Summary
Specific connectivity patterns among neurons create the basic architecture underlying parallel processing in our nervous system. Here we focus on the visual system’s first synapse to examine the structural and functional consequences of sensory deprivation on the establishment of parallel circuits. Dark rearing reduces synaptic strength between cones and cone bipolar cells, a previously unappreciated effect of sensory deprivation. In contrast, rod bipolar cells, which utilize the same glutamate receptor to contact rods, are unaffected by dark rearing. Underlying the physiological changes, we find the localization of metabotropic glutamate receptors within cone bipolar, but not rod bipolar, cell dendrites is a light-dependent process. Furthermore, although cone bipolar cells share common cone partners, each bipolar cell type we examined depends differentially on sensory input to achieve mature connectivity. Thus, visual experience differentially affects maturation of rod versus cone pathways and of cell types within the cone pathway.
Introduction
Recognition of the importance of sensory input to the development of neural circuits originated with findings that ocular dominance columns in primary visual cortex are shaped by visual experience (Hubel et al., 1977; LeVay et al., 1980). Since that seminal work, findings across sensory systems have uncovered further how sensory deprivation impinges on the normal development of cortical circuits (Chen et al., 2001; Cummings and Belluscio, 2010; Hofer et al., 2009; Lu et al., 2008; Philpot et al., 2001; Shepherd et al., 2003; Tyler et al., 2007; Zuo et al., 2005). In visual cortex, monocular or binocular deprivation increases the rate of spine formation (Hofer et al., 2009), alters connectivity between inhibitory and pyramidal neurons (reviewed in Espinosa and Stryker, 2012), and disrupts normal developmental changes in receptor subunits (Chen et al., 2001; Lu et al., 2008; Philpot et al., 2001).
One mystery is where the effects of sensory deprivation originate along sensory pathways. Changes in cortex could occur independently of subcortical alterations or reflect modifications at earlier stages of sensory processing. For example, deprivation-induced changes in the receptive field properties of somatosensory cortical neurons cannot be attributed to plasticity of thalamic neurons (reviewed in Fox et al., 2002). In contrast, sensory deprivation affects response kinetics of olfactory sensory neurons potentially accounting for alterations in olfactory cortex (He et al., 2012). Indeed, earlier in the visual pathway, sensory deprivation prevents refinement of retinogeniculate synapses during a postnatal critical period (Hooks and Chen, 2006). Within the retina, ganglion cell spike responses (Di Marco et al., 2009; Tian and Copenhagen, 2001) and pruning of ganglion cell dendrites (Tian and Copenhagen, 2003) were abnormal in dark-reared mice. Here, we asked whether sensory experience regulates the development of the visual system’s first synapse between retinal photoreceptors and bipolar cells.
As in other sensory systems, information from primary sensory neurons is conveyed to specific cell types for parallel processing. A second mystery is whether sensory deprivation affects parallel channels equally. In the present study, we take advantage of the well-characterized connectivity patterns between photoreceptors and multiple bipolar cell types, allowing for the investigation of specific synaptic partners at a common processing level. Photoreceptors contact at least ten types of bipolar cells (Wässle et al., 2009). Bipolar cells fall into two broad categories: OFF bipolar cells, which use a variety of ionotropic glutamate receptors (Puller et al., 2007), and ON bipolar cells, which use metabotropic glutamate receptors (mGluR6; Nomura et al., 1994). Rod bipolar cells carry signals from rod photoreceptors, whereas the other bipolar cells carry signals predominantly from cone photoreceptors. Previous work demonstrated that connectivity between mouse cone photoreceptors and three types of ON cone bipolar cells matures at different rates, such that the type 6 chooses its synaptic cone partners before the time of eye-opening, and types 7 and 8 do not settle on their cone partners until weeks after eye-opening (Dunn and Wong, 2012). Such timing differences between bipolar cell types inspired us to examine whether the formation of correctly-wired and functioning circuits depends on sensory stimulation. We find that dark rearing disturbs cone, but not rod, pathways at the level of the visual system’s first synapse. Furthermore, sensory deprivation differentially affects cone bipolar cell types.
Results
Dark rearing diminishes cone-mediated but not rod-mediated responses
To examine the contribution of sensory experience to the development of the photoreceptor-to-bipolar cell synapse, we reared mice in darkness and recorded electroretinograms (ERGs) in isolated retina. We begin by comparing rod and cone pathways to examine how distinct channels may be differentially regulated by sensory input.
