Abstract
The diagnostic performance of canine serum amyloid A (SAA) was compared with that of C-reactive protein (CRP) in the detection of systemic inflammation in dogs. Sera from 500 dogs were retrospectively included in the study. C-reactive protein and SAA were measured using validated automated assays. The overlap performance, clinical decision limits, overall diagnostic performance, correlations, and agreement in the clinical classification between these 2 diagnostic markers were compared. Significantly higher concentrations of both proteins were detected in dogs with systemic inflammation (SAA range: 48.75 to > 2700 mg/L; CRP range: 0.4 to 907.4 mg/L) compared to dogs without systemic inflammation (SAA range: 1.06 to 56.4 mg/L; CRP range: 0.07 to 24.7 mg/L). Both proteins were shown to be sensitive and specific markers of systemic inflammation in dogs. Significant correlations and excellent diagnostic agreement were observed between the 2 markers. However, SAA showed a wider range of concentrations and a significantly superior overall diagnostic performance compared with CRP.
Résumé
Comparaison de la protéine amyloïde sérique A et de la protéine C réactive comme marqueurs diagnostiques de l’inflammation systémique chez les chiens. La performance diagnostique de l’amyloïde sérique canine A (SAA) a été comparée à celle de la protéine C réactive (PCR) dans la détection de l’inflammation systémique chez les chiens. Le sérum de 500 chiens a été inclus rétrospectivement dans l’étude. La protéine C réactive et la SAA ont été mesurées en utilisant des bioanalyses automatisées validées. La performance de chevauchement, les limites de décision cliniques, la performance diagnostique globale, les corrélations et la concordance dans la classification clinique entre ces 2 marqueurs diagnostiques ont été comparés. Des concentrations significativement supérieures des deux protéines ont été détectées chez les chiens avec une inflammation systémique (plage de la SAA : de 48,75 à > 2700 mg/L; plage de la PCR : de 0,4 à 907,4 mg/L) comparativement aux chiens sans inflammation systémique (plage de la SAA : de 1,06 à 56,4 mg/L; plage de la PCR : de 0,07 à 24,7 mg/L). Il a été démontré que les deux protéines étaient sensibles et des marqueurs spécifiques de l’inflammation systémique chez les chiens. Des corrélations significatives et une concordance diagnostique excellente ont été observées entre les deux marqueurs. Cependant, la SAA a indiqué un écart plus vaste pour les concentrations et une performance diagnostique significativement supérieure comparativement à la PCR.
(Traduit par Isabelle Vallières)
Introduction
C-reactive protein (CRP) and serum amyloid A (SAA) are major positive acute phase proteins in dogs and humans that show marked increases in concentration during systemic inflammation (1,2). The advantages of routine measurements of CRP for diagnostic and monitoring purposes in dogs have been well-documented (3–8). In addition, commercially available assays for human CRP have been validated for automated measurements of canine CRP (9–11), so that CRP has become the main acute phase protein used for routine measurements in dogs (2,12). In humans, SAA has been shown to be potentially comparable to CRP in terms of diagnostic value (13–15), and some studies have demonstrated that human SAA may be an even more sensitive marker of systemic inflammation than CRP (16–20). On the other hand, the results of studies comparing the diagnostic value of canine CRP and SAA have been equivocal. While some studies have shown comparable diagnostic performance between these 2 inflammatory markers (6,21–23), other studies have found canine SAA to be inferior to CRP as a diagnostic marker (24–26). Previous studies comparing canine SAA and CRP have used time-consuming methods to measure SAA, such as enzyme-linked immunoassays (22,23,26,27), which are not applicable to routine diagnostic use. However, a latex agglutination turbidimetric immunoassay (LAT) is now available for automated, routine measurements of canine SAA (28). Therefore, further investigation of the diagnostic potential of this acute phase protein, including comparative studies of canine SAA and CRP, has become relevant.
Previous studies comparing the diagnostic potential of canine SAA and CRP have focused only on specific disorders such as leishmaniosis (23,26), pyometra (21), or meningitis (6). However, more knowledge about diagnostic performance in a wider spectrum of disorders is needed for the results to be representative of a general clinical setting. Therefore, the aim of this study was to compare the diagnostic capacity of canine SAA and CRP in the detection of systemic inflammation in a wide range of clinically representative conditions with and without inflammation.
Materials and methods
Animals
Sera from 500 dogs were included in the study. There were 250 dogs with well-categorized inflammatory and noninflammatory conditions (group A), and 250 dogs with various or unknown degrees of systemic inflammation (group B). The 250 group A dogs were used to compare the diagnostic capacity of canine CRP and SAA, while all 500 dogs were used to investigate the diagnostic agreement and correlation between canine CRP and SAA. Serum was obtained by venipuncture, isolated by centrifugation, and stored in plastic vials between −20°C and −80°C for a maximum of 5 y. Sera stored in serum banks after diagnostic analyses or previous scientific studies (29–31) were retrospectively used in the present study. This approach was approved by the local ethical committee, Department of Clinical and Animal Sciences, University of Copenhagen, Denmark (CLINCopen). The samples were shipped on dry ice (with the exception of samples obtained after castration of male dogs or accidental trauma, which were shipped on wet ice for a maximum of 13 h) for analysis at the Central Laboratory, CLINCopen. The samples were brought to room temperature on the day of analysis, visible particulates were removed, and samples were thoroughly mixed prior to analysis.
