Abstract
Nutrient intake and avoidance of toxins are essential for survival and controlled by attractive and aversive feeding responses. Drosophila melanogaster presents one of the best characterized systems for studies on chemosensation, which is mediated by multigene families of chemoreceptors, including olfactory receptors, gustatory receptors, and odorant-binding proteins (OBPs). Although the response profiles of gustatory receptors have been well studied, the contribution of OBPs to food intake is largely unknown. As most aversive (“bitter”) tastants are hydrophobic, we hypothesized that OBPs may fulfill an essential function in transporting bitter tastants to gustatory receptors to modulate feeding behavior. Here, we used 16 RNAi lines that inhibit expression of individual target Obp genes and show that OBPs modulate sucrose intake in response to a panel of nine bitter compounds. Similar to their function in olfaction, OBPs appear to interact with bitter compounds in a combinatorial and sex-dependent manner. RNAi-mediated reduction in expression of individual Obp genes resulted either in enhanced or reduced intake of sucrose in the presence of bitter compounds, consistent with roles for OBPs in transporting tastants to bitter taste receptors, sequestering them to limit their access to these receptors, or interacting directly with gustatory neurons that respond to sucrose.
Key words: bitter taste, chemosensation, gustation, insects, proboscis extension reflex, RNAi
Introduction
Aversive taste responses have evolved to prevent ingestion of toxic compounds. Toxins comprise a wide variety of chemicals and large families of gustatory receptors have evolved both in insects and mammals to enable their detection. In humans such compounds evoke a bitter taste perception. In mouse, multiple bitter taste receptors are expressed in individual taste cells enabling an appropriate rejection response to bitter compounds with little discrimination in taste quality (Chandrashekar et al. 2000; Mueller et al. 2005). Bitter (i.e., aversive) taste perception is essential for insects to enable avoidance of plant toxins and unfavorable oviposition sites.
The gustatory system of Drosophila melanogaster has been studied extensively and gustatory receptors that detect sweet tastants (Dahanukar et al. 2001; Ueno et al. 2001; Slone et al. 2007), bitter tastants (Meunier et al. 2003; Thorne et al. 2004; Lee et al. 2009; Weiss et al. 2011), as well as acid (Charlu et al. 2013), water (Cameron et al. 2010; Chen et al. 2010), carbon dioxide (Fischler et al. 2007), and pheromones (Bray and Amrein 2003; Moon et al. 2009) have been identified. Taste representations for different modalities project to segregated regions of the suboesophageal ganglion (Scott et al. 2001; Wang et al. 2004; Marella et al. 2006). Gustatory neurons that mediate aversive taste responses in Drosophila also express multiple bitter taste receptors (Thorne et al. 2004; Lee et al. 2009; Weiss et al. 2011) with limited discrimination in taste quality, similar to the mouse bitter taste system (Masek and Scott 2010). A comprehensive study of taste responses in subclasses of small, intermediate and large sensilla of the labellum characterized the molecular response profiles of 33 bitter taste receptors in all 31 labellar taste sensilla against a panel of 16 bitter compounds and identified four classes of bitter taste neurons (Weiss et al. 2011).
Bitter compounds are comparable to odorants in that they are generally small poorly water soluble molecules, such as alkaloids or terpenoids. In the insect olfactory system, transport of hydrophobic odorants is facilitated by odorant-binding proteins (OBPs; Wojtasek and Leal 1999; Xu et al. 2005; Grosse-Wilde et al. 2006), which modulate olfactory behavioral responses (Swarup et al. 2011). There is evidence that OBPs may also play a role in gustatory perception. OBP57d and OBP57e in taste hairs on the tarsi mediate recognition of hexanoic acid and octanoic acid, plant-derived toxic compounds, and mutations in these OBPs enable host-specific adaptation of Drosophila sechellia to the fruit of Morinda citrifolia (Matsuo et al. 2007; Matsuo 2008). Furthermore, many OBPs are expressed in the labellum, the pharyngeal labral sense organ, the dorsal and ventral cibarial organs, and taste sensilla on the tarsi and wing margins (Galindo and Smith 2001).
Based on previous studies, it is reasonable to hypothesize that OBPs may function as transporters of hydrophobic tastants similar to their role in olfaction. To test this hypothesis we measured feeding behavior of flies exposed to a panel of bitter tastants, while suppressing the expression of individual Obp genes using RNA interference with the binary GAL4-UAS expression system (Brand and Perrimon 1993). Our results show that, similar to their roles in olfaction, OBPs modulate ingestion of bitter tastants in a combinatorial and sexually dimorphic manner.