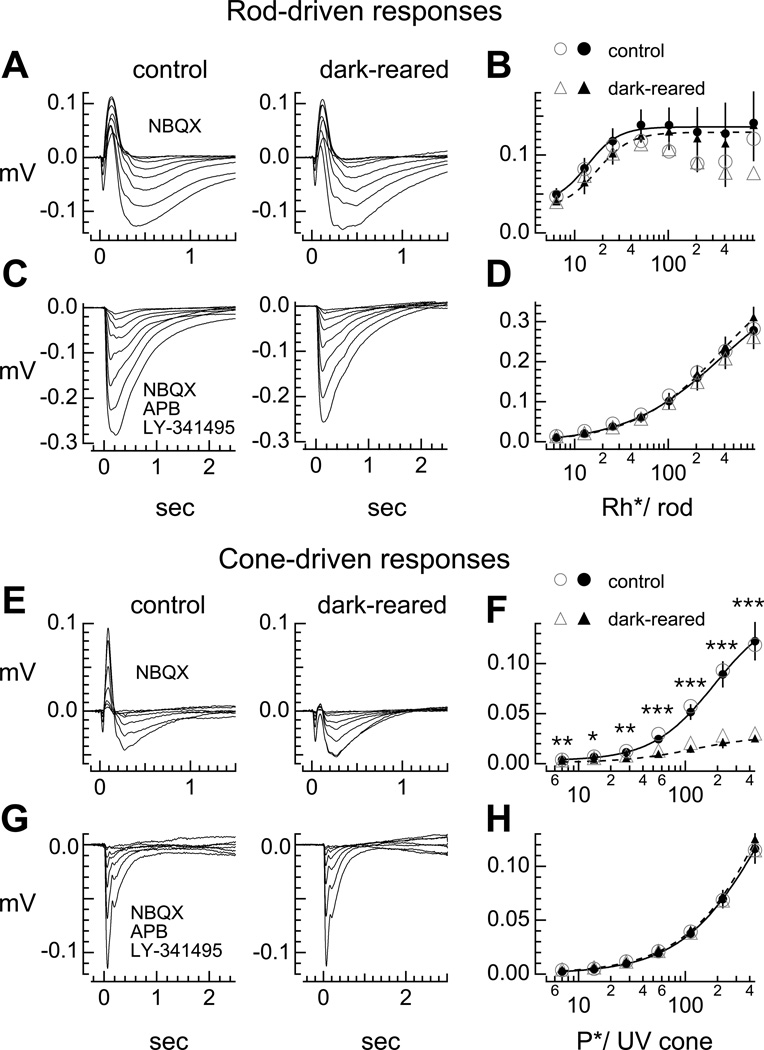
Dorsal halves of retina were stimulated from darkness with a light emitting diode (LED) which preferentially stimulates rods (see Experimental Procedures). Figure 1A shows example ERGs recorded under pharmacological isolation of responses generated by rods and Müller cells (a-wave) and rod bipolar cells (b-wave). The initial downward component is the a-wave; the subsequent upward component is the b-wave. Figure 1B plots the amplitudes of the b-waves from these individual retinas and across the population. Average intensity-response functions for the rod-driven b-waves lay on top of each other, suggesting no differences in the rod bipolar cell-driven responses between control and dark-reared animals. To examine the a-wave component of the ERG, we pharmacologically blocked the majority of ionotropic and metabotropic glutamate receptor-mediated transmission in the retina, preserving the rod and Müller cell contributions to the ERG (Figure 1C). Average intensity-response relationships of the rod-driven a-wave also reveal no differences between the two rearing conditions (Figure 1D).
Figure 1. Dark rearing diminishes the cone-mediated b-wave.
(A, C) Electroretinograms (ERGs) of isolated dorsal retina from (first column) control and (second column) dark-reared mice. Flashes delivered from darkness. (A) Individual retina responses from rods, Müller cells, and rod bipolar cells to a family of 10 ms flashes from a 470 nm LED, doubling in intensity. (C) Individual retina responses from rods and Müller cells. Summary of absolute values of (B) b-wave and (D) a-wave amplitudes as a function of flash strength in photoisomerizations per rod (Rh*/rod) from individual retinas (open symbols) and average across the population (closed symbols; n = 7 normally-reared; n = 6 dark-reared mice). (E, G) Electroretinograms (ERGs) of isolated ventral retina from (first column) control and (second column) dark-reared mice to a family of 10 ms flashes from a 395 nm LED superimposed on a constant background of 4000 R*/rod/sec with a 470 nm LED. (E) Individual retina responses of cones, Müller cells, and ON cone bipolar cells. (G) Individual retina responses of cone photoreceptors and Müller cells. Summary of absolute values of (F) b-wave and (H) a-wave amplitudes as a function of flash strength in photoisomerizations per UV cone (P*/UV cone) of individual retinas (open symbols) and average across the population (closed symbols; n = 6 normally-reared; n = 5 dark-reared mice). Closed symbols represent Mean ± SEM and are fit by the Hill equation. Fits to equation reported in Table S1. Mice spanned ages P69–P72.
To preferentially stimulate the cones, we used ventral retina, where the ultraviolet (UV)-sensitive opsin dominates in mouse retina (Wang et al., 2011). Retinas were stimulated with an LED with maximal emission at 395 nm superimposed on a constant background of 4000 photoisomerizations per rod per second (R*/rod/sec) produced by the 470 nm LED, to preferentially adapt down the rods. Rod contributions to the ERG were minimal under these conditions (data not shown). Figure 1E shows cone-driven ERGs, and Figure 1F summarizes the b-wave amplitudes. Significant differences between cone-driven b-wave amplitudes suggest that in dark-reared mice, voltage responses of ON cone bipolar cells have reduced gain compared to normally-reared mice. To determine whether the decreased gain is reflected in the a-wave, we pharmacologically isolated the a-wave (Figure 1G). On average, cone-driven a-wave amplitudes superimpose across flash strengths, revealing no significant differences between retinas from control and dark-reared mice (Figure 1H). However, cone-driven b-waves decreased significantly across light levels. Our results suggest that whereas dark rearing left intact transmission between rods and rod bipolar cells, the same manipulation compromised transmission between cones and ON cone bipolar cells.
