Abstract
The recent evidence that extant cycads are not living fossils triggered a renewed search for a better understanding of their evolutionary history. In this study, we investigated the evolutionary diversification history of the genus Encephalartos, a monophyletic cycad endemic to Africa. We found an antisigmoidal pattern with a plateau and punctual explosive radiation. This pattern is typical of a constant radiation with mass extinction. The rate shift that we found may therefore be a result of a rapid recolonization of niches that have been emptied owing to mass extinction. Because the explosive radiation occurred during the transition Pliocene–Pleistocene, we argued that the processes might have been climatically mediated.
Keywords: Climate change, Encephalartos, extinction, gymnosperms, adaptive radiation, subtropical Africa.
Introduction
Current biological diversity has been shaped through macro-evolutionary processes or ecological dynamics. For example, speciation events may initially occur in bursts, owing to the availability of empty niches, and later decline as niches become occupied (Simpson 1953; Schluter 2000; Gavrilets and Vose 2005). Investigating the process of species diversification could therefore shed light on the roles ecological versus stochastic forces play in shaping species accumulation (Ricklefs 1987, 1989; Ricklefs and Schluter 1993; McPeek and Brown 2000; Stoks and McPeek 2006). Fossil records provide the best opportunity to investigate the dynamics of species accumulation, but such records are often lacking for several taxonomic groups. The phylogenetic analysis of radiation events provides an alternative tool commonly used to reconstruct the history of species diversification (Harvey et al. 1994). Such analysis of lineage splitting has revealed an emerging pattern of early-explosive radiation (Harmon et al. 2003; Shaw et al. 2003; Kadereit et al. 2004; Machordom and Macpherson 2004; Morrison et al. 2004; Williams and Reid 2004; Xiang et al. 2005; Kozak et al. 2006; Weir 2006; Phillimore and Price 2008). A pattern of increasing diversification through time is unusual as this has been showed in only very few studies (e.g., Barraclough and Vogler 2002; Linder et al. 2003; Turgeon et al. 2005).
In general, increased attention has been devoted to the geographical regions (e.g., the Cape Floristic Region) and lineages (e.g., the genus Dianthus L.) that are theaters of spectacular evolutionary events (Baldwin and Sanderson 1998; Richardson et al. 2001; Verboom et al. 2003; Klak et al. 2004; Kay et al. 2005; Hughes and Eastwood 2006; Garcίa-Maroto et al. 2009; Valente et al. 2010). In contrast, taxonomic groups such as gymnosperms are a priori of secondary interest because they are characterized by morphological stasis and low interspecific genetic variation (Van der Bank et al. 2001; Vorster 2004). However, the limited genetic variation may be indicative of recent but rapid radiations, thus raising an interesting question of what might have triggered their recent and rapid diversification.
Gymnosperms in general have long been regarded as living fossils (Hill and Brodribb 1999; Liao et al. 2004; McLoughlin and Vajda 2005; Keppel et al. 2008; Xiao et al. 2010; Álvaréz-Yepiz et al. 2011). Recent studies challenged this view (Crisp and Cook 2011; Nagalingum et al. 2011; Burleigh et al. 2012) and consequently stimulated an increased interest into the reconstruction of their evolutionary history (Crisp and Cook 2011; Burleigh et al. 2012). In an earlier study, Crisp and Cook (2009) developed a unified framework describing alternative scenarios of species diversification. These scenarios can be broadly summarized into four patterns. First, the tempo and mode of species accumulation is constant over time. This is revealed by a linear semi-log lineages-through-time (LTT) plot, indicating a constant ratio birth/death through time. Second, the pattern can depart from a linear semi-log LTT plot showing a concave or convex line as a result of single rate decrease or increase, respectively. Third, a pattern of early rapid radiation that later slows down can also be observed, driven potentially by ecological opportunities. This is generally referred to as adaptive radiation and is expected to be accompanied by the development of key innovations. The main feature of the corresponding LTT plot is an early steep slope that later flattens. Finally, it is possible that the LTT plot shows a late upswing in slope. Such pattern is generally referred to as antisigmoidal and is driven by punctual mass extinctions (Crisp and Cook 2009). On an antisigmoidal LTT plot resulting from a phylogenetic tree of only extant species, the mass extinction translates into a plateau.