Materials and methods
Drosophila stocks
Sixteen lines expressing RNAi corresponding to Obp transcripts under UAS promoters inserted in the neutral phiC31 integration site along with the co-isogenic progenitor control line (y,w 1118 ;P{attP,y + ,w 3 ’}) were obtained from the Vienna Drosophila RNAi Center (http://www.vdrc.at; Dietzl et al. 2007). Each of these lines and the progenitor control was crossed to a tubulin-GAL4 driver line (y 1 w * ; P{tubP-GAL4}LL7/TM3; Sb 1) to suppress the expression of the target Obp gene. F1 offspring was used for both molecular and behavioral experiments. The efficiency and specificity of RNAi-mediated suppression of individual Obp genes in these lines has been reported previously (Swarup et al. 2011). Flies were grown on cornmeal-molasses-agar medium at 25°C and a 12h/12h light/dark cycle. The UAS-Obp RNAi lines provided viable offspring when crossed to the tubulin-GAL4 driver line with normal morphology, development time and fertility, except males of the tubulin-GAL4/UAS-Obp58b RNAi line, for which we could not obtain behavioral measurements due to poor viability.
Tastants
We selected 13 bitter tastants, which were obtained from Sigma-Aldrich (St Louis, MO, USA) and dissolved in 50mM sucrose for behavioral assays. Corresponding catalogue numbers are: sucrose, SigmaUltra (S7903); berberine chloride (B3251); caffeine (C0750); coumarin (C4261); denatonium benzoate (D5765); N,N-diethyl-meta-toluamide (PS902); escin (E1378); (-)-lobeline hydrochloride (141879); papaverine hydrochloride (P3510); N-phenylthiourea (P7629); quinine hydrochloride dehydrate (Q1125); D-(−)-salicin (S0625); D-(+)-sucrose octaacetate (252603); theophylline (T1633); and umbelliferone (H24003).
Behavioral assays
Capillary feeder (CAFE) assay
Three to four day-old flies were separated by sex and food deprived for 24h on starvation media with 1.5% agar to avoid dehydration. Eight individuals of the same sex were placed in each vial. Three capillaries were inserted through the foam cap and 50mM sucrose solution (control) or 50mM sucrose solution supplemented with bitter tastant was aspirated into each capillary. Mineral oil was placed on the top of the capillary to minimize evaporation and the initial level of the solution was marked on the capillary (Figure 1A). A control for evaporation without flies was included each time. Three replicates were run over 3 days with three replicates per day to account for environmental variation. The assay was conducted for 24h in a closed humid chamber with 80% humidity. The amount of solution consumed was calculated by measuring the difference in the levels of solution in the capillaries before and at the end of the assay and correcting for evaporation rate. All tubulin-GAL4/UAS-Obp RNAi lines were measured contemporaneously for each tastant along with a tubulin-GAL4/UAS-progenitor control line (i.e., flies that carry the tubulin-GAL4 driver without a UAS transgene in the same genetic background).