Dark rearing affects metabotropic glutamate receptor 6 (mGluR6) labeling associated with cone bipolar cells but not with rod bipolar cells
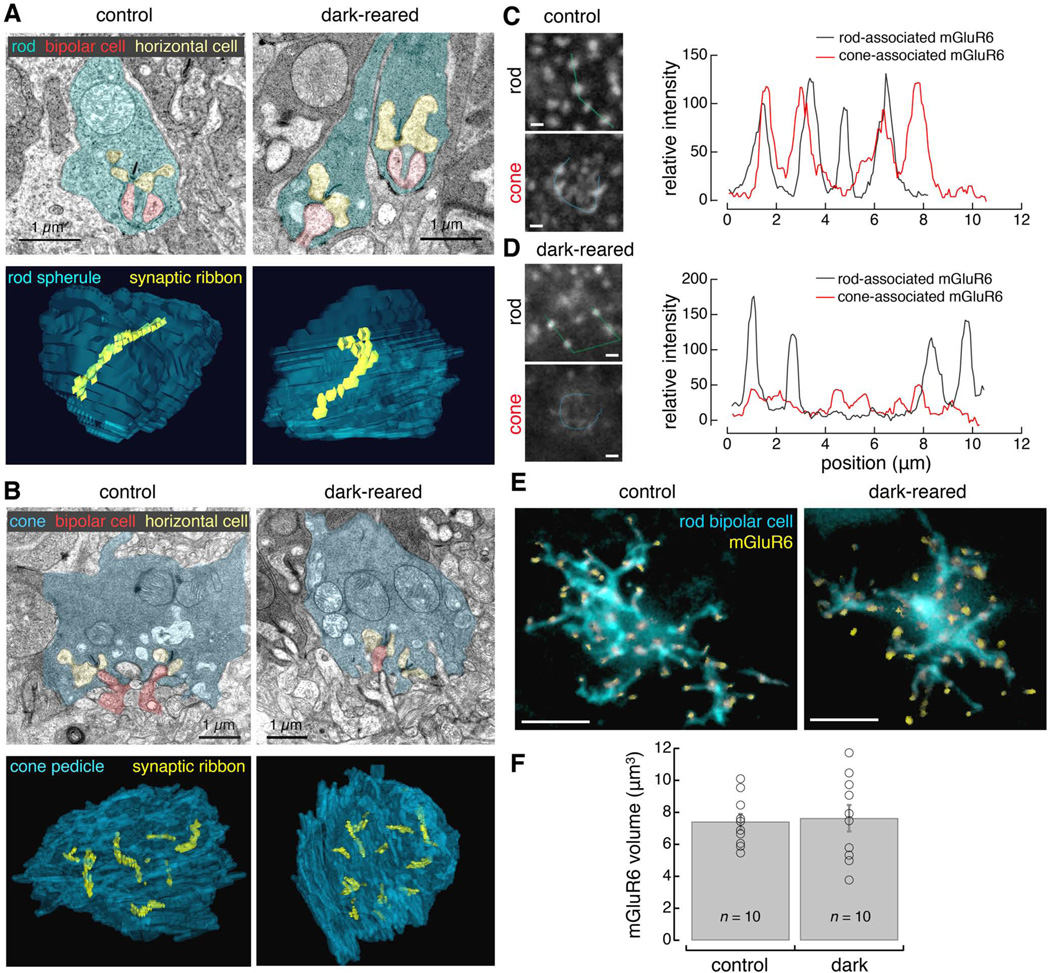
Having found differential functional effects of dark rearing on rod versus cone pathways, we investigated the effects of light deprivation on synapse structure. To determine whether dark rearing affected presynaptic release sites in photoreceptors, we reconstructed electron micrographs of serial sections through rod spherules (Figure 2A) and cone pedicles (Figure 2B). Within each rod we consistently observed a single ribbon. Within each cone pedicle, we identified individual synaptic ribbons and counted 12 ± 1 ribbons in control (n = 3 cones, mean ± SD; P30; similar to previously reported values by Tsukamoto et al., 2001) and 11.3 ± 3 ribbons (n = 7 cones, mean ± SD; P35) in dark-reared mice (example cone reconstructions in Figure 2B bottom). A two-sample t-test showed that the null hypothesis could not be rejected in comparing the total number of ribbons between cones in control and dark-reared mice (p = 0.72). We also found normal invaginating synapses at rod and cone ribbons with horizontal cells and ON bipolar cell dendrites in the dark-reared mice (Figure 2A and B, top). These ultrastructural data demonstrate that invaginating synapses between photoreceptors and ON bipolar cells remain intact in dark-reared mice.
Figure 2. Dark rearing affects the mGluR6 labeling associated with cone bipolar cells but not with rod bipolar cells.
Electron micrographs of (A) rod spherules (cyan) and (B) cone pedicles (blue), horizontal cell processes (yellow), and ON bipolar cell dendrites (red) in (left) control (P30) and (right) dark-reared (P35) mice. (A–B, bottom) Three-dimensional reconstructions of serial electron micrographs of a rod spherule (blue) and cone pedicle (blue) with all associated synaptic ribbons (yellow) in (left) control and (right) dark-reared mice. (C–D) Confocal images of mGluR6 labeling at the level of mGluR6 postsynaptic to rods (top) and to cones (bottom) in (C) control and (D) dark-reared conditions. Single planes from the same stack. Plots show intensity profiles of the line scan (indicated by the lines in the images) across four example rod bipolar mGluR6 puncta (black) and around the perimeter of cone bipolar mGluR6 (red). Scale bars, 1 µm. (E) En face view of rod bipolar cell dendrites labeled in the Grm6-tdTomato line (cyan) with mGluR6 (yellow) within the volume of the bipolar cell dendrites under (left) control and (right) dark-reared conditions. Scale bars, 5 µm. (F) Mean total volume of mGluR6 within the dendrites of rod bipolar cells at P30 for control and dark-reared conditions (error bars are SEM). Individual rod bipolar cells shown in open circles. See also Figure S1 and Table S2.
