observed that treatment with the diazobenzene compounds in a dose-dependent manner blocked NF-κB-directed NO production in RAW264.7 cells upon LPS stimulation (Figure 3B). In agreement with their in vitro binding affinity to the BRD4 BrD1, MS436 showed more profound inhibitory activity on NO production than MS435. Consistent with the fact that the BET proteins are functionally vital for macrophage inflammatory responses,23 these diazobenzene bromodomain inhibitors effectively block LPS-induced pro-inflammatory cytokine interleukin-6 expression in the macrophage cells as illustrated in an ELISA assay (Figure 3C), of which MS436 and MS363 showed most profound inhibitory activity similar to their activity in the NO inhibition study. Taken together, these results demonstrate that these lead diazobenzene bromodomain inhibitors, particularly MS436 can effectively modulate BRD4 functions in gene transcriptional activation in cells.
Figure 3. Characterization of effects of the diazobenzene BrD inhibitors on BRD4 function in gene transcription.
(A) Effects of cell viability on the BrD inhibitors in murine macrophage RAW264.7 cells. Mitochondrial respiration, an indicator of cell viability, was assayed by the mitochondrial-dependent reduction of MTT to formazan. (B) Effects of the BrD inhibitors on LPS-induced ELISA in murine macrophage RAW264.7 cells. (C) Effects of the BrD inhibitors on LPS-induced nitric oxide (NO) production in murine macrophage RAW264.7 cells. (D) The estimated efficiency of the four lead BrD inhibitors in the cellular assays as described in B and C
CONCLUSION
In this study, we report our structure-guided development of a new class of diazobenzene-based small molecule inhibitors for the BET bromodomains. MS436 is the best inhibitor yielded from our extensive lead optimization using a combined medicinal chemistry and structure-activity relationship study. We improved the affinity of the diazobenzene compounds toward the BRD4 BrD1 by over 100 fold. MS436 has an estimated Ki of 30–50 nM for the BRD4 BrD1 with a 10-fold selectivity over the BrD2. These lead diazobenzene compounds possess preferable drug-like properties. We further demonstrated the cellular efficacy of four lead diazobenzene BrD inhibitors in inhibiting BRD4 transcriptional activity in LPS-activated, NK-κB-directed production of nitric oxide and pro-inflammatory cytokine IL-6 in murine macrophage RAW264.7 cells. To our knowledge, MS436 is the first low nanomolar small-molecule bromodomain inhibitor that is selective between the two structurally highly similar BrDs of BRD4. It compares favorably to the other recently reported BRD4 BrD inhibitors such as 3,4-dihydro-3methyl-2(1H)-quinazolinones24 and 3,5-dimethoxyl-isoxazole,14 which do not show selectivity between the two BrDs of BRD4.
Our detailed high-resolution crystal structural analysis of the BRD4 BrD1 bound to the lead diazobenzene ligands reveals that the residues in the acetyl-lysine binding pocket adapt rather rigid conformation, not influenced significantly upon binding to different small molecule ligands, or the acetylated-lysine in a histone peptide (see Figure 2). Surprisingly, we found that a set of structurally bound water molecules work together to direct ligand recognition through bridging multiple water-mediated hydrogen bonding interactions between the key, conserved residues and a bound ligand. It is interesting to note that through such versatile and cooperative activity, bound water molecules play a direct role in ligand recognition by the bromodomains. These observations likely reflect the dynamic and transient nature of acetyl-lysine binding by the bromodomains, which is necessarily for directing protein-protein interactions required for gene transcriptional activation on-demand and in an ordered fashion in the context of chromatin.
Unlike other bromodomains of the HAT co-activators PCAF and CBP/p300, the BET bromodomains distinctively prefer to interact with multi-lysine acetylation sites in histones and transcription-associated proteins. Growing evidence from our studies and the others suggest that the two bromodomains of BRD4 are engaged in different molecular functions in control of gene transcriptional activation in chromatin10, 12, 13. Specifically, the first bromodomain of BRD4 binds to hyper-acetylated histone H4 by recognizing H4K5ac/K8ac, a dual-acetylation mark that signals for gene activation, whereas the second bromodomain functions to recruit activated and lysine-acetylated transcription factors to target gene promoter and enhancer sites, as well as to bridge tri-acetylated cyclin T1 of p-TEFb to RNA polymerase II to establish an activated transcriptional machinery complex for productive transcriptional elongation. Our new diazobenzene-based bromodomain inhibitors that are potent and selective for these two BrDs of BRD4, particularly MS436 should facilitate further mechanistic investigation of specific functions of the two bromodomains of BRD4 in various physiological and pathophysiological contexts. Such studies are needed to safe-guide future development of novel and safe small molecule BRD4 inhibitors as new targeted epigenetic therapies to treat human disorders including cancer and inflammation.
MATERIALS AND METHODS
Protein Preparation
Expression and purification of the recombinant bromodomains of various transcriptional proteins in poly-His tag form were performed using a procedure described previously10. The protein was purified by affinity chromatography on a nickel-IDA column (Invitrogen), followed by the removal of poly-His tag by thrombin cleavage.
Fluorescence Anisotropy Binding Assay
Binding affinity of the newly synthesized diazobenzene compounds for various bromodoamins was assessed in a fluorescence anisotropy competition assay using a fluorescein isothiocyanate (FITC)-labeled MS417 as an assay probe as described previously10. Competition experiments were performed with a BrD protein (0.25–1 µM) and the fluorescent probe (80 nM), and increasing concentration of unlabeled competing ligand in a PBS buffer (pH 7.4) in total volume of 80 µL Measurements were obtained after a 1 hour incubation of the fluorescent ligand and the protein at 25°C with Safire 2 microplate reader (Tecan). In a competition-binding assay, fluorescent ligand concentration was ≤ 2Kd, and protein concentration was set at which 50–80% of fluorescent ligand is bound. Dissociation constant of a competing ligand was calculated with the correction to Cheng-Prussoff equation introduced by Nicolovska-Coleska and colleagues25. Assuming one-site competitive binding model, the equation used to calculate Ki’s from IC50 values recovered from fitting data using Prism:
where [I50] is the concentration of free inhibitor at 50% inhibition, [L50], the concentration of free labeled ligand at 50% inhibition, and [P0], concentration of free protein at 0% inhibition. Note that Kd for each protein-probe pair is the limit of resolvable Ki in a competition assay.
Protein Crystallization, X-ray Diffraction Data Collection and Structure Determination
Purified BRD4-BrD1 protein (14 mg/mL) was mixed with a diazobenzene BrD inhibitor at 1:10 molar ratio of protein:ligand. The complex was crystallized using the sitting drop vapor diffusion method at 20°C by mixing 1 µL of protein solution with 1 µL of the reservoir solution that contains 15–30% PEG 4000, 0.2 M MgCl2, 0.1 M Tris-HCl pH 8.5. Crystals were soaked in the corresponding mother liquor supplemented with 20% ethylene glycerol as cryoprotectant before freezing in liquid nitrogen. X-ray diffraction data were collected at 100K at beamline X6A of the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory. Data were processed using the HKL-2000 suite26. The BRD4-BD1 structure was solved by molecular replacement using the program MOLREP27, and the structure refinement was done using the program Refmac28. Graphics program COOT29 was used for model building and visualization. Crystal diffraction data and refinement statistics for the structure are displayed in Supplemental Table S2 (see Supplemental Information).
Cell Viability Study of Murine Macrophage Cells
Murine macrophage RAW264.7 cells were plated at a density of 1 × 104 cells per well in a 96-well plate and incubated at 37 °C for 18 h. The cells were then treated with the diazobenzene bromodomain inhibitors up to 100 µM for 24 hours. At the end of the 24 hr incubation, 10 µL of the MTT solution (4 mg/ml) was added to each well and incubated at 37°C for 4 h. The supernatants were then removed and the cells were solubilized in 100 µl of 100% DMSO. The diazobenzene compounds were first dissolved in DMSO then diluted with culture medium to concentrations that ranged from 0.28 to 50000 nM. The final concentration of DMSO was adjusted to 0.05% (v/v). The extent of the reduction was measured by the absorbance at 570/630 nm using EnVison 2104 Multilabel Reader (PerkinElmer, Inc., Waltham, MA).