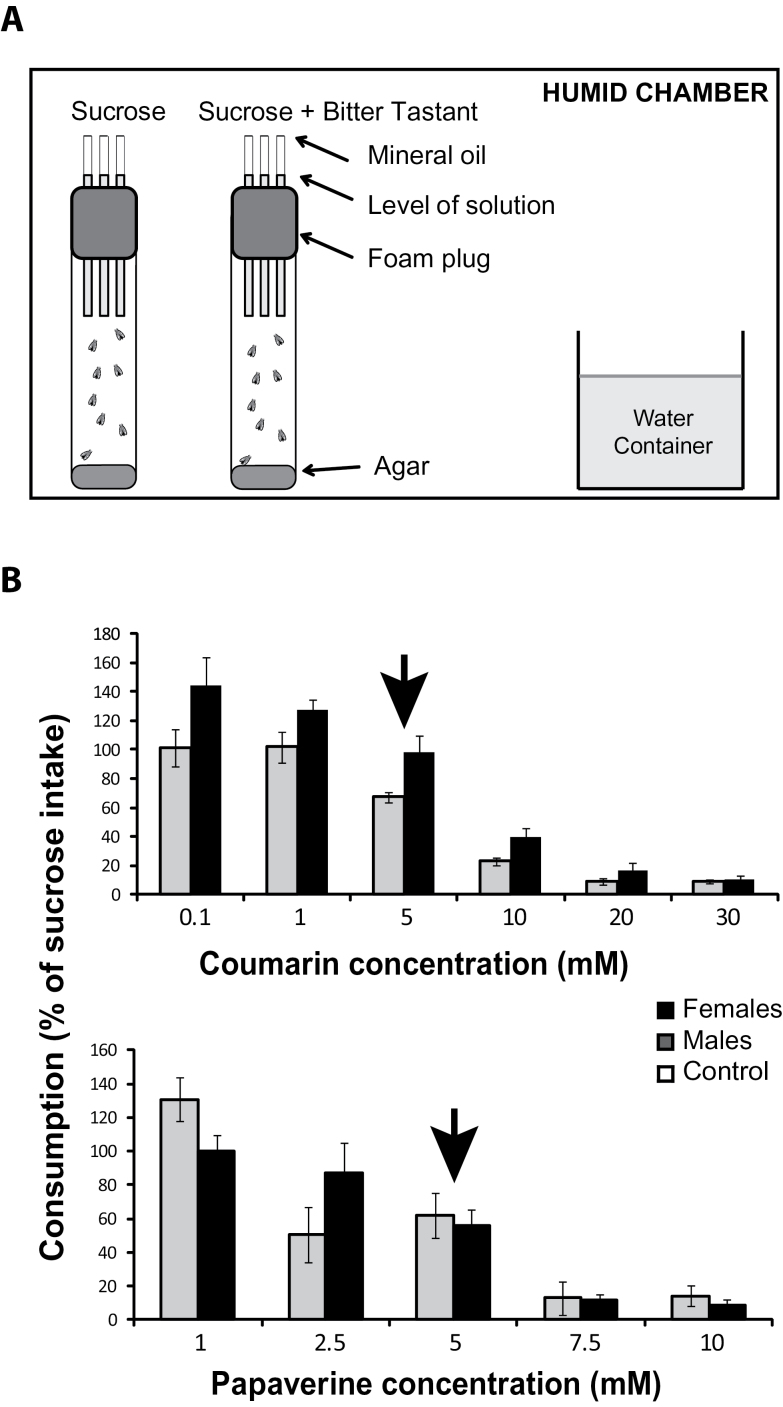
Figure 1.
Inhibition of nutrient intake by aversive tastants. (A) Schematic diagram of the Capillary Feeding (CAFE) assay. Eight individuals of the same sex are placed in each vial. Three capillaries are inserted through the foam cap and 50mM sucrose solution (positive control) or a 50mM sucrose solution supplemented with bitter tastant is aspirated into each capillary. Mineral oil is placed on the top of the capillary to prevent evaporation. Flies are allowed to feed for 24h in a closed humid chamber with 80% humidity. (B) The figure shows two representative examples for dose-dependent consumption of sucrose solution supplemented with bitter tastant, coumarin, and papaverine. Consumption of bitter tastants is represented as percentage of sucrose intake by offspring from the progenitor control line (y,w 1118 ; P{attP,y + ,w 3’}) crossed to the tubulin-GAL4 driver line. Arrows indicate the optimally discriminating bitter tastant concentrations selected for further experiments. Males are shown in grey bars and females in black bars.
Proboscis extension reflex
We performed PER assays on tubulin-GAL4/UAS-Obp28a RNAi and tubulin-GAL4/UAS-progenitor F1 offspring essentially as described by Shiraiwa and Carlson (2007). Three to 4 day-old flies were separated by sex and food deprived for 24h. Flies were immobilized in 200 μl micropipette tips with widened opening, with their heads protruding from the tip. We used fine paint brushes to deliver 50mM sucrose solution, distilled water, or 50mM sucrose solution supplemented with 0.8mM quinine solution, in the order: sucrose-water-tastant-water-sucrose. We discarded flies that did not respond to sucrose solution and flies that responded to water. Each fly was tested only once and the response was recorded as all (1) or none (0). Three sets of 15 flies per sex were measured for each line. Significant differences in PER responses between tubulin-GAL4/UAS-Obp28a and tubulin-GAL4/progenitor F1s were determined by two tailed Student’s t tests.
Assessment of Obp gene expression levels in proboscis
Independent triplicates of 100–150 probosces were manually collected from tubulin-GAL4/UAS-Obp RNAi and tubulin-GAL4/UAS-progenitor control F1s. RNA from probosces was extracted from F1 males and females separately. The efficiency of RNAi-mediated suppression of expression of Obp28a and Obp56h genes in probosces of tubulin-GAL4/UAS-Obp RNAi F1s was assessed by quantitative real time PCR using the SYBR green detection method, as described previously (Swarup et al. 2011). Statistically significant differences in Obp expression levels in probosces of tubulin-GAL4/UAS-Obp RNAi F1s and tubulin-GAL4/progenitor F1s were determined by two tailed Student’s t tests.