Next, to determine whether functional effects observed in the ERGs could be attributed to changes in postsynaptic receptor localization, we immunolabeled for mGluR6 (Figure 2C and D; Morgans et al., 2006; see Figure S1 for whole retina protein quantification). mGluR6 at the level of rod bipolar cell dendritic tips appeared as segregated puncta (Figure 2C top), whereas mGluR6 at the level of ON cone bipolar cell dendritic tips appeared as a contiguous string of clusters (Figure 2C bottom). In the control condition, rod- and cone-associated mGluR6 show similar intensity ranges and sizes of puncta. In contrast, intensity values of cone-associated mGluR6 were lower and less distinct than rod-associated mGluR6 in the dark-reared condition.
To examine glutamate receptors at the level of individual postsynaptic cells, we first focused on rod bipolar cells in the Grm6-tdTomato transgenic line (Kerschensteiner et al., 2009). We imaged individual rod bipolar cells (Figure 2E) and measured the volume of mGluR6 within their dendrites (Figure 2F). At P30, each rod bipolar cell dendritic tip contains mGluR6 puncta, suggesting that mGluR6 is properly located at synaptic sites. The null hypothesis could not be rejected in comparing the volume of mGluR6 within individual rod bipolar cells from control and dark-reared conditions (two-sample t-test, p = 0.82). Thus dark rearing has no significant effect on the mGluR6 levels in individual rod bipolar cells.
Dark rearing disrupts mGluR6 localization in ON cone bipolar cells
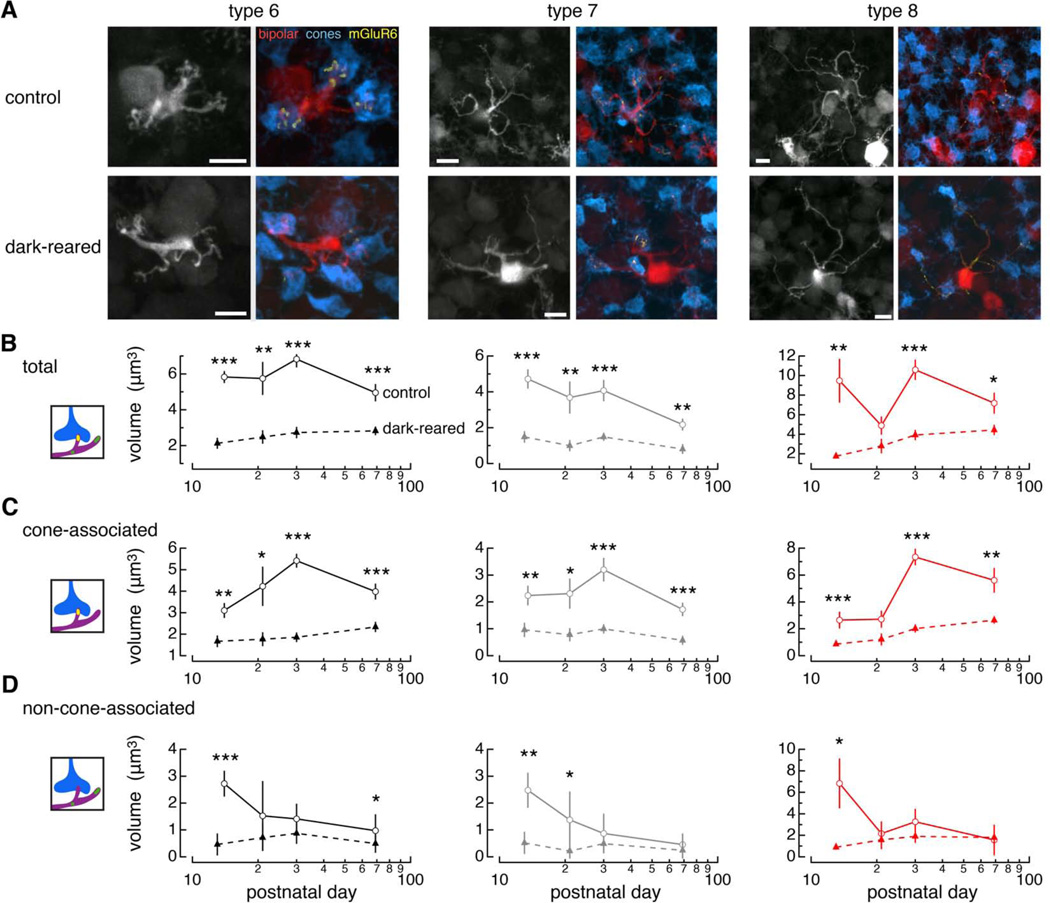
To explore the mGluR6 levels in individual ON cone bipolar cells, we focused on the type 6, 7, and 8 bipolar cells found in the Grm6-tdTomato line [Figure 3A; see Dunn and Wong (2012) for explanation of cell classification]. In addition to the mGluR6, presynaptic cone photoreceptors were labeled with an antibody against cone arrestin (Figure S2; Nikonov et al., 2008; Zhu et al., 2002). Figure 3A shows mGluR6 labeling within the dendrites of the bipolar cell of interest; glutamate receptor labeling exterior to the bipolar cell is digitally removed from the image (Experimental Procedures). We quantified the total volume of glutamate receptors within bipolar cell dendrites and also within cone photoreceptors to determine the location of mGluR6. The total volume of glutamate receptors within bipolar cell dendrites was significantly lower at all ages after dark rearing (Figure 3B; see Supplemental Text, Figure S1, and Table S2 for total mGluR6 in the retina). Likewise, the volume of glutamate receptors associated with cones decreased with dark rearing (Figure 3C). The volume of receptors exterior to cones was consistently lower at the earliest time points across bipolar cell types in the dark-reared condition (Figure 3D). Under normal rearing conditions, the volume of non-cone-associated receptor declines during development, and this trend is lost upon dark rearing (Figure 3D). At the time of eye-opening (postnatal day 13; P13), mGluR6 levels in the dark-reared condition are less than control, suggesting that initial mGluR6 expression depends on light entering through closed eyelids. Such a mechanism is consistent with light responses present in the retina by P10. Thus, we find that mGluR6 localization at sites of cone contacts requires light stimulation.