Comparison of the diagnostic capacity of canine CRP and SAA
Samples from 250 group A dogs were divided into 7 groups according to clinical diagnosis and the presence or absence of systemic inflammation: clinically healthy dogs (n = 76), dogs with various disorders not accompanied by systemic inflammation (n = 36), dogs exposed to elective surgery (n = 26), dogs exposed to accidental traumas (n = 21), snake-envenomed dogs (n = 45), dogs with miscellaneous systemic inflammatory disorders (n = 36), and dogs that developed aspiration pneumonia during hospitalization (n = 10). The first 2 groups represented dogs without systemic inflammation, while the remaining groups represented those with systemic inflammation. The dogs had a mean age of 5.3 y (range: 0.5 to 14 y); 82% of the dogs were purebreds from 67 breeds; 48% of the dogs were males and 52% were females.
Clinically healthy dogs
Clinically healthy dogs (n = 76) were client-owned dogs with no history of clinical illness and no signs of illness on clinical or hematologic examinations. Twenty-nine of these dogs were presented to CLINCopen, during 2010–2011 for various reasons, such as health screening prior to blood donation or breeding, and were retrospectively included in the study. Forty-seven dogs were included as healthy controls from serum banks. These dogs were previously included in prospective, ethically approved studies at the Department of Clinical Sciences of Companion Animals, University of Utrecht, Netherlands (CLINUtrecht) (n = 28) (29); CLINCopen (n = 8); Blue Star Animal Hospital, Gothenburg, Sweden (CLINGothen) (n = 6); and Department of Clinical Studies, School of Veterinary Medicine, University of Pennsylvania, United States (CLINPenn) (n = 5) (31).
Diseased dogs without systemic inflammation
Thirty-six dogs were diagnosed with cardiovascular, hepatic, dental, or other disorders not previously reported to be associated with systemic inflammation (3,32). No clinical signs of systemic involvement were observed in these animals. Dogs with uncomplicated local inflammatory disorders such as cystitis, local dermatitis, or anal sacculitis were also included in this group, since systemic involvement was unlikely in such localized processes without systemic clinical signs (33). All dogs in this group were presented to CLINCopen, during 2010–2012, and were retrospectively included in the study. A general clinical examination was performed, and basic hematologic and biochemical profiles were analyzed for all dogs (34) with no signs of inflammation observed by hematologic examination. Additional diagnostic tests were performed at the discretion of the attending clinician, including urinalysis, radiography, ultrasonography, endocrine testing, and cytology. Conclusions about the diagnoses were based on written information from the attending clinicians.
Dogs with systemic inflammation induced by aseptic elective surgery
Twenty-one healthy bitches were included in a study at CLINUtrecht; the serum was collected 24 h following completion of ovariohysterectomy or ovarioectomy without complications (29). In addition, 5 healthy dogs were included in a study at the Department of Animal Medicine and Surgery, University of Murcia, Spain; the serum was collected 24 h following uncomplicated surgical castration (30).
Dogs with systemic inflammation induced by accidental trauma
Nine dogs were presented to the San Marco Veterinary Clinic, Padova, Italy, during the summer of 2011; 8 dogs, to CLINPenn, during the summer of 2011 (31); and 4 dogs to CLINCopen, during 2010–2012. All dogs were hospitalized for treatment after accidental traumas (e.g., car accidents or dog bites) and the serum was collected 12 to 24 h after the primary event.
Dogs with systemic inflammation induced by snake envenomation
Since snake envenomation has been reported to be associated with a systemic inflammatory response (35,36), the serum of 45 dogs, collected 24 h after snake envenomation, was retrospectively included in the study. Twenty-five dogs were hospitalized at CLINGothen, following envenomation by the European viper (Vipera berus). Twenty dogs were hospitalized at the Department of Companion Animal Clinical Sciences, University of Pretoria, South Africa, during 2010–2011 after envenomation by the African puff adder (Bitis arietans, n = 8), snouted cobra (Naja annulifera, n = 10), or the Mozambique spitting cobra (Naja mossambica, n = 2).
Dogs with systemic inflammation induced by various miscellaneous causes
Thirty-six dogs that suffered from polyarthritis, peritonitis, pyometra, or other disorders associated with systemic inflammation (2,3,21,37), were included. All of these dogs were lethargic suggesting systemic involvement. Twenty-five of the dogs were presented to CLINCopen, during 2010–2012. Additionally, 11 dogs were hospitalized at CLINPenn during the summer of 2011 (31). General clinical examinations were performed, and basic hematologic and biochemical profiles were analyzed for all dogs (34). Additional diagnostic tests were performed at the discretion of the attending clinicians, including radiography, ultrasonography, cytology, histopathology, and analysis of pancreatic lipases. The final diagnoses were based on written information from the attending clinicians.
Dogs with systemic inflammation induced by aspiration pneumonia
Ten dogs developed aspiration pneumonia during hospitalization at CLINPenn during the summer of 2011 (31). The presence of aspiration pneumonia was suspected because of a history of vomiting or regurgitation, and confirmed by radiography. Serum collected 24 h after the aspiration was used in the study.