Assessing LPS-induced IL-6 and Nitric Oxide Levels in Murine Macrophage Cells
Murine macrophage RAW264.7 cells were cultivated in DMEM (Hyclone, Logan, UT) supplemented with 10% FBS (Hyclone, Logan, UT) at 37°C in a humidified atmosphere of 5% CO2. Cells in 96-well plates (0.1 mL, 3 × 105 cells/mL) were treated with lead diazobenzene inhibitors. After 30 min, all supernatants were removed and cells were treated with LPS (1 µg/mL) (Sigma-Aldrich Chemical Co., St. Louis, MO) and lead diazobenzene inhibitors. After 24 h, the supernatant was collected and measured using mouse IL-6 ELISA assay kit (Thermo Scientific, Pittsburgh, PA). The lead diazobenzene bromodomain inhibitors were first dissolved in DMSO (Sigma-Aldrich Chemical Co., St. Louis, MO) then diluted with culture medium to concentrations that ranged from 0.28 to 50,000 nM. The final concentration of DMSO was adjusted to 0.05% (v/v). The assay was measured by an absorption reading at 570 nm using EnVison 2104 Multi-label Reader (PerkinElmer, Inc., Waltham, MA). For assessing LPS-induced nitric oxide release, the nitrite production was measured by spectrophotometry at 520 nm using EnVison 2104 Multilabel Reader (PerkinElmer, Inc., Waltham, MA). Each experiment was performed in triplicate and plotted using Prism 5.0 (GraphPad Software, Inc.). The curve-fitting equation used was “log(inhibitor) vs. response – variable slope (four parameters)”.
Chemical Synthesis
The core of the diazobenzene compounds was constructed by azo coupling30 of the diazonium chloride intermediate 2 with appropriately functionalized phenol (Scheme 1).31 For C ring diazo analogs, 4-aminobenzenesulfonyl chloride precursors were first coupled to functionalized aromatic amines. The protective groups of the aromatic amines were either unmasked by sodium hydroxide aqueous solution or reduced with iron in ammonium chloride aqueous solution. The key intermediate A was accessed by treating the anilines with glacial acetic acid and isoamyl nitrite sequentially. Subsequent azo coupling with 2,6-dimethylphenol in the presence of potassium carbonate gave a variety of C ring diazobenzene analogs. A ring diazobenzene analogs were accessed through a simpler procedure. Sulfapyridine was treated with concentrated hydrogen chloride, and the resulting diazonium chloride reacted readily with various phenols under basic conditions to give diversified A ring diazobenzene analogs (Scheme 2).
Scheme 1.
General synthetic scheme for C ring analogs of diazobenzene
Scheme 2.
General synthesis of A ring analogs of diazobenzene
All non-aqueous reactions were carried out in oven-dried glassware under an atmosphere of argon. All solvents were purchased in anhydrous from Acros Organics and used without further purification. Automatic chromatography was performed on a Biotage Isolera system equipped with a variable wavelength detector and a fraction collector, using Biotage SNAP cartridge KP-Sil 10 g. Analytical thin layer chromatography (TLC) was performed employing Sigma-Aldrich 250 µm 60F-254 silica plates. The plates were visualized either by exposure to UV light, staining with iodine impregnated silica gel, or by staining with ceric ammonium molybdate (CAM). Preparative TLC was performed employing Silicycle 1000 µm SiliaPlate Prep silica plates. LCMS analysis was conducted on an Agilent Technologies G1969A high-resolution API-TOF mass spectrometer attached to an Agilent Technologies 1200 HPLC system. Samples were ionized by electrospray ionization (ESI) in positive mode. Chromatography was performed on a 2.1 × 150 mm Zorbax 300SB-C18 5 µm column with water containing 0.1% formic acid as solvent A and acetonitrile containing 0.1% formic acid as solvent B at a flow rate of 0.4 mL/min. The gradient program was as follows: 1% B (0–1 min), 1–99% B (1–4 min), 99% B (4–8 min). The temperature of the column was held at 50 °C for the entire analysis. NMR spectra were acquired on a Bruker DRX-600 spectrometer at 600 MHz for 1H and 150 MHz for 13C. Chemical shifts are expressed in parts per million downfield from tetramethylsilane (TMS), using either TMS or the solvent resonance as an internal standard (TMS, 1H: 0 ppm; chloroform, 13C: 77.0 ppm; DMSO-d6, 1H: 2.50 ppm; 13C: 39.5 ppm; methanol-d4, 1H: 3.31 ppm; 13C: 49.0 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), integration, and coupling constant. IR spectra were obtained on a Bruker TENSOR 27 series FT-IR spectrometer equipped with a Diamond ATR. Melting point was measured on an OptiMelt automatic melting point system MPA100 from Stanford Research System.
General experimental procedures
(E)-5-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-2,4-dimethylbenzenesulfonic acid (1)
A 100 mL round bottom flask was charged with concentrated HCl (5 mL) and crushed ice (1 g) and cooled to 0 °C. To this flask was added 5-amino-2,4-xylene- sulfonic acid (230 mg, 1.15 mmol, 1.0 eq.), and NaNO2 (1 N, 1 mL, 1.15 mmol, 1 eq.). The mixture was stirred at 0 °C for 2 h. 5-amino-2-methylphenol (155 mg, 1.26 mmol, 1.2 eq.) was dissolved in 10% NaOH aq solution (20 mL, 50 eq.). The previously prepared yellow color diazonium ion was added drop wise under argon over 10 min. The pH of the solution was maintained between 8–10 with the addition of small amount of 10% NaOH aq. The solution was allowed to stir at 0 °C for 1h and quenched with 1 N HCl and adjusted to pH 7. Filtration provided the product as a fine red powder (335 mg, 95%). 1H NMR (600 MHz, DMSO-d6) δ 10.20 (br s, 1H), 7.97 (s, 1H), 7.48 (s, 1H), 7.11 (s, 1H), 6.41 (s, 1H), 2.51 (s, 3H), 2.48 (s, 3H), 2.07 (s, 3H); 13C NMR (150 MHz, MeOD-d4) δ = 155.5, 148.0, 144.2, 141.5, 139.4, 139.2, 138.6, 134.0, 119.3, 117.5, 116.8, 114.4, 19.6, 16.0, 15.9. MS calculated for C15H18N3O4S+ [M+H]+ 336.10, found 336.10. Purity >95%, tR = 4.4 min.
(E)-5-(3,5-dimethyl-4-hydroxyphenylazo)-2,4-dimethylbenzenesulfonic acid (2)
Following a similar procedure as compound 1, compound 2 was obtained as a yellowish solid (74%). 1H NMR (600 MHz, Methanol-d4) δ = 8.33 (s, 1H), 7.71 (s, 2H), 7.40 (s, 1H), 2.84 (s, 3H), 2.82 (s, 3H), 2.45 (s, 6H); 13C NMR (150 MHz, MeOD-d4) δ = 151.1, 143.4, 137.7, 135.1, 133.7, 132.6, 130.4, 129.5, 110.5, 108.5, 29.3, 22.6, 21.1. MS calculated for C16H19N2O4S+ [M+H]+ 335.11, found 335.13.