Figure 3. Dark rearing disrupts glutamate receptor localization in ON cone bipolar cells.
(A) Confocal images of type 6, 7, and 8 cone bipolar cells (red), cone photoreceptors (blue), and mGluR6 within bipolar cell dendrites (yellow) from mice reared in (top) control and (bottom) dark conditions until P30. Scale bars, 5 µm. (B) Total mGluR6 volume within the dendrites of each bipolar cell type from control (open circles) and dark-reared (closed triangles) mice (Vcone+Vnon-cone). (C) Volume of mGluR6 within dendrites of each bipolar cell that is also associated with cones (Vcone). (D) Volume of mGluR6 within each bipolar cell that is not associated with cones (Vnon-cone). Cartoons to the left illustrate the measurement shown in graphs. Data reported as mean ± SEM. Number of cells analyzed at each age are reported in Table S3. See also Figure S2.
Dark rearing has different effects on the formation, maintenance, and elimination of cone contacts across bipolar cell types
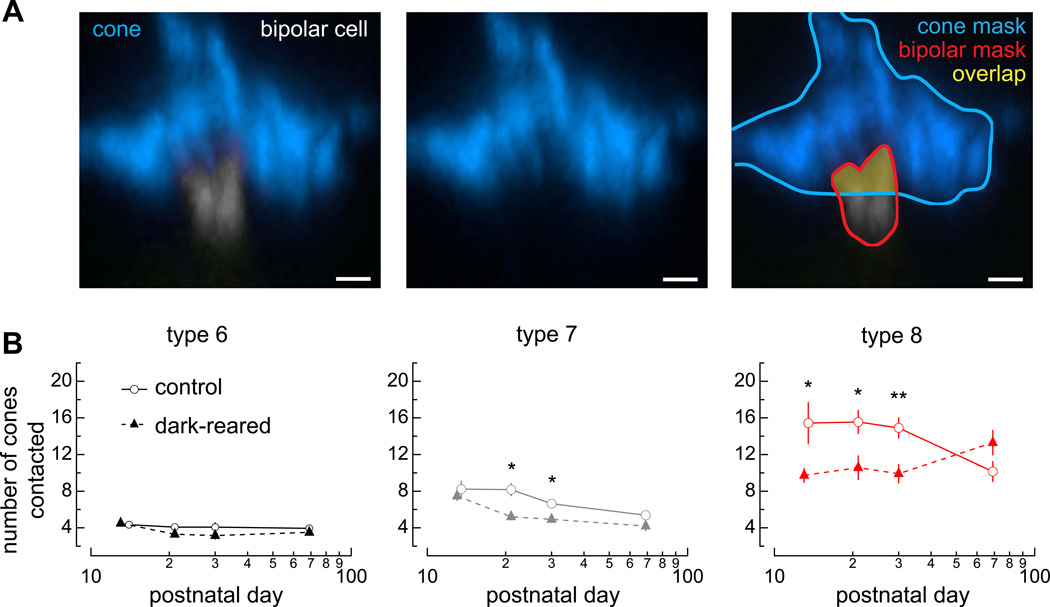
Next we asked whether the developmental processes of finding cone partners and forming synapses require light stimulation. We used volume overlap between the bipolar cell dendrites and cones as a criterion for contact to count the total number of cones contacted by each bipolar cell (Figure 4A; Experimental Procedures). Type 6 cone bipolar cells showed no significant difference between the number of cones contacted in control and dark-reared conditions across ages, suggesting that the formation and maintenance of cone contacts are independent of light stimulation (Figure 4B). The normal pruning process observed in type 7 cone bipolar cells was unaffected in the dark-reared condition as evidenced by the downward slope; however, the total number of cones contacted dropped at P21 and P30 (Figure 4B). Type 8 cone bipolar cells exhibited a decrease in the total number of cones contacted at P13, P21, and P30 and a change in the trajectory of settling on the appropriate number of partners, suggesting that light deprivation disrupts the formation, maintenance, and/or elimination of cone contacts.
Figure 4. Dark rearing differentially affects the number of cones contacted across bipolar cell types.
(A) (left) Confocal image of a side view of a cone pedicle (blue) and ON bipolar cell dendrite (gray), (middle) the cone pedicle alone, and (right) an example of how the structures are masked to determine the overlap region. (B) Plots summarize the number of cones contacted by bipolar cells of each type for mice reared in control (open circles) and dark-reared (closed triangles) conditions. Data represented as mean ± SEM. Number of cells analyzed at each age are reported in Table S3. Subset of control data previously shown in Dunn and Wong (2012). See also Figure S3.
Can differences in cone density or bipolar cell dendritic territory predict the observed changes in the number of cones contacted by each bipolar cell type? Predicted values for the number of cones contacted based on changes in cone density and bipolar cell territory between control and dark-reared conditions could account for the majority of observed values in the type 6 and 7, but failed to match the observed cone contacts in the dark-reared condition for the type 8 cone bipolar cells (Figure S3). Such results imply that any changes observed in the type 8 connectivity pattern upon dark rearing cannot be attributed to changes in the cone population or bipolar cell dendritic territory alone. Taken together, these results demonstrate that type 6 continue to establish the normal number of cone contacts; type 7 may contact fewer cones because of lower cone densities, and type 8 cone bipolar cells fail to establish the normal number of cone contacts upon dark rearing.