In this study, we reconstructed the temporal dynamics of phylogenetic diversification of the African cycads. We also investigated how the diversification rates vary across lineages. Cycads in general include about 300 extant species in 10 genera, of which the monophyletic genus Encephalartos (Nagalingum et al. 2011) and its 65 species (Hill and Stevenson 2004) are endemic to Africa. Members of the genus are unequally distributed across African regions. For example, only one species occurs in West Africa (E. barteri) while over 50% of the Encephalartos species are endemic to southern Africa, a geographical region considered as the center of diversity of the genus (Golding and Hurter 2003).
Specifically, we investigated three questions: What is the net speciation rate of the genus Encephalartos and how does it compare with other groups? Is diversification rate constant over time? How does the diversification rate compare across lineages within the group?
Methods
Compilation of DNA matrix
We compiled a matrix of DNA sequences for all the 65 Encephalartos species. These sequences were generated in a recent phylogenetic study of the genus (Rousseau 2012). The matrix includes three plastid regions (rbcLa, matK, and trnH-psbA) and one nuclear region (nrITS). All voucher information and GenBank/EBI accession numbers are presented as Supplementary Information (Table S1). Also, we included in the matrix, DNA sequences of the following species that we used as outgroups and for calibration purpose: Stangeria eriopus (Kunze) Baill., Macrozamia plurinervia (L.A.S.Johnson) D.L.Jones, Macrozamia communis L.A.S.Johnson, Macrozamia macdonnellii (F.Muell. ex Miq.) A.DC., Macrozamia pauli-guilielmi W.Hill & F.Muell., Lepidozamia peroffskyana Regel, and Lepidozamia hopei (W.Hill) Regel.
Tree reconstruction and estimation of divergence time
We first generated an XML file using the program BEAUTi (Bayesian Evolutionary Analysis Utility) implemented in the program BEAST (Bayesian Evolutionary Analysis by Sampling Trees; Drummond and Rambaut 2007). Then, the XML file was used to reconstruct the complete phylogeny and estimate the divergence times, using a Bayesian MCMC approach also implemented in the BEAST program. Each individual marker (matK, rbcLa, trnH-psbA, and nrITS) was given its own partition. We selected GTR + I + Γ as the best model of sequence evolution for each partition based on the Akaike information criterion evaluated using MODELTEST (Nylander 2004). A speciation model following a Yule process was selected as the tree prior, with an uncorrelated relaxed lognormal model for rate variation among branches. Further, we conducted simultaneous searches of topology and divergence times. For this purpose, we applied a normal prior distribution and the following secondary calibration points extracted from Nagalingum et al. (2011): Encephalartos crown node (11.3648 Myr), Macrozamia crown node (7.4836 Myr), Lepidozamia crown node (7.914 Myr), Encephalartos – Lepidozamia (39.7442 Myr) and (Encephalartos – Lepidozamia) – Macrozamia (49.037 Myr). Monte Carlo Markov Chains were run for 100 million generations with trees sampled every 10000 generations.
Log files, including prior and likelihood values, as well as the effective sample size (ESS) were examined using TRACER (Rambaut and Drummond 2007). ESS values varied between 449 and 7501 for the age estimates (Table S2), confirming stationarity. Of the resulting 10001 trees, we removed the first 2500 trees as burn-in and combined the remaining trees using TREEANNOTATOR (Rambaut and Drummond 2007) to generate a maximum clade credibility (MCC) tree. Three MCC trees were reconstructed: one based on plastid regions (rbcLa, matK, and trnH-psbA), one on the nuclear gene nrITS, and another tree based on the combination of all regions.
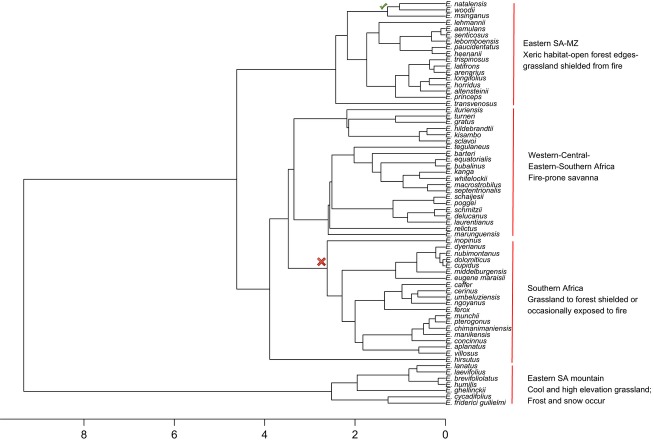
We tested for congruence between plastid and nuclear regions using the partitioned Bremer support test (DeSalle and Brower 1997) with 1000 heuristic searches, as implemented in TreeRot, version 3 (Sorenson and Franzosa 2007). A negative Bremer index is indicative of incongruence between the plastid and nuclear genes, whereas a positive score indicates congruence. Our Bremer scores were positive for all nodes, except for only one node (E. msinganus, E. woodii – E. natalensis). We therefore focused our statistical analyses on the MCC tree generated using the combined regions (Figs 1 & S1).