(E)-3-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (3)
A 250 mL round bottom flask was charged with 4-nitrobenzenesulfonyl chloride (500.0 mg, 2.26 mmol, 1.0 eq.) and was dissolved in DCM (40 mL). The solution was cooled down to 0 °C. 2-aminopyridine (212 mg, 2.26 mmol, 1.0 eq.) was dissolved in DCM (5 mL) and was added carefully at 0 °C dropwise over 20 min. After 1h, the solution was gradually warmed up to rt and stirred overnight. The reaction was quenched by water (30.0 mL). The organic layer was washed sequentially with water and brine and dried over MgSO4. The volatiles were removed under reduced pressure. The crude product was recrystallized in a mixture of DCM and MeOH. The pure product A appeared as a pale yellow powder (410.0 mg, 65%). A 100 mL pressure tube was charged with compound A (410.0 mg, 1.47 mmol, 1.0 eq.) and was dissolved 1:1 (v/v) ratio MeOH/H2O mixture (20 mL). To this mixture was added iron (240 mg, 4.33 mmol, 2.95 eq.) and NH4Cl aq. (400 mg, 7.34 mmol, 5.0 eq.). The mixture was heated up to 70 °C for 4h. The reaction mixture was cooled and filtered through a celite pad to remove the inorganic residues. The pad was washed (10 mL × 3) with acetone. The combined filtrate was concentrated in vacuo. DI water (40 mL) was added and the pH was adjusted to basic by the addition of NaHCO3. The solid was filtered and dried. The pure product B appeared as grey powder (250 mg, 68 %). A 100 mL round bottom flask was charged with B (100.0 mg, 0.41 mmol, 1.0 eq.) and concentrated HCl (0.16 mL, 0.41 mmol, 1.0 eq.). The mixture was dissolved in a MeOH/ACN mixture 1.5mL/1.5 mL. The reaction solution was stirred at 0 °C for 15 min. Isoamyl nitrite (55 µL, 0.41 mmol, 1.0 eq.) was added drop by drop under argon over 15 min. The bright yellow solution was stirred at 0 °C for 1 h and gradually warmed to rt for 1 h. 2,6-dimethylphenol (50.0 mg, 0.41 mmol, 1.0 eq.) and K2CO3 (280.6 mg, 2.03 mmol, 5.0 eq.) was premixed and deoxygenized by argon for 15 min. The previously prepared amber color diazonium ion C was added dropwise under argon. At the end of the addition, the pH of the solution was maintained between 8–10 using K2CO3. The solution was stirred at 0 °C for 1 h then rt overnight. The resultant solution was adjusted to pH 1. The solid were filtered and dried. The pure product 3 appeared as an orange powder (134.1 mg, 86%). 1H NMR (600 MHz, DMSO-d6) δ 9.29 (br s, 1H), 8.18 (s, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.97-7.92 (m, 2H), 7.76 (dt, J = 8.4, 1.5 Hz, 1H), 7.70 (t, J = 7.8 Hz, 1H), 7.59 (s, 2H), 7.20 (d, J = 9.0 Hz, 1H), 6.84 (t, J = 6.0 Hz, 1H), 2.24 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 160.9, 156.8, 155.2, 148.0, 146.9, 144.5, 133.5, 131.4, 130.8, 129.8, 128.3, 127.0, 122.3, 121.4, 117.7, 19.9. MS calculated for C19H19N4O3S+ [M+H]+ 383.11, found 383.11. Purity >99%, tR = 5.0 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (4, MS435)
Following a similar procedure as compound 3, compound 4 was prepared as an orange powder (80%). 1H NMR (600 MHz, DMSO-d6) δ 12.2 (br s, 1H), 9.33 (br s, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.98-7.93 (m, 2H), 7.87 (d, J = 8.4 Hz, 2H), 7.74 (t, J = 8.4 Hz, 1H), 7.57 (s, 2H), 7.20 (d, J = 9.0 Hz, 1H), 6.85 (t, J = 6.6 Hz, 1H), 2.14 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 158.3, 154.3, 154.0, 145.4, 143.7, 141.8, 141.6, 128.6, 125.6, 124.3, 122.8, 120.0, 114.8, 17.1. MS calculated for C19H19N4O3S+ [M+H]+ 383.11, found 383.11. Purity >99%, tR = 2.1 min (Chromatography was performed on a 2.1 × 50 mm Acquity BEH C-18 column using acetonitrile as solvent A and water containing 0.025% TFA as solvent B at a flow rate of 0.5 mL/min. The gradient program was as follows: 90% B (0–0.5 min), 90−10% B (0.5–3 min), 10% B (3–6 min).
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(4-(trifluoromethyl)benzyl)benzenesul-fonamide (5)
1H NMR (600 MHz, MeOD-d4) δ 5 7.93 (d, J = 8.4 Hz, 2H), 7.89 (d, J = 8.4 Hz, 2H), 7.62 (s, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 4.20 (s, 2H), 2.30 (s, 6H); 13C NMR (150 MHz, MeOD-d4) δ 160.5, 157.7, 148.6, 144.7, 144.0, 132.1, 131.9, 130.8, 130.5, 127.9, 127.6 (d), 126.6, 125.1, 48.7, 18.1. IR (neat): v 3498 (–OH), 3247 (-NH-), 1616 (–C=N–), 1591 (–N=N–), 1382 (–SO2–). MS calculated for C22H21F3N3O3S+ [M+H]+ 464.12, found 464.12. Purity >99%, tR = 6.5 min. Melting point: 195.7 °C.
The following compounds were synthesized following Scheme 1.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-methyl-N-(pyridin-2-yl)benzenesul- fonamide (6)
1H NMR (600 MHz, MeOD-d4) δ 8.16-8.12 (m, 1H), 8.10 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 8.4 Hz, 2H), 7.63 (s, 2H), 7.04 (t, J = 6.6 Hz, 1H), 6.86 (d, J = 7.2 Hz, 1H), 6.63 (t, J = 7.2 Hz, 1H), 2.30 (s, 6H), 2.18 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 161.1, 156.9, 155.6, 148.3, 146.7, 143.1, 140.9, 130.7, 127.8, 126.8, 122.1, 115.1, 114.1, 31.5, 18.1. MS calculated for C20H21N4O3S+ [M+H]+ 397.13, found 397.13. Yield: 31%, purity >99%, tR = 4.8 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(3-(trifluoromethyl)phenyl)benzenesul-fonamide (7)
1H NMR (600 MHz, CDCl3) δ 7.90 (m, 4H), 7.65 (s, 2H), 7.38 (m, 3H), 7.29 (br s, 1H), 6.82 (s, 1H), 5.10 (s, 1H), 2.34 (s, 6H). MS calculated for C21H19F3N3O3S+ [M+H]+ 450.11, found 450.11. Yield: 68%, purity >99%, tR = 6.5 min.
(E)-N-(4-chloropyridin-2-yl)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)benzenesulfon- amide (8)
1H NMR (600 MHz, DMSO-d6) δ 9.39 (br s, 1H), 8.18 (d, J = 1.8 Hz, 1H), 8.04 (d, J = 7.8 Hz, 2H), 7.89 (d, J = 7.8 Hz, 2H), 7.79 (dd, J = 9.0, 1.8 Hz, 1H), 7.56 (s, 2H), 7.08 (d, J = 9.0 Hz, 1H), 7.01 (d, J = 5.6 Hz, 1H), 2.23 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 161.2, 157.7, 153.2, 149.3, 148.2, 143.9, 141.7, 131.8, 128.3, 127.1, 125.6, 121.8, 116.7, 19.8. MS calculated for C19H18ClN4O3S+ [M+H]+ 417.08, found 417.08. Yield: 87%, purity >99%, tR = 5.3 min.
(E)-6-(4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)phenylsulfonamido)nicotinic acid (9)
1H NMR (600 MHz, DMSO-d6) δ 9.31 (br s, 1H), 8.24-8.14 (m, 2H), 8.03 (d, J = 8.4 Hz, 2H), 7.88 (d, J = 8.4 Hz, 2H), 7.72 (s, 1H), 7.57 (s, 2H), 7.50 (s, 1H), 7.27 (d, J = 4.8 Hz, 2H), 2.24 (s, 6H); 13C NMR (150 MHz, MeOD-d4) δ 169.0, 161.2, 157.3, 156.1, 148.1, 145.0, 131.4, 128.4, 127.1, 125.5, 122.3, 117.7, 114.8, 19.9. MS calculated for C20H18N4O5S [M]+ 426.11, found 426.11. Yield: 24%, purity >99%, tR = 5.1 min.
(E)-N-(5-chloropyridin-2-yl)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)benzenesulfon- amide (10)
1H NMR (600 MHz, DMSO-d6) δ 9.36 (br s, 1H), 8.03 (d, J = 7.8 Hz, 4H), 7.89 (d, J = 7.8 Hz, 2H), 7.57 (s, 2H), 7.16 (s, 1H), 7.01 (d, J = 5.6 Hz, 1H), 2.24 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 161.1, 157.4, 156.6, 149.2, 148.2, 145.1, 131.7, 131.3, 128.3, 127.1, 125.6, 119.9, 115.8, 19.8. MS calculated for C19H18ClN4O3S+ [M+H]+ 417.08, found 417.07. Yield: 87%, purity >99%, tR = 5.3 min.