Data analysis
Statistical significance of sucrose consumption between tubulin-GAL4/UAS-Obp RNAi lines and the tubulin-GAL4/UAS-progenitor was estimated using a fixed two-way ANOVA model, Y = μ + G + S + E, where μ is the overall mean, G the effect of genotype, that is the tubulin-GAL4/UAS-Obp RNAi lines versus the tubulin-GAL4/UAS-progenitor lines, S the fixed effect of sex and E the environmental variance. Bitter tastant consumption data were analyzed using a three-way fixed ANOVA model, Y = μ + L + S + T + L × S + L × T + S × T + L × S × T + E, where μ is the overall mean, L the effect of UAS-Obp RNAi lines, S the effect of sex, T the effect of bitter tastant, and E the environmental variance. Next, the data were analyzed by tastant using a reduced fixed two-way ANOVA model, Y = μ + L + S + E, where μ is the overall mean, L the effect of the tubulin-GAL4/UAS-Obp RNAi line versus the tubulin-GAL4/UAS-progenitor line, S the fixed effect of sex and E the environmental variance. The data were further analyzed by tastant and sex using a reduced one-way ANOVA model, Y = μ + L+ E, where μ is the overall mean, L the fixed effect of the tubulin-GAL4/UAS-Obp RNAi lines, and E the environmental variance. Analyses of variance and tests of significance were calculated using the Proc GLM procedure in SAS (SAS Institute, Cary, NC, USA).
Results
Assessment of reduction in feeding to a panel of bitter tastants
To assess the effects of bitter tastants on sucrose consumption we measured dose-dependent responses to 13 tastants of offspring from the progenitor control line (y,w 1118 ; P{attP,y + ,w 3’ }) crossed to the tubulin-GAL4 driver line, for males and females separately, over concentrations ranging from 1–30mM. These tastants include naturally occurring alkaloids, terpenoids, and phenolic compounds, as well as three synthetic compounds. Many of them are toxic, perceived as bitter by humans, and have been tested in Drosophila previously (Meunier et al. 2003; Hiroi et al. 2004; Thorne et al. 2004; Wang et al. 2004; Marella et al. 2006; Lee et al. 2009). We selected concentrations that resulted in a decline of about 50% of sucrose consumption compared to control for subsequent studies. These concentrations enable detection of both reductions and increases in consumption during inhibition of expression of target Obp genes with RNAi. Examples of dose–response profiles for two bitter tastants, coumarin and papaverine, are shown in Figure 1B. In each case ~50% reduction in sucrose consumption occurs at 5mM bitter tastant for both sexes. Optimal discriminatory concentrations for the other tastants were 7.5mM for berberine chloride, 5mM for caffeine, 0.5mM for denatonium benzoate, 1mM for escin, 2.5mM for N-phenylthiourea and theophylline, and 0.8mM for quinine hydrochloride dehydrate (Supplementary Figure 1). We did not observe reductions in sucrose intake for N,N-diethyl-meta-toluamide, d-(-)-salicin, sucrose octaacetate, and umbelliferone, which, therefore, appear not to be perceived as aversive. Hence, these four tastants were excluded from further studies.
Effects of inhibition of expression of Obp genes by RNAi on feeding behavior
We determined feeding response profiles to 9 bitter tastants and sucrose for each tubulin-GAL4/UAS-Obp RNAi line and the control. Since we did not find significant differences between sucrose intake without bitter tastant among the tubulin-GAL4/UAS-Obp RNAi lines and the control line (Table 1), we analyzed the data without normalization for sucrose consumption and used a three way ANOVA to assess statistically significant differences between tubulin-GAL4/UAS-Obp RNAi lines and the control. We found significant effects for the Line, Line-by-Tastant, and Line-by-Sex terms for all tubulin-GAL4/UAS-Obp RNAi lines (Table 2). We then used a reduced ANOVA model to identify tastant-specific and sex-specific effects for each tubulin-GAL4/UAS-Obp RNAi line.
Table 1.
Analysis of variance of sucrose consumption by tubulin-GAL4/UAS-Obp RNAi and progenitor control RNAi lines
| Source of variation | df | MS | F | P value |
|---|---|---|---|---|
| Genotype | 1 | 13.42 | 1.79 | 0.18 |
| Sex | 1 | 9.06 | 1.21 | 0.27 |
| Genotype × Sex | 1 | 4.44 | 0.59 | 0.44 |
| Error | 216 | 7.50 |
df, degrees of freedom; MS, mean squares
Table 2.