Discussion
In the present study, functional measures of the photoreceptor-to-bipolar cell synapse show a selective decrease in the population voltage responses of ON cone bipolar cells in dark-reared animals. Dark rearing had no effect on responses from rod photoreceptors or rod bipolar cells. Additionally, we found that dark rearing differentially affects the development of cone contacts by the types 6, 7, and 8 ON cone bipolar cells. Taken together, our results suggest that developing cone-to-cone bipolar cell contacts, localizing glutamate receptors, and establishing proper function of parallel channels at the visual system’s first synapse depend on sensory stimulation.
Functional changes from visual deprivation
Our findings on the effects of sensory deprivation at the visual system’s first synapse follow a long history of work on visual system development. While previous studies suggested that b-waves at brighter flash intensities were diminished in dark-reared cats (Baxter and Rissen, 1961) and in dark-reared mice (He et al., 2011), other studies in mice have reported an enhancement or minimal effect of dark rearing on the b-wave (Tian and Copenhagen, 2001). We designed our experimental conditions to preferentially stimulate either rods or cones. In the rod-driven b-wave, we reported a minimal effect on rod bipolar cell responses, consistent with ERGs recorded in dark-adapted mice stimulated from darkness (Tian and Copenhagen, 2001). In our cone-driven responses, dark rearing diminished b-waves, consistent with previous findings that b-wave responses to brighter flash intensities and higher temporal frequencies, thus presumably cone-driven, were compromised (Baxter and Rissen, 1961; He et al., 2011). Here we reconcile the differences in previously reported results by directly measuring both rod- and cone-driven responses in retinas from the same mice. We speculate that dark rearing alters normal spontaneous release from cones more severely than from rods, thus explaining differences between rod and cone pathways. mGluR6 in ON cone bipolar cell dendrites could be reduced in response to the constant barrage of transmitter release from cones in darkness.
Further along the visual pathway, changes caused by dark rearing or monocular deprivation have been attributed to cortical alterations for functions arising in cortex, e.g., ocular dominance selectivity (LeVay et al., 1980) and orientation selectivity (Crair et al., 1998). However, all features in visual cortex rely on passage through earlier stages of processing, and thus we propose the effects of sensory deprivation on the cone-to-bipolar cell synapse must be considered as a potential contributor. Previous results in cat and monkey suggest that cortical changes occur independently of effects on retina (Hendrickson and Boothe, 1976; Sherman and Stone, 1973). Results reported here raise the possibility that effects of light deprivation on the development of mouse visual cortex, retinogeniculate projections (Hooks and Chen, 2006), and retinal amacrine and ganglion cells (Di Marco et al., 2009; Giovannelli et al., 2008; Tian and Copenhagen, 2001; 2003; Vistamehr and Tian, 2004) could relay changes from earlier stages of processing. Previous reports demonstrate that dark rearing disrupts the normal developmental increase in synaptic inputs to ganglion cells (Tian and Copenhagen, 2001). Such changes could arise partially from a diminished responsiveness of ON cone bipolar cells: dark-reared mice with less mGluR6 in the dendrites of cone bipolar cells would convey less spontaneous activity from cones to ON ganglion cells. Dark rearing reduces the spike responses of ganglion cells (Tian and Copenhagen, 2001) and ERG responses of amacrine and ganglion cells to light increments (Vistamehr and Tian, 2004), but not of light-evoked currents in amacrine and ganglion cells (He et al., 2011). Such contrasting observations could be reconciled by differences in cell types and circuits probed in each of these studies.
Implications for cell type-specific effects across parallel circuits
Prior and present work demonstrate the importance of considering the developmental mechanisms of individual circuits and cell types within a system. Reverse suturing three weeks after monocular deprivation reversed geniculocortical projections from parvocellular layers of the dorsal lateral geniculate nucleus but not from magnocellular layers, suggesting asynchronous critical periods for plasticity (LeVay et al., 1980). Furthermore, several studies have examined excitatory and inhibitory circuits in visual cortex following monocular deprivation and have found that inhibitory circuits are altered even when excitatory circuits are not (Chen et al., 2012; van Versendaal et al., 2012; reviewed in Espinosa and Stryker, 2012). Within the retina, dark rearing caused an imbalance of excitatory and inhibitory inputs to ganglion cells (Di Marco et al., 2009). At the first synapse examined here, the differences in timing and strategies of choosing presynaptic partners by the type 6, 7, and 8 ON cone bipolar cells (Dunn and Wong, 2012) also emerged in the effects of dark rearing. These findings in retina suggest that sharing a common presynaptic partner does not dictate a common mechanism for creating synapses. Instead, proper synapse formation requires several mechanisms, working over different periods and within specific circuits. We imagine that such diversity in mechanisms confers the diversity in convergence and synapse structure found across cone bipolar cells and ultimately enables the development of unique pathways for encoding information. Results from our sensory deprivation experiments imply that different bipolar cell types may retain plasticity later in development than other bipolar cell types. Diversity during normal development suggests the possibility for differences among parallel pathways in the degeneration or in the potential for regeneration of circuits, if such developmental mechanisms can be recapitulated following damage or disease.