(E)-N-(5-bromopyridin-2-yl)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)benzenesulfon-amide (11)
1H NMR (600 MHz, MeOD-d4) δ 7.89-7.80 (m, 4H), 7.58 (s, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.04 (d, J = 8.4 Hz, 2H), 2.27 (s, 6H); 13C NMR (150 MHz, MeOD-d4) δ 160.6, 157.9, 148.5, 142.6, 139.6, 134.6, 130.7, 127.5, 126.6, 125.5, 125.0, 120.2, 18.1. MS calculated for C19H18BrN4O3S+ [M+H]+ 462.03, found 462.00. Yield: 55%, purity >99%, tR = 5.6 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(5-methoxypyridin-2-yl)benzenesulfon- amide (12)
1H NMR (600 MHz, DMSO-d6) δ 10.85 (s, 1H), 9.35 (s, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.92-7.85 (m, 3H), 7.59 (s, 2H), 7.37 (m, 1H), 7.11 (m, 1H), 3.73 (s, 3H), 2.26 (s, 6H). MS calculated for C20H21N4O4S+ [M+H]+ 413.13, found 413.12. Yield: 57%, purity >99%, tR = 6.2 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(4-methoxyphenyl)benzenesulfonamide (13)
1H NMR (600 MHz, CDCl3) δ 7.86 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H), 7.65 (s, 2H), 6.98 (d, J = 9.0 Hz, 2H), 6.77 (d, J = 9.0 Hz, 1H), 6.28 (s, 1H), 5.10 (s, 1H), 3.76 (s, 3H), 2.34 (s, 6H). MS calculated for C21H22N3O4S+ [M+H]+ 412.13, found 412.13. Yield: 57%, purity >99%, tR = 6.3 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-N-(4-(trifluoromethyl)phenyl)benzenesul-fonamide (14)
1H NMR (600 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.38 (s, 1H), 7.98 (d, J = 8.4 Hz, 2H), 7.92 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.59 (s, 2H), 7.31 (d, J = 8.4 Hz, 2H), 2.26 (s, 6H). MS calculated for C21H19F3N3O3S+ [M+H]+ 450.11, found 450.13. Purity >95%, tR = 6.5 min.
(E)-4-(4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)phenylsulfonamido)benzamide (15)
1H NMR (600 MHz, DMSO-d6) δ 10.81 (br s, 1H), 8.36 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.84-7.77 (m, 2H), 7.75-7.66 (m, 2H), 7.28-7.20 (m, 2H), 7.14 (d, J = 8.4 Hz, 2H), 2.48 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 170.3, 160.0, 153.0, 149.5, 143.5, 142.6, 132.8, 132.0, 131.3, 130.9, 128.6, 126.8, 122.1, 121.8, 16.5. MS calculated for C21H21N4O4S+ [M+H]+ 425.13, found 425.14. Yield: 24%, purity >99%, tR = 5.4 min.
(E)-N-(5-fluoropyridin-2-yl)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)benzenesulfon- amide (16)
1H NMR (600 MHz, DMSO-d6) δ 9.47 (br s, 1H), 8.15 (d, J = 2.4 Hz, 1H), 8.02 (d, J = 7.8 Hz, 2H), 7.88 (d, J = 7.8 Hz, 2H), 7.64 (dt, J = 7.8, 2.4 Hz, 1H), 7.56 (s, 2H), 7.11 (dd, J = 7.8, 2.4 Hz, 1H), 2.23 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 161.2, 159.9, 158.3, 157.6, 150.9, 148.1, 144.0, 131.7, 129.4, 128.3, 127.1, 125.6, 117.1, 19.8. MS calculated for C19H18FN4O3S+ [M+H]+ 401.11, found 401.08. Yield: 78%, purity >99%, tR = 5.3 min.
(E)-N-(4-fluorophenyl)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)benzenesulfonamide (17)
1H NMR (600 MHz, CDCl3) δ 10.31 (br s, 1H), 9.40 (br s, 1H), 8.00–7.79 (m, 4H), 7.59 (s, 2H), 7.21-7.07 (m, 4H), 2.25 (s, 6H). MS calculated for C20H19FN3O3S+ [M+H]+ 400.11, found 400.12. Yield: 76%, purity >99%, tR = 6.4 min.
(E)-4-((4-hydroxy-3,5-dimethylphenyl)diazenyl)-2-methoxy-N-(pyridin-2-yl)benzenesulfon-amide (18)
1H NMR (600 MHz, DMSO-d6) δ 9.31 (br s, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.96 (d, J = 4.8 Hz, 1H), 7.70 (t, J = 7.8 Hz, 1H), 7.58 (s, 2H), 7.47-7.41 (m, 2H), 7.18 (d, J = 8.4 Hz, 1H), 6.85 (t, J = 6.6 Hz, 1H), 3.8 (s, 3H), 2.24 (s, 6H); 13C NMR (150 MHz, DMSO-d6) δ 160.6, 158.7, 156.6 (m), 148.0, 143.2, 140.9, 133.9, 131.4, 128.4, 127.1, 122.3, 117.5, 117.0, 114.9, 114.1, 31.5, 18.1. MS calculated for C20H21N4O4S+ [M+H]+ 413.13, found 413.13. Yield: 31%, purity >99%, tR = 4.8 min.
The following compounds were synthesized following Scheme 2.
(E)-4-((3,5-dichloro-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (19)
1H NMR (600 MHz, MeOD-d4) δ 8.08 (d, J = 8.4 Hz, 1H), 8.02–7.94 (m, 3H), 7.93 (s, 2H), 7.74 (t, J = 7.8 Hz, 2H), 7.30 (d, J = 9.0 Hz, 1H), 6.89 (t, J = 6.6 Hz, 1H); 13C NMR (150 MHz, DMSO-d6) δ 158.0, 156.5, 156.0, 146.7, 146.5, 142.9, 142.5, 131.3, 131.0, 126.8 (m), 125.9, 118.1, 115.7. MS calculated for C17H13Cl2N4O3S+ [M+H]+ 423.01, 425.01, found 423.01, 425.01. Yield: 75%, purity >95%, tR = 5.9 min.
(E)-4-((3-bromo-4-hydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (20)
1H NMR (600 MHz, DMSO-d6) δ 10.12 (s, 1H), 8.13-7.98 (m, 3H), 7.97-7.87 (m, 3H), 7.83-7.72 (m, 2H), 7.35-7.10 (m, 1H), 6.95-6.75 (m, 1H), 2.34 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 158.9, 157.8, 156.6, 153.9, 148.6, 147.2, 144.5, 132.6, 131.0, 130.9, 130.3, 128.3, 125.8, 124.9, 124.5, 123.4, 117.7, 115.8, 20.1. MS calculated for C18H16BrN4O3S+ [M+H]+ 447.01 and 449.01, found 446.99, 448.98. Yield: 94%, purity >99%, tR = 6.0 min.
(E)-4-((3-chloro-4-hydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (21)
1H NMR (600 MHz, DMSO-d6) δ 10.22 (s, 1H), 9.08 (s, 1H), 8.02 (d, J = 8.4 Hz, 2H), 8.00–7.94 (m, 1H), 7.91 (d, J = 8.4 Hz, 2H), 7.79-7.68 (m, 3H), 7.21 (d, J = 8.4 Hz, 1H), 6.85 (t, J = 6.6 Hz, 1H), 2.30 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 157.9, 156.6, 153.9, 148.1, 147.2, 144.4, 132.6, 131.0, 130.9, 130.3, 128.3, 125.8, 124.9, 124.5, 123.4, 117.7, 115.8, 20.1. MS calculated for C18H16ClN4O3S+ [M+H]+ 403.06, found 403.05. Purity >99%, tR = 6.0 min.