Analysis of variance of bitter tastant consumption by tubulin-GAL4/UAS-Obp RNAi and progenitor control RNAi lines
| Source of variation | df | MS | F | P value |
|---|---|---|---|---|
| Line | 16 | 425.56 | 81.89 | <0.0001 |
| Tastant | 8 | 1034.63 | 199.11 | <0.0001 |
| Line × Tastant | 128 | 68.29 | 13.14 | <0.0001 |
| Sex | 1 | 401.60 | 77.29 | <0.0001 |
| Line × Sex | 15 | 61.60 | 11.85 | <0.0001 |
| Sex × Tastant | 8 | 354.79 | 68.28 | <0.0001 |
| Line × Sex × Tastant | 120 | 38.97 | 7.50 | <0.0001 |
| Error | 1888 | 5.20 |
df, degrees of freedom; MS, mean squares
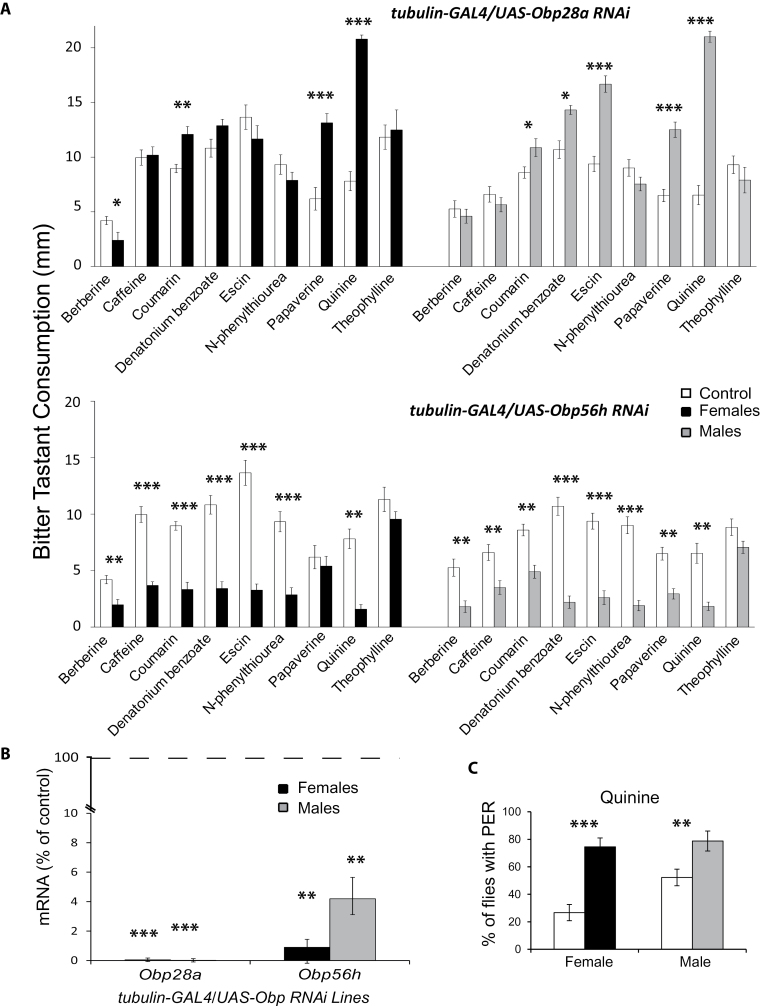
Disruption of expression of individual Obp genes resulted in altered feeding responses to multiple, but not all tastants (Figure 2A; Supplementary Figure 2), indicating that modulation of consumption of bitter tastants by OBPs is combinatorial, similar to their effects on olfactory behavior (Swarup et al. 2011). Inhibition of Obp expression could result either in increased or reduced consumption depending on the odorant or OBP. For example, suppression of expression of Obp28a led to increases in consumption of bitter tastants, whereas inhibition of expression of Obp56h led to dramatic reductions in bitter taste consumption (Figure 2A). We isolated probosces from tubulin-GAL4/UASObp28a RNAi, tubulin-GAL4/UASObp56h RNAi and control flies and showed that mRNA for Obp28a was virtually eliminated and mRNA levels for Obp56h were reduced by approximately 95% and 98% in males and females, respectively (Figure 2B). The largest increase in bitter tastant intake in tubulin-GAL4/UASObp28a RNAi flies was observed for quinine (Figure 2A). To confirm that this increase in quinine intake was due to gustation rather than postingestive effects, we measured probosic extension responses for tubulin-GAL4/UAS-Obp28a RNAi flies and controls. The observed increase in quinine intake in the CAFE assay was paralleled by a substantial increase in the proboscis extension response (Figure 2C). Thus, gustatory perception contributes to modulation of consumption of bitter tastants by OBPs.
