Abstract
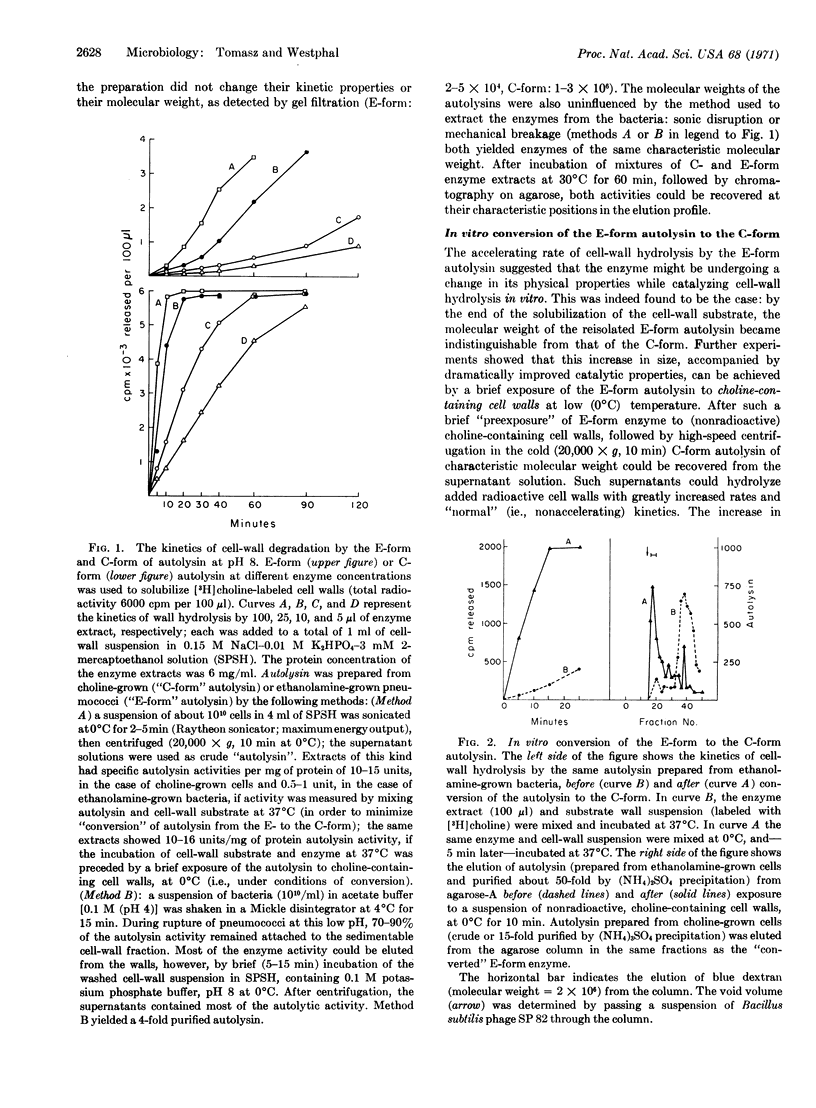
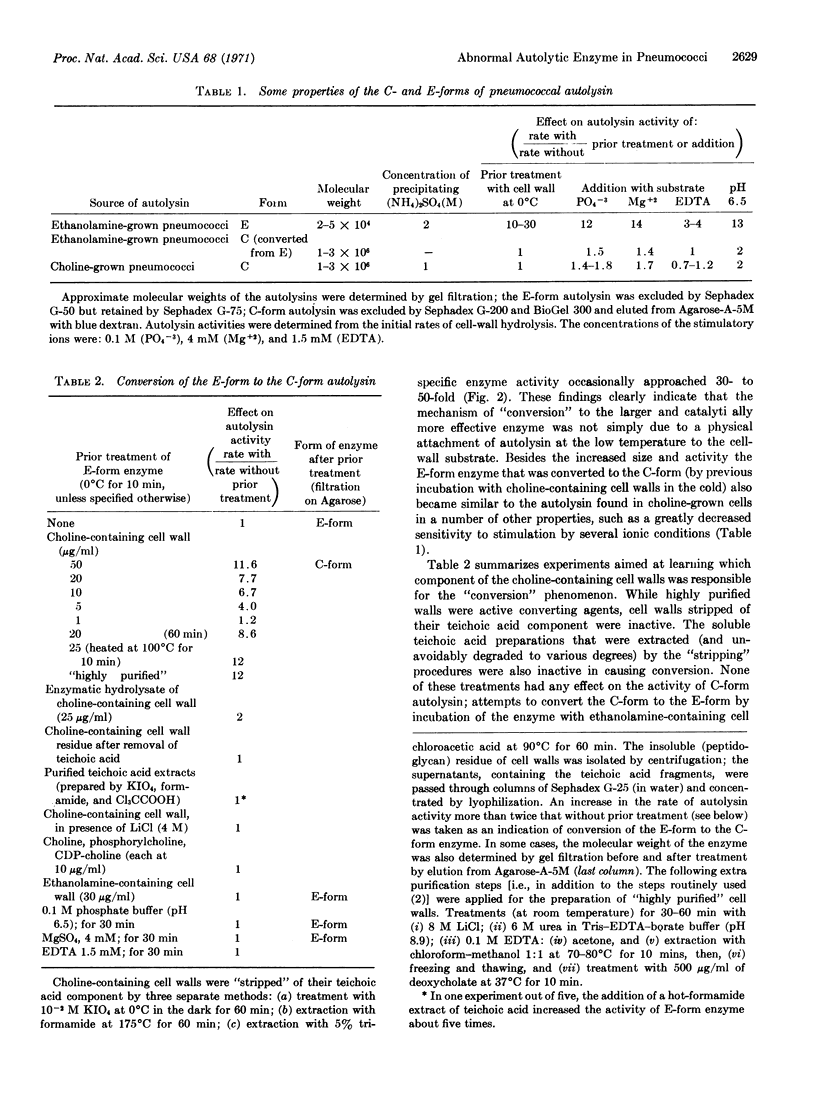
Pneumococci in which the choline component of the cell wall teichoic acid was replaced by ethanolamine contain an abnormal autolytic enzyme that has a low molecular weight and low activity in contrast to the enzyme typical of choline-containing bacteria that has a high molecular weight and high activity. The abnormal autolysin can be converted to the normal (cholinetype) enzyme by incubation in vitro with choline-containing cell walls.
Keywords: cell wall, lysis, enzyme activation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Brock T. D. Effect of teichoic acid on resistance to the membrane-lytic agent of Streptococcus zymogenes. J Bacteriol. 1966 Dec;92(6):1623–1631. doi: 10.1128/jb.92.6.1623-1631.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y. Structural and immunological studies on the pneumococcal C polysaccharide. J Biol Chem. 1967 Feb 10;242(3):463–470. [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1969;38:389–414. doi: 10.1146/annurev.bi.38.070169.002133. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]