Diagnostic agreement and correlation between canine SAA and CRP
Apart from the dogs previously mentioned, an additional 250 dogs were included in the study to contribute to the assessment of the correlation and diagnostic agreement between canine SAA and CRP. These dogs were presented to CLINCopen (n = 198), during 2007–2012 or CLINPenn (n = 20), during the summer of 2011 (31). These dogs presented with disorders of varying degrees of systemic involvement (e.g., neoplastic, neurologic, or gastrointestinal diseases), or with nonspecific symptoms treated without a diagnosis. The remaining 32 dogs presented to private Danish veterinary clinics, and serum was collected as part of a diagnostic work-up and sent for analysis at the Central Laboratory, University of Copenhagen, Denmark, during 2010–2011. Information about the specific diagnoses of these patients was not available to the authors.
Procedures
Canine serum amyloid A concentrations were measured by an automated LAT (SAA-1; Eiken Chemical Company, Tokyo, Japan) using heterologous human SAA for calibration. C-reactive protein concentrations were measured with a commercial human turbidimetric immunoassay (TIA; High linearity CRP, Randox, Crumlin, County Antrim, United Kingdom) using canine CRP for calibration (Canine CRP; LifeDiagnostics, West Chester, Pennsylvania, USA). Both parameters were analyzed using an automated clinical chemical analyzer (Advia 1800; Siemens, Munich, Germany); both assays have been validated for diagnostic use in dogs (10,11,28,38). An automated 1:6 reflex dilution of samples with CRP concentrations above 160 mg/L resulted in a linear range up to 960 mg/L. Measurements exceeding the documented range of linearity for the SAA assay (2700 mg/L) (38) were assigned the value of this limit.
The SAA and CRP concentrations determined for the 7 groups of dogs (n = 250) were visualized graphically on a scatter plot using a logarithmic scale. The overall diagnostic capacity of SAA and CRP was assessed by receiver operating characteristic analysis (ROC) based on the measurements of both parameters in the 5 groups of dogs with systemic inflammation and in the 2 groups of dogs without systemic inflammation. The parameters were deemed to be efficient markers of systemic inflammation if the 95% confidence interval (CI) of the area under the ROC curve (AUC) exceeded 0.8. The diagnostic potential of SAA and CRP as markers of systemic inflammation was compared by use of the AUCs obtained by ROC analysis (39,40). The significance level was set to P < 0.05.
The optimal clinical decision limit of CRP was established by the ROC analysis through the use of the maximal differential positive rate, where the differential positive rate = sensitivity − (1 − specificity), as previously done for SAA (41).
The overlap performance of CRP was evaluated as previously done for SAA (41). The Kruskal-Wallis test was used to test for significant differences in concentrations among the 7 groups of dogs, and Dunn’s multiple comparison test was used for further characterization of observed differences. Because of the heterogeneous nature of the group of dogs envenomed by snakes (envenomation was caused by 4 different types of snakes), an additional investigation of the overlap performance was conducted for this particular group. The significance level was set to P < 0.05.
The agreement in clinical classification was evaluated through visualization on a scatter plot of the 500 paired CRP and SAA measurements and through dichotomization by using the clinical decision limits established above. Weighted kappa was used for the statistical assessment of agreement in the clinical classification. Weighted kappas between 0.4 and 0.75 or above 0.75 were set as the criteria for good or excellent agreement, respectively (42).
The correlation between canine SAA and CRP measurements was evaluated by calculating Pearson’s correlation coefficient; a correlation coefficient exceeding 0.3 was set as the criterion for positive correlation (42). All assumptions for statistical tests were formally tested and found to be met.
Results
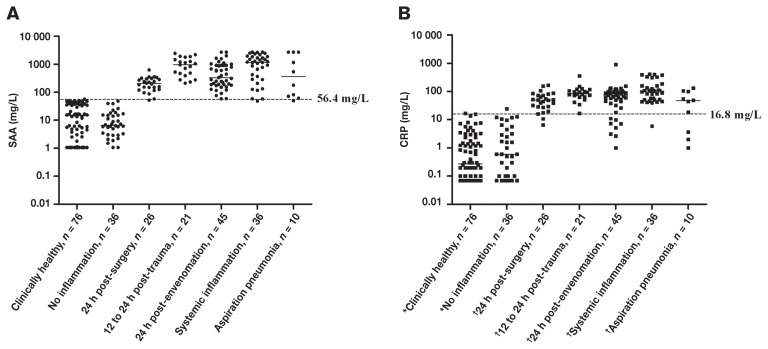
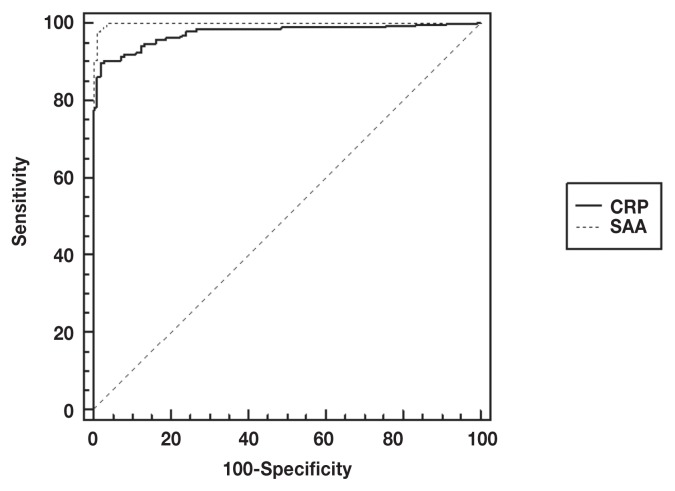
A wide range of concentrations was measured for both CRP and SAA. However, a wider range of values was observed for the SAA measurements (range: 1.06 to > 2700 mg/L than for the CRP measurements (range: 0.07 to 902 mg/L) (Figure 1, Table 1). Both proteins showed efficient discriminative capacity, with the 95% CIs of the AUCs above 0.8, indicating that both proteins are useful markers of systemic inflammation for the groups of dogs included in this study (Figure 2). However, the AUC for SAA was significantly higher than that for CRP, indicating the diagnostic capacity of SAA is superior to that of CRP (Figure 2).