Figure 2.
Behavioral responses of tubulin-GAL4/UAS-Obp28a and tubulin-GAL4/UAS-Obp56h RNAi lines to bitter tastants. (A) Consumption of 9 bitter tastants by tubulin-GAL4/UAS-Obp28a RNAi and tubulin-GAL4/UAS-Obp56h RNAi lines represented as solution intake in millimeters summed over three capillaries. Asterisks denote significant changes in consumption of bitter tastants between females, shown in black bars; males, shown in grey bars, and corresponding consumption of the progenitor control line, shown in open bars, as determined by Tukey’s test (*P < 0.05; **P < 0.005; ***P < 0.0001). (B) Changes in mRNA levels in the probosces of tubulin-GAL4/UAS-Obp28a and tubulin-GAL4/UAS-Obp56h RNAi flies, shown as a percentage of the expression level in the control line (dotted line). Asterisks denote significant changes determined by two tailed Student t tests (**P < 0.005; ***P < 0.0001). (C) Proboscis extension response (PER). Tubulin-GAL4/UAS-Obp28a RNAi flies, represented by black and grey bars for females and males, respectively, and tubulin-GAL4/progenitor control flies (open bars) were stimulated with a 2µl droplet of 0.8mM quinine for up to 5 s. Full proboscis extension in response to the stimulus was counted as a positive response. Asterisks denote significant changes determined by two tailed Student’s t tests (**P < 0.001; ***P < 0.0001).
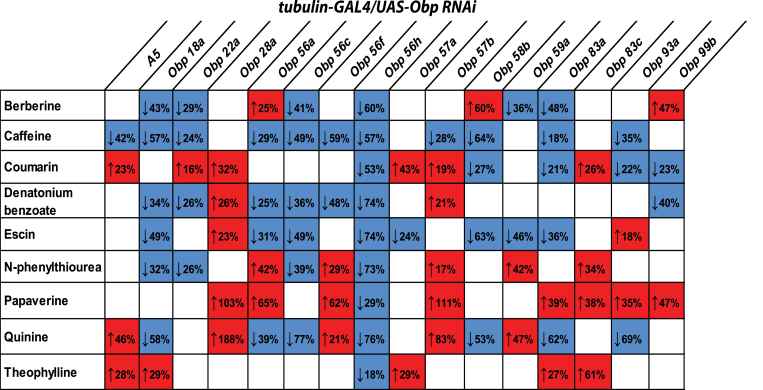
Feeding response profiles for all other tubulin-GAL4/UAS-Obp RNAi lines are illustrated in Supplementary Figure 2 and summarized in Figure 3, which illustrates that inhibition of individual Obp genes modulates feeding behavior with different bitter tastants and each bitter tastant is affected by a different subset of Obp RNAi targets. When feeding responses are considered pooled across sexes, inhibition of some OBPs consistently, albeit with minor exceptions, leads to increased consumption of the cognate bitter tastants (e.g., A5, OBP28a, OBP57b, OBP83c), whereas others lead to a decrease in bitter taste consumption (e.g., OBP18a, OBP22a, OBP56c, OBP56h, OBP58b, and OBP59a; Figure 3).
Figure 3.
Combinatorial response profiles for intake of bitter tastants of tubulin-GAL4/UAS-Obp RNAi lines. The red boxes and “up” arrow sign (↑) indicate more consumption of bitter tastant compared to the control, while the blue boxes and “down” arrow sign (↓) indicate less consumption of aversive tastant compared to the control. Numbers represent the magnitude of changes in consumption level as percentage compared to control. Note, that only female flies for the tubulin-GAL4/UAS-Obp58b RNAi line were available for testing.