(E)-4-((3-bromo-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (22)
1H NMR (600 MHz, DMSO-d6) δ 8.10 (s, 1H), 8.00–7.93 (m, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.82 (d, J = 9.0 Hz, 1H), 7.73 (t, J = 7.2 Hz, 2H), 7.29 (d, J = 8.4 Hz, 1H), 7.00 (d, J = 9.0 Hz, 1H), 6.88 (t, J = 6.0 Hz, 1H); 13C NMR (150 MHz, MeOD-d4) δ 158.8, 154.1, 153.9, 145.8, 144.2, 141.7, 128.3, 126.9, 126.3, 123.1, 117.0, 114.9 (br), 111.2. MS calculated for C17H14BrN4O3S+ [M+H]+ 432.99 and 434.99, found 432.99 and 434.99. Yield: 80%, purity >95%, tR = 5.9 min.
(E)-4-((3-chloro-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (23)
1H NMR (600 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.09 (d, J = 8.4 Hz, 1H), 8.03 (d, J = 7.8 Hz, 2H), 7.95-7.89 (m, 3H), 7.87-7.81 (m, 2H), 7.77 (m, 1H), 7.24 (d, J = 9.0 Hz, 1H), 7.20 (d, J = 9.0 Hz, 1H), 6.86 (s, 1H). MS calculated for C17H14ClN4O3S+ [M+H]+ 389.05, found 389.05. Yield: 100%, purity >99%, tR = 5.9 min.
(E)-4-((4-hydroxy-3-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (24)
1H NMR (600 MHz, MeOD-d4) δ 8.05 (d, J = 8.4 Hz, 2H), 7.95 (d, J = 5.3 Hz, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.77-7.70 (m, 2H), 7.68 (d, J = 8.4 Hz, 1H), 7.29 (d, J = 8.7 Hz, 1H), 6.94-6.87 (m, 2H), 2.26 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 162.0, 156.6, 155.9, 147.9, 144.9, 144.0, 143.0, 129.9, 127.5, 127.3, 125.7, 124.3, 117.4, 116.8, 116.7, 17.3. IR (neat): v 3326 (−OH), 1641 (−C=N−), 1593 (−N=N−), 1396 (−SO2−). MS calculated for C18H17N4O3S+ [M+H]+ 369.10, found 369.11. Purity >99%, tR = 5.7 min. Melting point: 248.1 °C.
(E)-4-((3,5-dibromo-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (25)
1H NMR (600 MHz, MeOD-d4) δ 8.12 (s, 1H), 8.08 (d, J = 8.4 Hz, 2H), 8.00–7.92 (m, 4H), 7.74 (t, J = 7.8 Hz, 2H), 7.30 (d, J = 9.0 Hz, 2H), 6.85 (t, J = 6.6 Hz, 1H); 13C NMR (150 MHz, DMSO-d6) δ169.6, 157.8, 157.2, 146.1, 143.0, 141.3, 140.8, 139.8, 131.3, 130.7, 130.4, 126.2, 124.0, 117.9, 115.5. MS calculated for C17H13Br2N4O4S+ [M+H]+ 510.90, 512.90, 514.90, found 510.90, 512.90, 514.90. Yield: 47%, purity >99%, tR = 6.0 min.
(E)-4-((4-hydroxy-3-(trifluoromethyl)phenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (26)
1H NMR (600 MHz, MeOD-d4) δ 8.17-8.13 (m, 1H), 8.08 (d, J = 9.0 Hz, 2H), 8.05 (d, J = 8.4 Hz, 1H), 8.03–7.95 (m, 3H), 7.74 (t, J = 7.8 Hz, 1H), 7.30 (d, J = 8.4 Hz, 2H), 7.09(d, J = 9.0 Hz, 1H), 6.88 (t, J = 6.6 Hz, 1H). MS calculated for C18H14F3N4O3S+ [M+H]+ 423.07, found 423.08. Yield: 39%, purity >99%, tR = 5.8 min.
(E)-4-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (27, MS436)
1H NMR (600 MHz, DMSO-d6) δ 10.28 (br s, 1H), 7.98 (m, 1H), 7.90 (d, J =8.4 Hz, 2H), 7.82 (d, J = 8.4 Hz, 2H), 7.71 (t, J = 7.2 Hz, 2H), 7.37 (s, 1H), 7.17 (d, J = 8.4 Hz, 1H), 7.11 (br s, 2H), 6.85 (t, J = 6.0 Hz, 1H), 6.22 (s, 1H), 2.00 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 162.0, 155.4, 153.7, 147.0, 141.0, 138.2, 133.2, 131.3, 128.7, 128.2, 121.9, 115.4, 114.4, 103.4, 100.4, 15.7. MS calculated for C18H18N5O3S+ [M+H]+ 384.11, found 384.12. Yield: 87%, purity >99%, tR = 5.6 min.
(E)-4-((2-amino-4-hydroxy-3,5-dimethylphenyl)diazenyl)N-(pyridin-2-yl)benzenesulfon- amide (28, MS267)
1H NMR (600 MHz, DMSO-d6) δ 8.01 (d, J = 4.8 Hz, 1H), 7.85 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 8.4 Hz, 2H), 7.70 (t, J = 7.8 Hz, 1H), 7.46 (s, 1H), 7.15 (d, J = 7.8 Hz, 1H), 6.86 (t, J = 6.0 Hz, 1H), 3.90-3.70 (m, 4H), 2.00 (s, 3H), 1.89 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 156.2, 154.9 (br), 153.0 (br), 146.8, 143.5, 140.1 (br), 134.7, 131.2, 122.9, 120.7, 119.0, 116.8, 116.4, 109.9, 19.9, 12.7. MS calculated for C19H20N5O3S+ [M+H]+ 398.13, found 398.12. Yield: 89%, purity >99%, tR = 5.4 min.
(E)-4-((2-amino-3-chloro-4-hydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesul-fonamide (29, MS363)
A 50 mL round bottom flask was charged with sulfapyridine (100.0 mg, 0.40 mmol, 1.0 eq.) and concentrated HCl (87.5 mg, 160 µL, 2.40 mmol, 5.98 eq.). The mixture was dissolved in a MeOH/ACN mixture (3 mL/3 mL). The solution was cooled to 0 °C and stirred for 15 min. Isoamyl nitrite (47.0 mg, 54 µL, 0.40 mmol, 1.0 eq.) was added drop by drop under argon over 10 min. The solution was stirred at 0 °C for 45 min. Meanwhile, another 50 mL round bottom flask 3-amino-2-chloro-6-cresol (63.0 mg, 0.40 mmol, 1.0 eq.) and potassium carbonate (276.3 mg, 2.0 mmol, 5.0 eq.). To this mixture was added methanol (1.0 mL) and DI H2O (8.0 mL). The solution was deoxygenated for 15 min by argon. The resultant solution was cooled to 0 °C. The previously prepared amber color diazonium ion was added drop wise under argon over 15 min. At the end of the addition, the pH of the solution was maintained between 8−10. The solution was allowed to stir at 0 °C for 1 h and then quenched with 1 N HCl to reach pH 1. The product was filtered and dried under vacuum. The pure product appeared as a fine red powder (167.0 mg, 99%). 1H NMR (600 MHz, DMSO-d6) δ 11.51 (s, 1H), 8.04 (s, 1H), 7.97-7.78 (m, 3H), 7.78-7.62 (m, 3H), 7.53 (s, 1H), 7.15 (s, 1H), 6.89 (s, 1H), 6.73 (br s, 2H), 1.98 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 158.1, 156.1, 155.4, 146.8, 146.3, 143.3, 142.0, 133.5, 132.0, 131.3, 130.6, 119.0, 116.7, 115.9, 115.7, 115.4, 107.4, 19.7. MS calculated for C18H17ClN5O3S+ [M+H]+ 418.07, found 418.08. Purity >99%, tR = 5.5 min.