Figure 1.
Overlap performance of serum amyloid A (A) and C-reactive protein (B) measured by automated assays in 5 groups of dogs suffering from disorders with systemic inflammation; 1 group of clinically healthy dogs, and 1 group of diseased dogs without systemic inflammation (‘No inflammation’). Measurements below the detection limit were plotted as 1.06 mg/L for SAA and 0.07 mg/L for CRP, which were the previously calculated detection limits of the assays used in the study (9,28). Concentrations above the linear range of the SAA assay were assigned the value of the limit (2700 mg/L) (38). Solid lines — median concentrations; dotted lines — clinical decision limits of canine SAA and CRP obtained by receiver operating-characteristic analyses. n = number of dogs in each group. Groups with different symbols (*, †) had significantly different concentrations of CRP (P < 0.01, Kruskal-Wallis and Dunn’s multiple comparison test). Parts of this figure have previously been used in another study from our group (41).
Table 1.
Serum amyloid A (SAA) and C-reactive protein (CRP) measured in 7 groups of dogs with different conditions with systemic inflammation (shaded area) or without it. Minimum, median, and maximum concentrations are shown for each group, n = number of dogs in each group. Measurements above the linear range of the SAA assay were assigned the value of this limit (>2700 mg/L) (38), whereas measurements below the detection limit of the assays were assigned the values of these limits (9,28)
| n | Minimum | Median | Maximum | ||
|---|---|---|---|---|---|
| Clinically healthy | 76 | SAA | 1.06 | 14.6 | 56.4 |
| CRP | 0.07 | 0.28 | 16.8 | ||
| No inflammation | 36 | SAA | 1.06 | 6.5 | 48.4 |
| CRP | 0.07 | 1.0 | 24.7 | ||
| 24 hours post-surgery | 26 | SAA | 52.3 | 204.2 | 625.9 |
| CRP | 6.6 | 49.9 | 169 | ||
| 12–24 hours post-trauma | 21 | SAA | 209.5 | 945.3 | 2434 |
| CRP | 17.05 | 81.8 | 360.1 | ||
| 24 hours post-envenomation | 45 | SAA | 58.4 | 334.1 | > 2700 |
| CRP | 0.07 | 73.6 | 902.4 | ||
| Systemic inflammation | 36 | SAA | 48.8 | 1174 | > 2700 |
| CRP | 6.0 | 103 | 411.6 | ||
| Aspiration pneumonia | 10 | SAA | 49.6 | 359.3 | > 2700 |
| CRP | 0.4 | 47.0 | 134.8 |
Figure 2.
Receiver operating characteristic analysis curves comparing the diagnostic sensitivity and specificity of serum amyloid A (SAA) and C-reactive protein (CRP) for the detection of systemic inflammation in dogs. The 95% confidence intervals of the area under the curve (AUC) was 0.999 (0.983 to 1.0) for SAA and 0.976 (0.948 to 0.991) for CRP. The difference between the AUCs was 0.023 (0.004 to 0.041) and was statistically significant (P = 0.015).
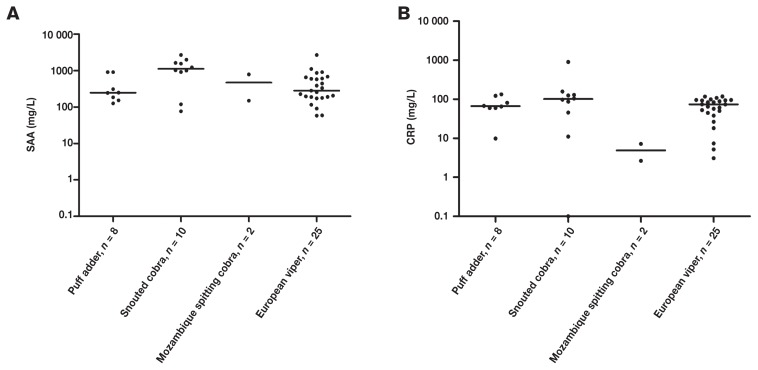
The optimal clinical decision limit was estimated to be 16.8 mg/L for CRP using ROC analysis. In a previous study in this laboratory, this value was determined to be 56.4 mg/L for SAA (41). An acceptable overlap performance was observed with higher concentrations of both CRP and SAA in dogs with systemic inflammation relative to dogs without systemic inflammation. The differences in CRP concentrations between dogs with and without inflammation were significant (Figure 1) and were comparable to previous results for SAA (41). However, in 4 of the 5 groups with systemic inflammation, several dogs had CRP levels below the clinical decision limit, even though SAA concentrations exceeded the clinical decision limit (Figure 1, Table 1). Particularly for snake envenomation, several dogs had CRP concentrations below the clinical decision limit of 16.8 mg/L, despite elevated concentrations of SAA (Figure 1). These findings could not be explained by differences in the acute phase response following envenomation by the different types of snakes, since no significant differences in either SAA or CRP concentrations were observed across the snake species included in the present study (P > 0.05, Figure 3).