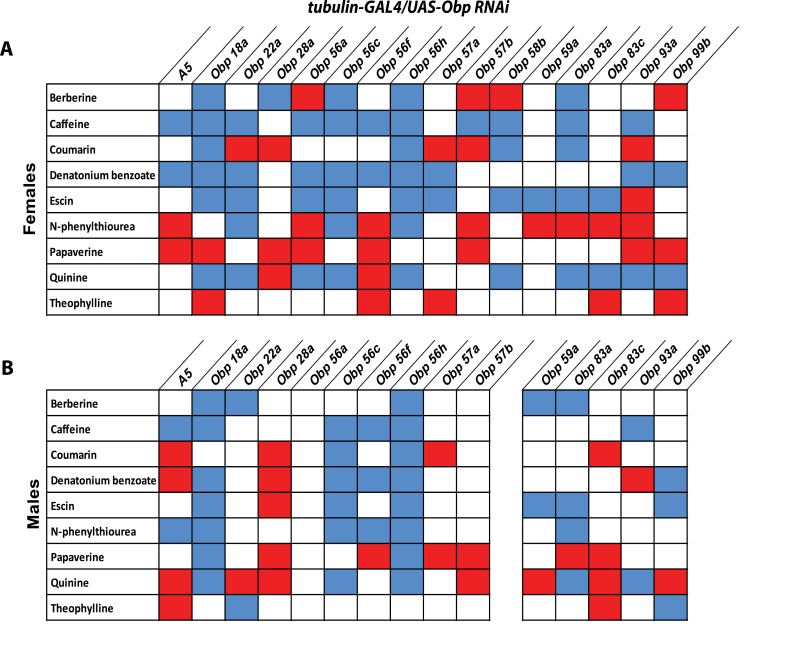
Since our ANOVA revealed significant effects by sex and all sex interaction terms (Table 2), we examined feeding responses of the tubulin-GAL4/UAS-Obp RNAi for males and females separately. This analysis reveals extensive sexual dimorphism (Figure 4), similar to that observed previously for combinatorial effects of OBPs on olfactory behavior (Swarup et al. 2011), indicating that inhibition of Obp gene expression affects feeding behavior differently in males and females.
Figure 4.
Sexually dimorphic combinatorial response profiles for intake of bitter tastants of tubulin-GAL4/UAS-Obp RNAi lines. (A) Females, (B) Males. The red boxes indicate more consumption of bitter tastant compared to the control, while the blue boxes indicate less consumption of aversive tastant compared to the control.
Discussion
OBPs and gustation
In recent years, substantial evidence has accumulated that OBPs function as carriers of hydrophobic ligands in the olfactory system (Wojtasek and Leal 1999; Xu et al. 2005; Grosse-Wilde et al. 2006; Swarup et al. 2011). Association of some OBPs with host plant selection (Matsuo et al. 2007; Matsuo 2008) and their expression in multiple taste organs (Galindo and Smith 2001) has led us to hypothesize that, in addition to modulating olfactory behavior, OBPs modulate feeding behavior, especially the avoidance of bitter tastants, which are mostly hydrophobic compounds. Results from our experiments consolidate this notion and show that suppression of individual OBPs affects intake of bitter substances. There are two caveats to this interpretation. First, we cannot exclude that olfactory cues may contribute to feeding behavior in the CAFE assay and that part of the aversive perception of bitter tastants may be mediated via olfactory receptors upon contact with the antenna. Second, we cannot exclude postingestive effects rather than gustatory input on feeding behavior. To evaluate these caveats we chose a more detailed follow-up on OBP28a. We selected this particular OBP, because 1) suppression of OBP28a consistently shows increased intake across the panel of bitter tastants, suggesting that OBP28a functions as a transporter of bitter tastants to gustatory receptors; 2) Obp28a mRNA is relatively abundant in isolated probosces; 3) RNAi that targets Obp28a results in nearly complete obliteration of its message; and, 4) RNAi-mediated inhibition of Obp28a results in an exceptionally large increase (188%) in intake of quinine, a standard bitter tastant. We showed that in the case of Obp28a increases in proboscis extension responses paralleled increases in intake of quinine. Thus, at least OBP28a contributes directly to gustation.
Sexually dimorphic effects of OBPs on feeding behavior
The interaction diagrams between OBPs and bitter tastants are overlapping, but distinct for males and females (Figure 4). This is perhaps not surprising, as previous studies have shown profound differences in expression levels of Obp genes between the sexes (Zhou et al. 2009) and combinatorial interactions between OBPs and odorants also show extensive sexual dimorphism (Swarup et al. 2011). These sex differences may have ecological significance as females evaluate oviposition sites, whereas males use, among others, gustatory cues to evaluate females as mating partners by detecting cuticular pheromones (Bray and Amrein 2003; Moon et al. 2009). The observed sexual dimorphism of the effects of OBPs on feeding behavior is in line with the previous assessment that males and females utilize the repertoire of chemosensory genes differently (Zhou et al. 2009; Swarup et al. 2011). This type of sex dependent phenotypic plasticity may be related to different toxins males and females are likely to encounter during distinct activities of their life cycle (e.g., only females are concerned with identifying suitable oviposition sites).