(E)-4-((2-amino-4-hydroxy-5-methoxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfon-amide (30)
1H NMR (600 MHz, MeOD-d4) δ 8.00 (d, J = 4.8 Hz, 1H), 7.88 (d, J = 8.4 Hz, 2H), 7.66 (dt, J = 7.2 Hz, 1.2, 2H), 7.55 (d, J = 8.4 Hz, 2H), 7.20-7.15 (m, 1H), 6.93 (t, J = 6.6 Hz, 1H), 6.89-6.84 (m, 2H), 6.76 (s, 1H), 3.84 (s, 3H). MS calculated for C18H18N5O4S+ [M+H]+ 400.11, found 400.11. Yield: 15%, purity >99%, tR = 5.0 min.
(E)-4-((5-hydroxy-4,6-dimethylpyridin-2-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (31)
1H NMR (600 MHz, MeOD-d4): δ 8.11 (d, J = 8.5 Hz, 2H), 8.04 (d, J = 8.4 Hz, 2H), 7.94 (d, J = 5.3 Hz, 1H), 7.78-7.74 (m, 2H), 7.32 (d, J = 8.8 Hz, 1H), 6.89 (t, J = 6.4 Hz, 1H), 2.59 (s, 3H), 2.39 (s, 3H). HRMS calculated for C18H18N5O3S+ [M+H]+ 384.113, found 384.112. Purity >96%, tR = 4.3 min.
(E)-4-((5-hydroxy-3,4,6-trimethylpyridin-2-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfon- amide (32)
1H NMR (600 MHz, MeOD-d4): δ 8.11 (d, J = 8.2 Hz, 2H), 8.01 (d, J = 4.9 Hz, 2H), 7.94 (d, J = 5.3 Hz, 1H), 7.75 (t, J = 7.9 Hz, 1H), 7.31 (d, J = 8.8 Hz, 1H), 6.89 (t, J = 6.4 Hz, 1H), 2.70 (s, 3H), 2.62 (s, 3H), 2.38 (s, 3H). HRMS calculated for C19H20N5O3S+ [M+H]+ 398.128, found 398.127. Purity >90%, tR = 4.7 min.
(E)-4-((3,4-dihydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (33)
1H NMR (600 MHz, DMSO-d6) δ 10.25 (s, 1H), 9.95 (s, 1H), 8.70 (s, 1H), 8.20-7.95 (m, 3H), 7.90 (d, J = 8.4 Hz, 2H), 7.82-7.70 (m, 2H), 7.30-7.13 (m, 2H), 6.98-6.80 (m, 1H), 2.22 (s, 3H). MS calculated for C18H17N4O4S+ [M+H]+ 385.10, found 389.05. Yield: 79%, purity >99%, tR = 5.5 min.
(E)-4-((3,4-dihydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (34)
1H NMR (600 MHz, DMSO-d6) δ 8.11-7.96 (m, 3H), 7.87 (d, J = 7.8 Hz, 2H), 7.77-7.70 (m, 2H), 7.39 (d, J = 8.4 Hz, 1H), 7.34 (s, 1H), 7.27-7.10 (m, 2H), 6.93 (d, J = 8.4 Hz, 1H), 6.91-6.80 (m, 2H). MS calculated for C17H15N4O4S+ [M+H]+ 371.08, found 371.10. Yield: 65%, purity >99%, tR = 5.4 min.
(E)-4-((4-hydroxy-3-methylphenyl)diazenyl)-N-(4-(trifluoromethyl) benzyl)benzenesulfon- amide
(35). 1H NMR (600 MHz, MeOD-d4) δ 7.94 (d, J = 8.4 Hz, 2H), 7.91 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 1.9 Hz, 1H), 7.70 (dd, J = 7.8, 1.9 Hz, 1H), 7.54 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 7.8 Hz, 1H), 4.21 (s, 2H), 2.28 (s, 3H); 13C NMR (150 MHz, MeOD-d4) δ 162.7, 157.7, 148.7, 144.6, 144.0, 132.1, 131.9, 130.8, 130.5, 128.2, 128.0, 127.6, 126.3, 125.1, 117.2, 48.7, 17.6. IR (neat): v 3442 (−OH), 3268 (-NH-), 1615 (−C=N−), 1591 (−N=N−), 1377 (−SO2−). MS calculated for C21H19F3N3O3S+ [M+H]+ 450.11, found 450.11. Purity >99%, tR = 6.5 min. Melting point: 181.8 °C.
(E)-4-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-N-(4-(trifluoromethyl)benzyl)benzene sulfonamide (36)
1H NMR (600 MHz, DMSO-d6) δ 10.22 (s, 1H), 8.30 (t, J = 3.9 Hz, 1H), 7.90-7.80 (m, 4H), 7.64 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.41 (s, 1H), 7.19 (br s, 2H), 6.23 (s, 1H), 4.17 (d, J = 3.9 Hz, 2H), 2.02 (s, 3H). IR (neat): v 3479 (−OH), 3233 (−NH−), 1631 (−C=N−), 1596 (−N=N−), 1389 (−SO2−). MS calculated for C21H20F3N4O3S+ [M+H]+ 465.12, found 465.12. Purity >99%, tR = 6.3 min. Melting point: 171.7 °C.
(E)-4-((4-methoxy-3,5-dimethylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (37)
1H NMR (600 MHz, DMSO-d6) δ 8.10-7.96 (m, 2H), 7.92-7.85 (m, 2H), 7.80-7.68 (m, 1H), 7.16-7.02 (m, 2H), 6.80-6.68 (m, 1H), 4.11 (s, 3H), 2.13 (s, 6H). MS calculated for C20H21N4O3S+ [M+H]+ 397.13, found 397.15. Yield: 3%, purity >99%, tR = 6.2 min.
(E)-N-(2,6-dimethyl-4-((4-(N-(pyridin-2-yl)sulfamoyl)phenyl)diazenyl)phenyl)acetamide (38)
1H NMR (600 MHz, DMSO-d6) δ 9.23 (s, 1H), 7.85-7.67 (m, 2H), 7.67-7.30 (m, 3H), 7.20-6.90 (m, 1H), 6.85-6.60 (m, 1H), 2.12 (s, 6H), 2.03 (s, 3H). MS calculated for C21H22N5O3S+ [M+H]+ 446.14, found 446.13. Yield: 5%, purity >99%, tR = 5.0 min.
(E)-4-((4-hydroxy-3-isopropylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (39)
1H NMR (600 MHz, DMSO-d6) δ 10.46 (s, 1H), 8.08-7.99 (m, 3H), 7.91 (d, J = 7.8 Hz, 2H), 7.76 (m, 2H), 7.67 (d, J = 8.4 Hz, 2H), 7.22 (m, 1H), 6.98 (d, J = 8.4 Hz, 1H), 6.86 (m, 1H), 3.25 (m, 1H), 1.22 (d, J = 7.2 Hz, 6H). MS calculated for C20H21N4O3S+ [M+H]+ 397.13, found 397.13. Yield: 24%, purity >99%, tR = 6.0 min.
(E)-4-((3-(tert-butyl)-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (40)
1H NMR (600 MHz, DMSO-d6) δ 10.51 (s, 1H), 8.20-7.97 (m, 2H), 7.90 (d, J = 8.4 Hz, 2H), 7.84-7.65 (m, 4H), 7.40-7.10 (m, 1H), 6.98 (d, J = 8.4 Hz, 1H), 1.40 (s, 9H). MS calculated for C21H23N4O3S+ [M+H]+ 411.15, found 411.14. Yield: 20%, purity >99%, tR = 6.2 min.
(E)-4-((4-hydroxy-3,5-diisopropylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (41)
1H NMR (600MHz, MeOD-d4) δ 8.07 (d, J = 8.4 Hz, 2H), 8.01–7.96 (m, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.74 (t, J = 7.8 Hz, 2H), 7.70 (s, 2H), 7.30 (d, J = 8.4 Hz, 2H), 6.89 (t, J = 6.6 Hz, 1H), 3.45-3.60 (m, 2H), 1.28 (d, J = 7.2 Hz, 12H). MS calculated for C23H27N4O3S+ [M+H]+ 439.18, found 439.22. Yield: 16%, purity >99%, tR = 6.3 min.