Figure 3.
Overlap performance of serum amyloid A (A) and C-reactive protein (B) in dogs following envenomation by African puff-adder (Bitis arietans), snouted cobra (Naja annulifera), Mozambique spitting cobra (Naja mossambica), or European viper (Vipera berus). No significant differences in measured concentrations were observed for either SAA or CRP. Concentrations above the linear range of the SAA assay were assigned the value of the limit [2700 mg/L (38)].
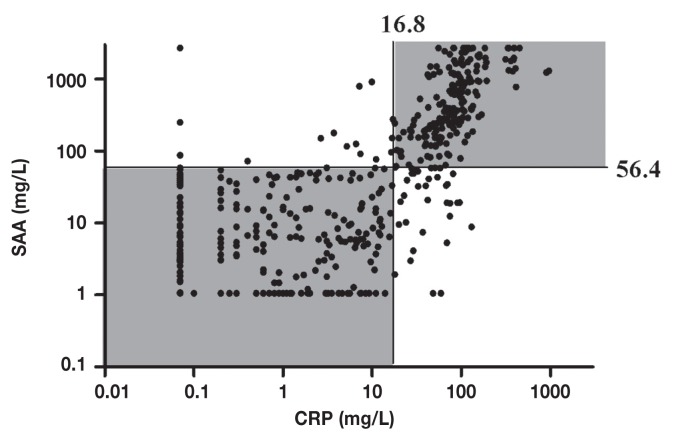
The diagnostic agreement in the clinical classification had a weighted kappa of 0.8, as determined using the estimated clinical decision levels for CRP and SAA (Figure 4) (42), and was therefore excellent. In addition, the Pearson correlation coefficient between canine SAA and CRP was 0.64 (95% CI: 0.58 to 0.69), indicating a positive correlation between these 2 diagnostic markers (42).
Figure 4.
Diagnostic measurements of serum amyloid A (SAA) and C-reactive protein (CRP) in 500 dogs plotted to illustrate the agreement in clinical classification. Measurements below detection limits were plotted as 1.06 mg/L for SAA and 0.07 mg/L for CRP, which were the previously calculated detections limits of the assays used in the study (9,28). Concentrations above the linear range of the SAA assay were assigned the value of the limit [2700 mg/L (38)]. Horizontal and vertical lines: clinical decision limits of canine SAA and CRP, respectively, as obtained by ROC analyses. Note that agreement in clinical classification was observed in 90% (450 out of 500) of the cases (shaded areas).
Discussion
In the present study, we found that SAA and CRP were both useful as diagnostic markers of systemic inflammation in dogs, and that both markers show comparable diagnostic capacity, overlap performance, and excellent agreement in clinical classification. Nonetheless, despite the utility of both markers, SAA may have greater diagnostic potential compared to CRP. Because the study included 500 dogs of both genders and a wide range of breeds, ages, and disorders, the results can be expected to be representative of a general clinical setting. Since the study was based on automated methods, the results also reveal the advantages of routine diagnostic measurements of the proteins, as the methods used are applicable for routine diagnostic use.
Samples were included following varying conditions of storage and transport, and a potential limitation to the study is the prolonged storage of up to 5 y that may influence results, as stability has not been documented for such a period. However, excellent stability for several months has been documented for both canine CRP and SAA at more harsh conditions, and for human CRP up to 34 mo at conditions similar to this study (43–45). Our group has experience with long-term stability (up to 36 mo) of canine internal control material for CRP and has demonstrated retained dynamics in concentrations of both canine SAA and CRP in serial post-operative samples stored for 5 y (unpublished).
The positive correlation between SAA and CRP in our study was comparable to those of previous studies in humans, which showed correlations of between 0.64 and 0.86 (14,17,20,46). A clear diagnostic potential was observed for both proteins, as demonstrated by an acceptable overlap performance and an effective discriminative capacity. Hence, both SAA and CRP can serve as useful markers of systemic inflammation, as reported in comparative studies of these proteins in dogs (6,21–23). Some studies have demonstrated that SAA may be an inferior marker compared to CRP (24–26). However, these studies used manual methods (e.g., enzyme-linked immunoassays), which are probably less sensitive and more prone to increased imprecision than the LAT. By contrast, the results of our study indicate that SAA measured by LAT is useful as a routine parameter in the assessment of the canine acute phase response, at least comparable to CRP measured by TIA.