Experimental Procedures
Mice and rearing conditions
The transgenic mouse lines, hLM-GFP (Fei and Hughes, 2001) and Grm6-tdTomato (Kerschensteiner et al., 2009), were crossed for visualization of cone photoreceptors and a sparse population of ON bipolar cells. Cell types were distinguished by previously reported methods including morphology, immunolabeling, and crosses to other transgenic lines (Dunn and Wong, 2012). All transgenic lines were back crossed into the C57Bl6 strain. Male and female mice were either raised in a 12 hour light-dark cycle or in a dark-rearing facility where mice were born and raised within light-tight cabinets. Dark-reared mice were cared for using infrared illumination and viewed with night vision goggles (B.E. Meyers, Redmond, WA).
Tissue preparation
All procedures were performed in accordance with the University of Washington Institutional Animal Care and Use Committee protocols. Control-reared mice were sacrificed either with cervical dislocation or 5% isoflurane in the light. Dark-reared mice were sacrificed with cervical dislocation, enucleated, and retinas isolated under infrared light. Retinas were dissected and mounted flat onto filter paper (Millipore, Billerica, MA) in bicarbonate-based Ames solution (Sigma, St. Louis, MO) equilibrated with 5% CO2 / 95% O2, fixed in 4% paraformaldehyde for 15 min at room temperature and rinsed in phosphate buffered saline, pH 7.42, then removed from the filter paper before immunoprocessing.
The following primary antibodies were used: cone arrestin/Arr4 (1:500 and 1:1000; gift of Cheryl Craft) and mGluR6 (1:100; gift of Catherine Morgans). The following secondary antibodies were used: Dy-Light-488 (1:1000; Jackson Laboratory, Bar Harbor, ME) and Alexa-633 (1:500; Invitrogen, Grand Island, NY). Retinas were mounted with Vectashield (Vector Labs, Burlingame, CA) underneath a coverslip.
Imaging
Retinas were imaged on a FV-1000 Olympus laser scanning microscope with an oil immersion Olympus 60× objective (1.35 NA). Imaging parameters were set for voxel sizes between 0.066–0.069 µm/pixel (x-axis, y-axis) and 0.2–0.3 µm/pixel (z-axis). Each plane was acquired 3–4 times with Kalman filtering.
Analysis
Images were analyzed as described previously. Briefly, cone photoreceptor and bipolar cell dendritic contacts were determined by nonzero volume overlap between binary masks of the three-dimensional confocal image stacks (Dunn and Wong, 2012). Bipolar cell somas were excluded from the masks. All mGluR6 puncta were labeled with a single identity. Because cone-associated mGluR6 was not punctate like rod-associated mGluR6, we determined the absence or presence and volume of the postsynaptic receptor marker, rather than counting the number of puncta.
Statistical Analysis
A two-sample t-test was used to test for significant differences between control and dark-reared conditions at each comparable light level (Figure 1) or age (Figures 2, 3, 4). Resulting p-values less than 0.05, 0.01, and 0.001 are indicated by single (*), double (**), and triple (***) asterisks.
Electron microscopy
Retinas were fixed by immersion of the eyecup in 4% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. The retinas were washed in buffer, postfixed in 1% osmium tetroxide for 90 min, rinsed, en bloc stained in 1% uranyl acetate, dehydrated in ethanol solutions, and embedded in epoxy resin (Epon Araldite). Serial sections (80 nm) of photoreceptor pedicles were aligned and labeled with TrakEM2 (Cardona et al., 2012).
Electroretinograms
Mice used in physiology experiments were dark adapted overnight and sacrificed by cervical dislocation. Eyes were dissected under infrared illumination, vitreous was removed, and eyecups were hemisected to separate the dorsal and ventral portions. Isolated half retinas were mounted in a chamber as previously described (Azevedo and Rieke, 2011). Retinas were perfused from top and bottom with bicarbonate-based Ames solution (Sigma, St. Louis, MO) equilibrated with 5% CO2 / 95% O2 and heated to 32–35°C. Protocols for the ERGs were designed to separate rod and cone light responses. Light was delivered to the photoreceptor side through at 550 µm2 aperture.
To isolate the photoreceptor, ON bipolar cell (b-wave), and Müller cell components of the ERG, we perfused 5 µM NBQX to eliminate the OFF cone bipolar, amacrine, and ganglion cell contributions. We found a minimal contribution of the NMDA receptor to the b-wave, thus we focused on blocking AMPA-receptor-mediated responses (data not shown). Amplitudes of the b-wave were taken as the sum of the absolute values of the peak of the first local minimum and b-wave (global maximum). To isolate the photoreceptor (a-wave) and Müller cell components of the ERG, we used 5 µM NBQX, 3.25 µM LY-341495, 2.5 µM L-APB, which eliminated the majority of glutamatergic transmission from bipolar, amacrine, and ganglion cells. Amplitudes of the a-wave were taken as the absolute value of the global minimum. Amplitudes as a function of flash strengths were fit with a Hill equation (see legend of Table S1). Values of the fits are reported in Table S1.