(E)-4-((3,5-di-tert-butyl-4-hydroxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (42)
1H NMR (600 MHz, MeOD-d6) δ 8.01 (d, J = 6.6 Hz, 2H), 7.95 (d, J = 8.4 Hz, 1H), 7.73 (s, 2H), 7.57 (t, J = 7.2 Hz, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 9.0 Hz, 2H), 6.89 (t, J = 6.6 Hz, 1H), 1.37 (s, 9H), 1.31 (s, 9H); 13C NMR (150 MHz, MeOD-d4) δ 156.6, 156.4, 153.6, 144.6, 144.5, 143.6, 136.6, 135.0, 131.3, 129.3, 126.8 (m), 125.9, 118.0, 117.5, 116.5, 37.1, 31.5, 31.3. MS calculated for C25H31N4O3S+ [M+H]+ 467.21, found 467.21. Yield: 2%, purity >99%, tR = 6.8 min.
(E)-4-((4-hydroxynaphthalen-1-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (43)
1H NMR (600 MHz, DMSO-d6) δ 10.13 (s, 1H), 8.96-8.88 (m, 1H), 8.55-8.43 (m, 1H), 8.37-8.20 (m, 1H), 8.16-8.00 (m, 2H), 7.99-7.84 (m, 1H), 7.83-7.70 (m, 2H), 7.69-7.54 (m, 1H), 7.53-7.40 (m, 1H), 7.40-7.28 (m, 1H), 7.27-6.99 (m, 1H), 6.97-6.69 (m, 1H), 5.75 (s, 1H). MS calculated for C21H17N4O3S+ [M+H]+ 405.10, found 405.12. Yield: 67%, purity >99%, tR = 5.0 min.
(E)-4-((4-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl)diazenyl)-N-(pyridin-2-yl)benzene sulfonamide (44)
1H NMR (600 MHz, MeOD-d4): δ 8.04 (d, J = 8.6 Hz, 2H), 7.95 (d, J = 5.1 Hz, 1H), 7.87 (d, J = 8.5 Hz, 2H), 7.73 (td, J = 8.0, 1.9 Hz, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 6.88 (t, J = 6.4 Hz, 1H), 6.65 (d, J = 8.8 Hz, 1H), 3.31-3.25 (m, 2H), 2.99 (s, 1H), 2.86 (s, 1H), 2.73-2.67 (m, 2H), 1.89-1.83 (m, 4H). HRMS calculated for C21H21N4O3S+ [M+H]+ 409.133, found 409.129. Purity >99%, tR = 5.1 min.
(E)-4-((5-hydroxy-1,2,3,4-tetrahydroquinolin-8-yl)diazenyl)-N-(pyridin-2-yl)benzene sulfonamide (45)
1H NMR (600 MHz, DMSO-d6): δ 8.03–7.95 (m, 1H), 7.87 (d, J = 8.5 Hz, 2H), 7.71 (td, J = 8.1, 1.9 Hz, 1H), 7.61 (d, J = 8.1 Hz, 2H), 7.15 (d, J = 7.9 Hz, 1H), 7.04 (d, J = 8.9 Hz, 1H), 6.86 (t, J = 6.4 Hz, 1H), 6.30 (d, J = 8.9 Hz, 1H), 3.32-3.24 (m, 2H), 3.16-3.14 (m, 2H), 1.76 (qt, J = 6.6 Hz, 2H). HRMS calculated for C20H20N5O3S+ [M+H]+ 410.128, found 410.126. Purity >96%, tR = 4.7 min.
(E)-4-((7-hydroxy-2,2-dimethyl-2,3-dihydrobenzofuran-4-yl)diazenyl)-N-(pyridin-2-yl)benzene sulfonamide (46)
1H NMR (600 MHz, MeOD-d4): δ 8.05 (d, J = 8.5 Hz, 2H), 7.95 (d, J = 4.8 Hz, 1H), 7.88 (d, J = 8.5 Hz, 2H), 7.74 (td, J = 8.1, 1.7 Hz, 1H), 7.37 (d, J = 8.6 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 6.89 (t, J = 6.4 Hz, 1H), 6.80 (d, J = 8.6 Hz, 1H), 3.42 (s, 2H), 1.52 (s, 6H). HRMS calculated for C21H21N4O4S+ [M+H]+ 425.128, found 425.124. Purity >99%, tR = 4.8 min.
(E)-4-((5-hydroxy-3-(hydroxymethyl)-4,6-dimethylpyridin-2-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (47)
1H NMR (600 MHz, MeOD-d4): δ 8.10 (d, J = 8.5 Hz, 2H), 8.03 (d, J = 8.4 Hz, 2H), 7.95 (d, J = 5.1 Hz, 1H), 7.76 (ddd, J = 8.9, 7.2, 1.8 Hz, 1H), 7.31 (d, J = 8.9 Hz, 1H), 6.89 (t, J = 6.5 Hz, 1H), 5.18 (d, J = 0.8 Hz, 2H), 2.57 (s, 3H), 2.46 (s, 3H). HRMS calculated for C19H20N5O4S+ [M+H]+ 414.123, found 414.121. Purity >95%, tR = 4.0 min.
(E)-4-((4-(aminomethyl)-5-hydroxy-3-(hydroxymethyl)-6-methylpyridin-2-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (48)
1H NMR (600 MHz, MeOD-d4): δ 8.04 (d, J = 7.1 Hz, 2H), 7.96 (d, J = 5.0 Hz, 1H), 7.92-7.79 (m, 2H), 7.74 (td, J = 8.0, 1.9 Hz, 1H), 7.28 (d, J = 8.7 Hz, 1H), 6.89 (t, J = 6.4 Hz, 1H), 5.17 (s, 2H), 4.28 (s, 2H), 2.51 (s, 3H). HRMS calculated for C19H21N6O4S+ [M+H]+ 429.135, found 429.136. Purity >95%, tR = 2.2 min.
(E)-4-((4-formyl-5-hydroxy-3-(hydroxymethyl)-6-methylpyridin-2-yl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (49)
1H NMR (600 MHz, DMSO-d6): δ 7.99 (d, J = 4.4 Hz, 1H), 7.85 (d, J = 8.6 Hz, 2H), 7.64 (dd, J = 17.8, 8.0 Hz, 3H), 7.08 (d, J = 8.5 Hz, 1H), 6.79 (t, J = 5.8 Hz, 1H), 6.11 (s, 2H), 5.14 (d, J = 15.1 Hz, 1H), 4.95 (d, J = 15.2 Hz, 1H), 2.23 (s, 3H). HRMS calculated for C19H18N5O5S+ [M+H]+ 428.103, found 428.105. Purity >96%, tR = 4.0 min.
(E)-4-((4-hydroxy-3-methoxyphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (50)
1H NMR (600 MHz, DMSO-d6) δ 10.19 (s, 1H), 8.09-7.95 (m, 3H), 7.92 (d, J = 9.0 Hz, 2H), 7.77 (t, J = 6.0 Hz, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.46 (s, 1H), 7.24 (d, J = 9.0 Hz, 1H), 7.22 (m, 1H), 7.00 (d, J = 8.4 Hz, 1H), 6.87 (s, 1H), 3.83 (s, 3H). MS calculated for C18H17N4O4S+ [M+H]+ 385.10, found 385.05. Yield: 60%, purity >99%, tR = 5.9 min.
(E)-3-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-N-(pyridin-2-yl)benzenesulfonamide (51)
1H NMR (600 MHz, DMSO-d6) δ 8.15 (s, 1H), 8.04–7.92 (m, 2H), 7.76 (d, J =7.8 Hz, 1H), 7.73 (t, J = 7.8 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.44 (s, 1H), 7.20 (d, J = 8.4 Hz, 1H), 6.84 (t, J = 6.0 Hz, 1H), 6.29 (s, 1H), 2.02 (s, 3H); 13C NMR (150 MHz, DMSO-d6) δ 165.6, 156.6, 154.9, 149.9, 146.5, 144.2, 140.1, 133.8, 133.1, 131.1, 128.6, 127.8, 121.9, 119.9, 118.3, 117.5, 103.5, 18.5. MS calculated for C18H18N5O3S+ [M+H]+ 384.11, found 384.14. Yield: 86%, purity >99%, tR = 4.7 min.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the use of the NMR facility at the New York Structural Biology Center and wish to thank the staff at the X6A beamline of the National Synchrotron Light Sources at the Brookhaven National Laboratory for facilitating X-ray data collection. This work was supported in part by research grants from the National Institutes of Health (to M.-M.Z.).