Figure 1.
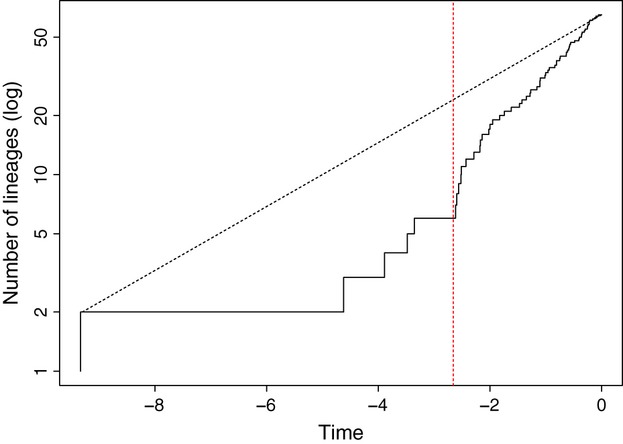
Log-lineage-through-time plot (LTT plot, full line) describing the dynamics of cladogenesis within the genus Encephalartos over time. The black dashed line indicates the null expectation (pure-birth model); the red dashed line indicates the period of shift in diversification around 2.6 million years before present day.
Statistical analyses
All statistical analyses were conducted in the program R (R Core Team, 2011). First, we estimated the net diversification rate using Magallon and Sanderson's whole-clade method (Magallon and Sanderson 2001) implemented in the R library GEIGER (Harmon et al. 2008). The net rate was calculated assuming ε = 0 (i.e., no extinction) and ε = 0.9 (high extinction rate; Magallon and Sanderson 2001).
Second, we investigated the temporal patterns of clades formation, based on the lineage-through-time plot (LTT plot) and the γ-statistics of Pybus and Harvey (2000). These analyses were conducted using the R package LASER (Rabosky 2007). The value of γ describes the temporal shift in speciation along a phylogenetic tree as follows: γ < 0 indicates a pattern of decreasing speciation over time, whereas γ > 0 corresponds to acceleration in speciation toward the present day (Pybus and Harvey 2000).
Third, we conducted a model fitting analysis, testing two rate-constant models (pure-speciation and birth–death models), and four rate-variable models, including the density-dependent exponential (DDX) model, the density-dependent linear (DDL) model, the Yule2rate model and the Yule3rate model. These models were fitted under the maximum-likelihood criterion, and the best of the competing models was selected using the Akaike information criterion (AIC). We computed the ΔAICRC statistics: ΔAICRC = AICH0 - AICH1, where AICH0 is the AIC score of the best rate-constant model and AICH1 is the AIC score of the best rate-variable model (DDX, DDL, Yule2rate, or Yule3rate). This model comparison was performed using the function fitdAICrc in the R package LASER. If ΔAICRC > 0, then the best of the rate-variable models is also the best model for the observed diversification pattern; if ΔAICRC < 0, the best rate-constant model would be favored (Rabosky and Lovette 2008). The significance of the observed ΔAICRC value was tested using the function fitdAICrc.batch (also implemented in the R package LASER) with which we simulated 5000 trees of 65 tips (total number of extant Encephalartos species) under a pure-birth process, allowing us to generate a null distribution for ΔAICRC values.
Fourth, we tested for rate heterogeneity across lineages using the Δ1 statistic test of Moore et al. (2004). This test is based on the whole tree topology to detect nodes associated with significant shifts in diversification rate. The Δ1 statistic test was performed using the R package apTreeshape (Bortolussi et al. 2006).
Finally, as the Δ1 statistic test indicated a rate shift around 2.66 MYA (see Results below), we analyzed, using the γ-statistics, the pattern of temporal clade accumulation from 2.66 MYA to the present day.