Supplementary Material
Acknowledgements
We thank John Campbell for assistance with care of dark-reared mice; Ethan Buhr, Angela Sandt, Russ Van Gelder for providing the dark-rearing facility; Anthony Azevedo, Juan Angueyra, Greg Schwartz, Sidney Kuo, Mark Cafaro, Fred Rieke (F.R.) for resources used in the ERGs; Mrinalini Hoon, Sachihiro Suzuki, Takeshi Yoshimatsu, Ximena Opitz-Araya, Waldo Cerpa, Andres Barria for assistance with Western blots; Maureen McCall, Ron Gregg, Nazarul Hasan for providing the mGluR6 knockout retina and technical help with Western blots; Paul Newman, Eric Martinson and Jonathan Linton, Jing Huang, Daniel Possin (EY-01730) for technical assistance; Thomas Hughes for the hLMcone-GFP mouse; Cheryl Craft for cone arrestin antibody; Catherine Morgans and Kirill Martemyanov for mGluR6 antibodies; Deda Gillespie, Mrinalini Hoon, Haruhisa Okawa, Fred Rieke, Huat Chye Lim for critical reading of the manuscript; Adam Bleckert, Florence D’Orazi for helpful discussions. This work was supported by NIH through grants EY-022910 (F.A.D.), EY-01730 (E.D.P.), EY-017101 (R.O.L.W.), the Howard Hughes Medical Institute (F.R.), and the Helen Hay Whitney Foundation (F.A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
F.A.D. and R.O.L.W. conceived the study and wrote the manuscript. F.A.D. and L.D.S. designed ERG experiments. E.D.P. and R.O.L.W. performed electron microscopy. F.A.D. carried out experiments and data analysis.
References
- Azevedo AW, Rieke F. Experimental protocols alter phototransduction: the implications for retinal processing at visual threshold. Journal of Neuroscience. 2011;31:3670–3682. doi: 10.1523/JNEUROSCI.4750-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter BL, Rissen AH. Electroretinogram of the visually deprived cat. Science. 1961;134:1626–1627. doi: 10.1126/science.134.3490.1626. [DOI] [PubMed] [Google Scholar]
- Cardona A, Saalfeld S, Schindelin J, Arganda-Carreras I, Preibisch S, Longair M, Tomancak P, Hartenstein V, Douglas RJ. TrakEM2 software for neural circuit reconstruction. PLoS ONE. 2012;7:e38011. doi: 10.1371/journal.pone.0038011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PTC, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang C, Mower GD. Developmental changes in the expression of GABAA receptor subunits (α1, α2, α3) in the cat visual cortex and the effects of dark rearing. Molecular Brain Research. 2001;88:135–143. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Belluscio L. Continuous neural plasticity in the olfactory intrabulbar circuitry. Journal of Neuroscience. 2010;30:9172–9180. doi: 10.1523/JNEUROSCI.1717-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco S, Nguyen VA, Bisti S, Protti DA. Permanent functional reorganization of retinal circuits induced by early long-term visual deprivation. Journal of Neuroscience. 2009;29:13691–13701. doi: 10.1523/JNEUROSCI.3854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Wong ROL. Diverse strategies engaged in establishing stereotypic wiring patterns among neurons sharing a common input at the visual system's first synapse. Journal of Neuroscience. 2012;32:10306–10317. doi: 10.1523/JNEUROSCI.1581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y, Hughes TE. Transgenic expression of the jellyfish green fluorescent protein in the cone photoreceptors of the mouse. Vis Neurosci. 2001;18:615–623. doi: 10.1017/s0952523801184117. [DOI] [PubMed] [Google Scholar]
- Fox K, Wallace H, Glazewski S. Is there a thalamic component to experience-dependent cortical plasticity? Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2002;357:1709–1715. doi: 10.1098/rstb.2002.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli A, Di Marco S, Maccarone R, Bisti S. Long-term dark rearing induces permanent reorganization in retinal circuitry. Biochem. Biophys. Res. Commun. 2008;365:349–354. doi: 10.1016/j.bbrc.2007.10.204. [DOI] [PubMed] [Google Scholar]
- He J, Tian H, Lee AC, Ma M. Postnatal experience modulates functional properties of mouse olfactory sensory neurons. Eur J Neurosci. 2012;36:2452–2460. doi: 10.1111/j.1460-9568.2012.08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wang P, Tian N. Light-evoked synaptic activity of retinal ganglion and amacrine cells is regulated in developing mouse retina. Eur J Neurosci. 2011;33:36–48. doi: 10.1111/j.1460-9568.2010.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Boothe R. Morphology of the retina and dorsal lateral geniculate nucleus in dark-reared monkeys (Macaca nemestrina) Vision Res. 1976;16:517–521. doi: 10.1016/0042-6989(76)90033-x. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong ROL. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Lu J, Cui Y, Cai R, Mao Y, Zhang J, Sun X. Early auditory deprivation alters expression of NMDA receptor subunit NR1 mRNA in the rat auditory cortex. J. Neurosci. Res. 2008;86:1290–1296. doi: 10.1002/jnr.21577. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci. 2006;23:1163–1171. doi: 10.1111/j.1460-9568.2006.04647.x. [DOI] [PubMed] [Google Scholar]
- Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, Okamoto N, Mizuno N, Nakanishi S. ScienceDirect - Cell : Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994 doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Puller C, Haverkamp S, Grünert U. OFF midget bipolar cells in the retina of the marmoset, Callithrix jacchus, express AMPA receptors. J Comp Neurol. 2007;502:442–454. doi: 10.1002/cne.21315. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Stone J. Physiological normality of the retinal in visually deprived cats. Brain Res. 1973;60:224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. Journal of Neuroscience. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Petzold GC, Pal SK, Murthy VN. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. Journal of Neuroscience. 2007;27:9427–9438. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer J-P, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 2012;74:374–383. doi: 10.1016/j.neuron.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Vistamehr S, Tian N. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci. 2004;21 doi: 10.1017/s0952523804041033. [DOI] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. Journal of Neuroscience. 2011;31:7670–7681. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. Journal of Neuroscience. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ma B, Babu S, Murage J, Knox BE, Craft CM. Mouse cone arrestin gene characterization: promoter targets expression to cone photoreceptors. FEBS Lett. 2002;524:116–122. doi: 10.1016/s0014-5793(02)03014-4. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan W-B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.