ABBREVIATIONS
- BrD
bromodomain
- NOS
nitric oxide synthase
- iNOS
inducible nitric oxide synthase
Footnotes
Supporting Information
Supporting Information includes 2 tables can be found with this article online. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
G.T.Z. and M.-M. Z. conceived and designed the experiments for this project. G.T.Z. and M.O. designed the synthetic schemes, and G.T.Z performed the chemical synthesis. J.J. prepared the protein samples and carried out crystallization. A.N.P. solved the crystal structures. E.R., K.M. and L.Z. performed the biochemical binding study. T.S. and S.M. performed the cell biology study. G.T.Z. and M.-M.Z. wrote the manuscript.
ACCESSION CODES
Structure factors and coordinates for the first bromodomain of BRD4 in complex with the lead diazobenzene inhibitors, MS436, MS435 and MS267 are being deposited at the Protein Data Bank.
REFERENCES
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez R, Zhou MM. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Devel. 2009;12:659–665. [PMC free article] [PubMed] [Google Scholar]
- 4.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, Gingras AC, Arrowsmith CH, Knapp S. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidler LR, Brown N, Knapp S, Hoelder S. Druggability Analysis and Structural Classification of Bromodomain Acetyl-lysine Binding Sites. J. Med. Chem. 2012;55:7346–7359. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang YC, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuber J, Shi JW, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Liu R, Zhong Y, Plotnikov AN, Zhang W, Zeng L, Rusinova E, Gerona-Nevarro G, Moshkina N, Joshua J, Chuang PY, Ohlmeyer M, He JC, Zhou MM. Down-regulation of NF-kappaB transcriptional activity in HIV-associated kidney disease by BRD4 inhibition. J. Biol. Chem. 2012;287:28840–28851. doi: 10.1074/jbc.M112.359505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang X, Peng R, Phillips JE, Deguzman J, Ren Y, Apparsundaram S, Luo Q, Bauer CM, Fuentes ME, Demartino JA, Tyagi G, Garrido R, Hogaboam CM, Denton CP, Holmes AM, Kitson C, Stevenson CS, Budd DC. Assessment of Brd4 Inhibition in Idiopathic Pulmonary Fibrosis Lung Fibroblasts and in Vivo Models of Lung Fibrosis. Am. J. Pathol. 2013;183:470–479. doi: 10.1016/j.ajpath.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell. Biol. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder S, Cho S, Zeng L, Zhang Q, Kaehlcke K, Mak L, Lau J, Bisgrove D, Schnolzer M, Verdin E, Zhou MM, Ott M. Two-pronged binding with bromodomain-containing protein 4 liberates positive transcription elongation factor b from inactive ribonucleoprotein complexes. J. Biol. Chem. 2012;287:1090–1099. doi: 10.1074/jbc.M111.282855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewings DS, Fedorov O, Filippakopoulos P, Martin S, Picaud S, Tumber A, Wells C, Olcina MM, Freeman K, Gill A, Ritchie AJ, Sheppard DW, Russell AJ, Hammond EM, Knapp S, Brennan PE, Conway SJ. Optimization of 3,5-Dimethylisoxazole Derivatives as Potent Bromodomain Ligands. J. Med. Chem. 2013;56:3217–3227. doi: 10.1021/jm301588r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seal J, Lamotte Y, Donche F, Bouillot A, Mirguet O, Gellibert F, Nicodeme E, Krysa G, Kirilovsky J, Beinke S, McCleary S, Rioja I, Bamborough P, Chung CW, Gordon L, Lewis T, Walker AL, Cutler L, Lugo D, Wilson DM, Witherington J, Lee K, Prinjha RK. Identification of a novel series of BET family bromodomain inhibitors: Binding mode and profile of I-BET151 (GSK1210151A) Bioorg. Med. Chem. Lett. 2012;22:2968–2972. doi: 10.1016/j.bmcl.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, Beinke S, Chapman TD, Roberts EJ, Soden PE, Auger KR, Mirguet O, Doehner K, Delwel R, Burnett AK, Jeffrey P, Drewes G, Lee K, Huntly BJP, Kouzarides T. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamborough P, Diallo H, Goodacre JD, Gordon L, Lewis A, Seal JT, Wilson DM, Woodrow MD, Chung CW. Fragment-Based Discovery of Bromodomain Inhibitors Part 2: Optimization of Phenylisoxazole Sulfonamides. J. Med. Chem. 2012;55:587–596. doi: 10.1021/jm201283q. [DOI] [PubMed] [Google Scholar]
- 18.Borah JC, Mujtaba S, Karakikes I, Zeng L, Muller M, Patel J, Moshkina N, Morohashi K, Zhang WJ, Gerona-Navarro G, Hajjar RJ, Zhou MM. A Small Molecule Binding to the Coactivator CREB-Binding Protein Blocks Apoptosis in Cardiomyocytes. Chem. Biol. 2011;18:531–541. doi: 10.1016/j.chembiol.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perola E. An Analysis of the Binding Efficiencies of Drugs and Their Leads in Successful Drug Discovery Programs. J. Med. Chem. 2010;53:2986–2997. doi: 10.1021/jm100118x. [DOI] [PubMed] [Google Scholar]
- 20.Tarcsay A, Keseru GM. Contributions of Molecular Properties to Drug Promiscuity Miniperspective. J. Med. Chem. 2013;56:1789–1795. doi: 10.1021/jm301514n. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JD, Blagg J, Price DA, Bailey S, DeCrescenzo GA, Devraj RV, Ellsworth E, Fobian YM, Gibbs ME, Gilles RW, Greene N, Huang E, Krieger-Burke T, Loesel J, Wager T, Whiteley L, Zhang Y. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008;18:4872–4875. doi: 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Kim DG, Shin NY, Song WK, Kwon H, Chung CH, Kang MS. NF-kappa B-dependent expression of nitric oxide synthase is required for membrane fusion of chick embryonic myoblasts. Biochem. J. 1997;324:237–242. doi: 10.1042/bj3240237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkina AC, Nikolajczyk BS, Denis GV. BET Protein Function Is Required for Inflammation: Brd2 Genetic Disruption and BET Inhibitor JQ1 Impair Mouse Macrophage Inflammatory Responses. J. Immunol. 2013;190:3670–3678. doi: 10.4049/jimmunol.1202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fish PV, Filippakopoulos P, Bish G, Brennan PE, Bunnage ME, Cook AS, Federov O, Gerstenberger BS, Jones H, Knapp S, Marsden B, Nocka K, Owen DR, Philpott M, Picaud S, Primiano MJ, Ralph MJ, Sciammetta N, Trzupek JD. Identification of a chemical probe for bromo and extra C-terminal bromodomain inhibition through optimization of a fragment-derived hit. J. Med. Chem. 201255:9831–9837. doi: 10.1021/jm3010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K, Saito NG, Stuckey JA, Wang S. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 2004;332:261–273. doi: 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Otwinowski Z, Minor W. [20] Processing of X-ray diffraction data collected in oscillation mode. In: Carter Charles W., Jr, editor. Methods in Enzymology. Vol. Volume 276. Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 27.Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. J. Appl. Cryst. 1997;30:1022–1025. [Google Scholar]
- 28.Murshudov GN, Vagin AA, Dodson EJ. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 29.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 30.Hunger K, Mischke P, Rieper W. Ullmann’s Encyclopedia of Industrial Chemistry. KGaA: Wiley-VCH Verlag GmbH & Co; 2000. Azo Dyes. [Google Scholar]
- 31.Zhou MM, Ohlmeyer M, Mujtaba S, Plotnikov A, Kastrinsky D, Zhang G, Borah J. Inhibitors of Bromodomains as Modulators of Gene Expression. 2012 WO2012116170. [Google Scholar]
- 32.Wallace A, Laskowski R, Thornton J. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