Several studies in humans have indicated that SAA may be a more sensitive marker of systemic inflammation than CRP, since increased concentrations of SAA can sometimes be detected despite normal levels of CRP (16–19,47). Similar results were observed in the investigation of the overlap performance in the present study. In particular, several dogs with CRP levels below the clinical decision limit were observed in 4 of the 5 groups with systemic inflammation, despite SAA concentrations exceeding the clinical decision limit. Because of the common etiology within each group of dogs, these findings cannot be explained by different causes of inflammatory stimulation and a more plausible explanation is that SAA and CRP show differences in diagnostic sensitivity. The potentially greater diagnostic capacity of SAA compared to CRP was supported by the comparative ROC analysis showing a significantly greater area under the ROC curve for the SAA measurements relative to CRP. The ROC analysis indicated an overall superiority over the entire range of measurements, independent of the chosen clinical decision limits (40). Differences in sensitivity to induction by cytokines have been suggested as a possible explanation for the observed differences in humans (20,46,48). However, additional studies will be needed to explore the mechanisms underlying these differences in dogs.
As noted, a wider range of concentrations was observed for SAA compared to CRP. This finding could be a result of the differences in the calibration materials used for the 2 assays. In particular, more accurate measurements can be expected when canine-specific material is used for the calibration of the CRP TIA (10) compared with the heterologous calibration of the SAA LAT using human SAA (28). Although several studies have shown less distinct differences in the ranges for SAA and CRP measurement in dogs (21,22,27), similar results have been demonstrated in a study measuring canine SAA (range: 0 to > 2000 mg/L) and CRP (range: 0 to 329 mg/L) (6). Comparable differences in ranges of concentrations have also been observed in several studies of human SAA and CRP (13,14,17,18,20,48,49). As long as specific clinical decision limits are calculated for each parameter individually, the differences in concentration ranges should not influence the clinical classification of the patients. In our study, this principle was demonstrated by the excellent agreement in clinical classification between the 2 parameters.
Serum amyloid A showed a wider range of concentrations in clinically healthy dogs than did CRP, as demonstrated in humans (19,49). The high baseline levels of human SAA have been emphasized as an advantage, because slight elevations in wide ranges are easier to detect compared to slight changes in tight baseline concentrations (18,50). For monitoring inflammation, the measurement of acute phase proteins within baseline levels is normally not necessary (18). However, if such measurements should become of interest, the relatively higher imprecision within these concentration ranges should be considered (9,28). In human medicine, this concentration range may be of interest, since low-grade inflammation with baseline values of SAA and CRP appears, for instance, to be associated with future risk of coronary heart disease (51). However, further studies are needed to investigate whether similar advantages can be expected for baseline measurements of canine acute phase proteins.
In conclusion, SAA and CRP measurements above basal levels are clearly indicative of systemic inflammation in dogs, and the automated assays used to determine SAA and CRP levels are useful for routine detection of systemic inflammation in a general clinical setting. Serum amyloid A seems to possess greater diagnostic potential for systemic inflammation compared with serum CRP.
Acknowledgments
The authors thank those who helped in the collection of samples for the study, including Tommaso Furlanello, Marco Caldin, and Samantha Guerrero, San Marco Veterinary Clinic, Padova, Italy; Björn Aablad and Bert-Jan Reezigt, Blue Star Animal Hospital, Gothenburg, Sweden; and Professors Lesley G. King, and Marc A. Oyama, Department of Clinical Sciences, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, USA. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was supported in part by EIKEN Chemical Company, Tokyo, Japan.
References
- 1.Malle E, DeBeer FC. Human serum amyloid A (SAA) protein: A prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 2.Ceron JJ, Eckersall PD, Martýnez-Subiela S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet Clin Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura M, Takahashi M, Ohno K, et al. C-reactive protein concentration in dogs with various diseases. J Vet Med Sci. 2008;70:127–131. doi: 10.1292/jvms.70.127. [DOI] [PubMed] [Google Scholar]
- 4.Fransson BA, Lagerstedt A, Bergstrom A, et al. C-reactive protein, tumor necrosis factor alpha, and interleukin-6 in dogs with pyometra and SIRS. J Vet Emerg Crit Care. 2007;17:373–381. [Google Scholar]
- 5.Bathen-Noethen A, Carlson R, Menzel D, Mischke R, Tipold A. Concentrations of acute-phase proteins in dogs with steroid responsive meningitis-arteritis. J Vet Intern Med. 2008;22:1149–1156. doi: 10.1111/j.1939-1676.2008.0164.x. [DOI] [PubMed] [Google Scholar]