Mechanisms by which OBPs and bitter tastants may modulate sucrose intake
Recognition of bitter tastants by OBPs appears to be combinatorial with any given OBP being able to interact with more than one tastant and each tastant with multiple OBPs (Figure 3). The pattern of interactions suggests a distinct, albeit not perfect, grouping of OBPs into two categories: OBPs that increase the consumption of bitter tastants when their expression is compromised, and OBPs that reduce the ingestion of bitter compounds when their expression is suppressed. The most parsimonious explanation for this phenomenon is that OBPs fulfill different functions: transport of bitter tastants to their cognate receptors, and sequestration or clearance of bitter tastants. The former would lead to an increase in feeding behavior, when inhibited, as is the case for OBP28a, whereas the latter would lead to a reduction in feeding behavior, as is the case for OBP56h. Alternatively, it is possible that some OBPs upon binding bitter tastants may interact directly with sucrose sensitive neurons and modulate their activity, as has been demonstrated recently for OBP49a, which is expressed in thecogen cells (Jeong et al. 2013). Binding of bitter tastants to OBP49a does not affect bitter taste sensation, but leads to inhibition of sucrose sensing gustatory neurons likely by interacting with Gr64a, a component of the sucrose receptor complex (Jeong et al. 2013). However, the precise molecular mechanism of this interaction remains to be elucidated.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health [GM059469].
Supplementary Material
Acknowledgments
We would like to thank Dr Trudy F. C. Mackay for helpful advice regarding statistical analyses.
References
- Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415 [DOI] [PubMed] [Google Scholar]
- Bray S, Amrein H. 2003. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 39:1019–1029 [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. 2010. The molecular basis for water taste in Drosophila. Nature 465:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100:703–711 [DOI] [PubMed] [Google Scholar]
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. 2013. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster . Nat Commun. 4: 2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. 2010. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for Drosophila gustatory water reception. J Neurosci. 30:6247–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. 2001. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila . Nat Neurosci. 4:1182–1186 [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila . Nature. 448:151–156 [DOI] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. 2007. The detection of carbonation by the Drosophila gustatory system. Nature. 448:1054–1057 [DOI] [PubMed] [Google Scholar]
- Galindo K, Smith DP. 2001. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics.159:1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde E, Svatos A, Krieger J. 2006. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem Senses. 31:547–555 [DOI] [PubMed] [Google Scholar]
- Hiroi M, Meunier N, Marion-Poll F, Tanimura T. 2004. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila . J Neurobiol. 61:333–342 [DOI] [PubMed] [Google Scholar]
- Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. 2013. An odorant binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 79:725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. 2009. Multiple gustatory receptors required for the caffeine response in Drosophila . Proc Natl Acad Sci USA. 106:4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. 2006. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 49:285–295 [DOI] [PubMed] [Google Scholar]
- Masek P, Scott K. 2010. Limited taste discrimination in Drosophila . Proc Natl Acad Sci U S A. 107:14833–14838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y. 2007. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia . PLoS Biol. 5:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T. 2008. Genes for host-plant selection in Drosophila . J Neurogenet. 22: 195–210 [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. 2003. Peripheral coding of bitter taste in Drosophila . J Neurobiol. 56:139–152 [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. 2009. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 19: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. 2005. The receptors and coding logic for bitter taste. Nature. 434: 225–229 [DOI] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. 2001. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila . Cell. 104:661–673 [DOI] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. 2007. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H. 2007. Sugar receptors in Drosophila . Curr Biol. 17:1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S, Williams TI, Anholt RRH. 2011. Functional dissection of Odorant binding protein genes in Drosophila melanogaster . Genes Brain Behav. 10:648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. 2004. Taste perception and coding in Drosophila . Curr Biol. 14:1065–1079 [DOI] [PubMed] [Google Scholar]
- Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. 2001. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 11:1451–1455 [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. 2004. Taste representations in the Drosophila brain. Cell. 117:981–991 [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. 2011. The molecular and cellular basis of bitter taste in Drosophila . Neuron. 69:258–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasek H, Leal WS. 1999. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J Biol Chem. 274:30950–30956 [DOI] [PubMed] [Google Scholar]
- Xu P, Atkinson R, Jones DN, Smith DP. 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 45:193–200 [DOI] [PubMed] [Google Scholar]
- Zhou S, Stone EA, Mackay TFC, Anholt RRH. 2009. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster . PLoS Genet. 5: e1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.