- 6.Lowrie M, Penderis J, Eckersall PD, McLaughlin M, Mellor D, Anderson TJ. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet J. 2009;182:125–130. doi: 10.1016/j.tvjl.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt C, Hirschberger J, Rau S, et al. Use of C-reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care. 2009;19:450–458. doi: 10.1111/j.1476-4431.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan DL, Rozanski EA, Freeman LM. Relationship among plasma amino acids, C-reactive protein, illness severity, and outcome in critically ill dogs. J Vet Intern Med. 2009;23:559–563. doi: 10.1111/j.1939-1676.2009.0296.x. [DOI] [PubMed] [Google Scholar]
- 9.Kjelgaard-Hansen M, Jensen A, Kristensen A. Evaluation of a commercially available human C-reactive protein (CRP) turbidometric immunoassay for determination of canine serum CRP concentration. Vet Clin Path. 2003;32:81–87. doi: 10.1111/j.1939-165x.2003.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 10.Kjelgaard-Hansen M. Comments on measurement of C-reactive protein in dogs RID E-6342-2011. Vet Clin Path. 2010;39:402–403. doi: 10.1111/j.1939-165X.2010.00276.x. [DOI] [PubMed] [Google Scholar]
- 11.Klenner S, Bauer N, Moritz A. Evaluation of three automated human immunoturbidimetric assays for the detection of C-reactive protein in dogs. J Vet Diagn Invest. 2010;22:544–552. doi: 10.1177/104063871002200408. [DOI] [PubMed] [Google Scholar]
- 12.Kjelgaard-Hansen M, Jacobsen S. Assay validation and diagnostic applications of major acute-phase protein testing in companion animals. Clin Lab Med. 2011;31:51–70. doi: 10.1016/j.cll.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Lange U, Boss B, Teichmann J, Klor H, Neeck G. Serum amyloid A — An indicator of inflammation in ankylosing spondylitis. Rheumatol Int. 2000;19:119–122. doi: 10.1007/s002960050114. [DOI] [PubMed] [Google Scholar]
- 14.Sukenik S, Henkin J, Zimlichman S, et al. Serum and synovial-fluid levels of serum amyloid-a protein and C-reactive protein in inflammatory and noninflammatory arthritis. J Rheumatol. 1988;15:942–945. [PubMed] [Google Scholar]
- 15.Hogarth M, Gallimore J, Savage P, et al. Acute phase proteins, C-reactive protein and serum amyloid A protein, as prognostic markers in the elderly inpatient. Age Ageing. 1997;26:153–158. doi: 10.1093/ageing/26.2.153. [DOI] [PubMed] [Google Scholar]
- 16.Casl M, Rogina B, Glojnaricspasic I, Minigo H, Planincperaica A, Jaksic B. The differential diagnostic capacity of serum amyloid a protein between infectious and noninfectious febrile episodes of neutropenic patients with acute-leukemia. Leuk Res. 1994;18:665–670. doi: 10.1016/0145-2126(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 17.Maury C. Comparative-study of serum amyloid-a protein and C-reactive protein in disease. Clin Sci. 1985;68:233–238. doi: 10.1042/cs0680233. [DOI] [PubMed] [Google Scholar]
- 18.Chambers R, Hutton C, Dieppe P, Whicher J. Comparative-study of C-reactive protein and serum amyloid-a protein in experimental inflammation. Ann Rheum Dis. 1991;50:677–679. doi: 10.1136/ard.50.10.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grindulis K, Scott D, Robinson M, Bacon P, McConkey B. Serum amyloid a protein during the treatment of rheumatoid-arthritis with 2nd-line drugs. Br J Rheumatol. 1985;24:158–163. doi: 10.1093/rheumatology/24.2.158. [DOI] [PubMed] [Google Scholar]
- 20.Cunnane G, Grehan S, Geoghegan S, et al. Serum amyloid A in the assessment of early inflammatory arthritis. J Rheumatol. 2000;27:58–63. [PubMed] [Google Scholar]
- 21.Dąbrowski R, Kostro K, Lisiecka U, Szczubiał M, Krakowski L. Usefulness of C-reactive protein, serum amyloid A component, and haptoglobin determinations in bitches with pyometra for monitoring early post-ovariohysterectomy complications. Theriogenology. 2009;72:471–476. doi: 10.1016/j.theriogenology.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Bayramli G, Ulutas B. Acute phase protein response in dogs with experimentally induced gastric mucosal injury. Vet Clin Path. 2008;37:312–316. doi: 10.1111/j.1939-165X.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Subiela S, Strauss-Ayali D, Ceron JJ, Baneth G. Acute phase protein response in experimental canine leishmaniosis. Vet Parasitol. 2011;180:197–202. doi: 10.1016/j.vetpar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Tecles F, Spiranelli E, Bonfanti U, Ceron J, Paltrinieri S. Preliminary studies of serum acute-phase protein concentrations in hematologic and neoplastic diseases of the dog. J Vet Int Med. 2005;19:865–870. doi: 10.1892/0891-6640(2005)19[865:psosap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Jergens A, Schreiner C, Frank D, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Int Med. 2003;17:291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Subiela S, Bernal L, Ceron J. Serum concentrations of acute-phase proteins in dogs with leishmaniosis during short-term treatment. Am J Vet Res. 2003;64:1021–1026. doi: 10.2460/ajvr.2003.64.1021. [DOI] [PubMed] [Google Scholar]
- 27.Dabrowski R, Wawron W, Kostro K. Changes in CRP, SAA and haptoglobin produced in response to ovariohysterectomy in healthy bitches and those with pyometra. Theriogenology. 2007;67:321–327. doi: 10.1016/j.theriogenology.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Christensen M, Jacobsen S, Ichiyanagi T, Kjelgaard-Hansen M. Evaluation of an automated assay based on monoclonal anti-human serum amyloid A (SAA) antibodies for measurement of canine, feline, and equine SAA. Vet J. 2012;194:332–337. doi: 10.1016/j.tvjl.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Peeters ME, Kirpensteijn J. Comparison of surgical variables and short-term postoperative complications in healthy dogs undergoing ovariohysterectomy or ovariectomy. J Am Vet Med Assoc. 2011;238:189–194. doi: 10.2460/javma.238.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Tvarijonaviciute A, Martinez-Subiela S, Carrillo-Sanchez JD, Tecles F, Ceron JJ. Effects of orchidectomy in selective biochemical analytes in beagle dogs. Reprod Domest Anim. 2011;46:957–963. doi: 10.1111/j.1439-0531.2011.01765.x. [DOI] [PubMed] [Google Scholar]
- 31.Langhorn R, Oyama M, King L, et al. Prognostic importance of myocardial injury in critically ill dogs with systemic inflammation. J Vet Int Med. 2013;27:895–903. doi: 10.1111/jvim.12105. [DOI] [PubMed] [Google Scholar]
- 32.Buttke B, Shipper G, Delano E, Trope M. C-reactive protein and serum amyloid A in a canine model of chronic apical periodontitis. J Endod. 2005;31:728–732. doi: 10.1097/01.don.0000158008.34623.6c. [DOI] [PubMed] [Google Scholar]
- 33.Bauer N, Mensinger S, Daube G, Failing K, Moritz A. A moderate aseptic local inflammation does not induce a significant systemic inflammatory response. Res Vet Sci. 2012;93:321–330. doi: 10.1016/j.rvsc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Jensen AL, Bomholt M, Moe L. Preliminary evaluation of a particle-enhanced turbidimetric immunoassay (PETIA) for the determination of serum cystatin C-like immunoreactivity in dogs. Vet Clin Path. 2001;30:86–90. doi: 10.1111/j.1939-165x.2001.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 35.Aroch I, Harrus S. Retrospective study of the epidemiological, clinical, haematological and biochemical findings in 109 dogs poisoned by Vipera xanthina palestinae. Vet Rec. 1999;144:532–535. doi: 10.1136/vr.144.19.532. [DOI] [PubMed] [Google Scholar]
- 36.Nogueira RMB, Sakate M. Clinical and hematological alterations in dogs during experimental envenomation with crotalus durissus terrificus venom and treated with antiophidic serum. J Venom Anim Toxins Incl Trop Dis. 2006;12:285–296. [Google Scholar]
- 37.Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J. 2010;185:23–27. doi: 10.1016/j.tvjl.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Christensen MB, Ceron JJ, Tvarijonaviciute A, Kjelgaard-Hansen M. Diagnostic measurements of canine serum amyloid A using automated latex agglutination turbidimetry: An interlaboratorial comparison. Vet Clin Path; Proceedings of the 14th Conference of the European Society of Veterinary Clinical Pathology; 2012. pp. E31–E35. [Google Scholar]
- 39.Delong E, Delong D, Clarkepearson D. Comparing the areas under 2 or more correlated receiver operating characteristic curves — A non-parametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 40.Gardner I, Greiner M. Receiver-operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary pathology tests. Vet Clin Path. 2006;35:8–17. doi: 10.1111/j.1939-165x.2006.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 41.Christensen MB, Langhorn R, Goddard A, et al. Canine serum amyloid A (SAA) measured by automated agglutination turbidimetry is useful for routine sensitive and specific detection of systemic inflammation in a general clinical setting. J Vet Med Sci. 2013;75:459–466. doi: 10.1292/jvms.12-0404. [DOI] [PubMed] [Google Scholar]
- 42.Ersboll A, Bruun J, Toft N. Data analysis. In: Houe H, Ersboll A, Toft N, editors. Introduction to Veterinary Epidemiology. 1st ed. Copenhagen, Denmark: Biofolia; 2004. pp. 205–266. [Google Scholar]
- 43.Hillström A, Tvedten H, Lilliehöök I. Evaluation of an in-clinic serum amyloid A (SAA) assay and assessment of the effects of storage on SAA samples. Acta Vet Scand. 2010;52:8–13. doi: 10.1186/1751-0147-52-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kjelgaard-Hansen M, Jensen A, Kristensen A. Internal quality control of a turbidimetric immunoassay for canine serum C-reactive protein based on pooled patient samples. Vet Clin Path. 2004;33:139–144. doi: 10.1111/j.1939-165x.2004.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 45.Brindle E, Fujita M, Shofer J, O’Conner KA. Serum, plasma, and dries spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362:112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miwata H, Yamada T, Okada M, Kudo T, Kimura H, Morishima T. Serum amyloid A-protein in acute viral-infections. Arch Dis Child. 1993;68:210–214. doi: 10.1136/adc.68.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzini C, Mussap M, Plebani M, Fanos V. C-reactive protein and serum amyloid A protein in neonatal infections. Scand J Infect Dis. 2000;32:229–235. doi: 10.1080/00365540050165848. [DOI] [PubMed] [Google Scholar]
- 48.Smith J, Colombo J, McDonald T. Comparison of serum amyloid-a and C-reactive protein as indicators of lung inflammation in corticosteroid treated and non-corticosteroid treated cystic-fibrosis patients. J Clin Lab Anal. 1992;6:219–224. doi: 10.1002/jcla.1860060410. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda Y, Hoshino S, Tanaka I, et al. Examination of serum amyloid A protein in kidney transplant patients. Transplant Proc. 2000;32:1796–1798. doi: 10.1016/s0041-1345(00)01368-3. [DOI] [PubMed] [Google Scholar]
- 50.Yamada T. Serum amyloid A (SAA): A concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999;37:381–388. doi: 10.1515/CCLM.1999.063. [DOI] [PubMed] [Google Scholar]
- 51.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. Br Med J. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]