Results
We evaluated the diversification rate assuming no extinction and high extinction. We found a rate of 0.37 species per million years (sp. Myr−1) and 0.21 sp. Myr−1, respectively.
We reconstructed the LTT plot for a graphical representation of the temporal patterns of clade accumulation. Our LTT plot is best described by an antisigmoidal shape characterized by two periods of constant rate separated by a plateau (Fig. 1). The first rate constant occurs before ∼2.6 MYA, and the second after ∼2.6 MYA with a sudden rate shift at ∼2.6 MYA (Fig. 1).
If we ignore the punctual shift at ∼2.6 MYA, the overall diversification pattern would match that of a linear semi-log LTT plot that characterizes a constant temporal radiation. We tested this using the γ-statistics. We found a positive but nonsignificant value (γ = 0.63; P = 0.73), confirming an overall rate-constant diversification over time. Consequently, we would expect a rate-constant model to fit better the data. Surprisingly, our model fitting indicated that the rate-constant models were outcompeted by a rate-variable model. In particular, the Yule3rate model was favored by AIC (ΔAICRC = 7.27, P = 0.01; Fig. 2; Table 1). This model indicates an initial diversification rate r1 = 0.22 sp. Myr−1 from the origin until ∼2.66 MYA. At ∼2.66 MYA, there was a drastic shift to r2 = 3.28 sp. Myr−1 (almost 15 times greater than r1). However, this shift occurred only within a short period because, at 2.47 MYA, the rate decreased to r3 = 0.69 sp. Myr−1, which remained constant to the present day (Table 1).
Figure 2.
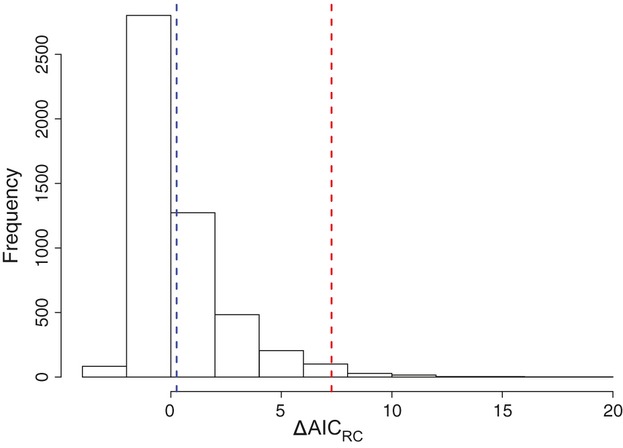
Histogram indicating the null distribution of ΔAICRC. ΔAICRC is the differences between the best rate-constant model and the best rate-variable model found for 5000 simulated trees of 65 species (total number of extant Encephalartos species) under a Yule process; red dashed line indicates the observed value of ΔAICRC, and blue dashed line indicates the mean of the null observations. The difference between observed and null is significant (P = 0.01).
Table 1.
Results of the test for temporal variation in diversification processes of Encephalartos. Prior to models fitting, outgroups were removed from the tree. Models were fitted to the Encephalartos chronograms generated from BEAST; RC = rate-constant model; AIC = Akaike information criterion; ΔAICRC = difference in AIC scores between the best rate-constant model (pure-birth) and each of the models. The best rate-variable model is Yule3rate; r = net diversification rate (speciation events per million years); a = extinction fraction; k = carrying capacity; x = rate change parameter; st = inferred time of rate shift in million years before present
| Diversification models | Log likelihood | AIC | ΔAICRC | Parameters estimates | ||
|---|---|---|---|---|---|---|
| Pure-birth (RC) | 114.799 | −227.598 | 0 | r1 = 0.647 | – | – |
| Birth–death (RC) | 115.115 | −226.231 | −1.367 | r1 = 0.520 | a = 0.317 | – |
| DDL | 114.799 | −225.598 | −2 | r1 = 0.647 | k = 1065642 | – |
| DDX | 115.658 | −227.317 | −0.281 | r1 = 0.389 | x = 0.164 | – |
| Yule2rate | 118.831 | −231.663 | 4.065 | r1 = 0.219 | r2 = 0.746; st = 2.633 | |
| Yule3rate | 122.434 | −234.870 | 7.271 | r1 = 0.22 | r2 = 3.28 st1=2.657 | r3 = 0.695 st2=2.474 |
Furthermore, we investigated whether the 15-time rate shift that occurred around 2.66 MYA was significant compared with the rate across all nodes in the phylogeny. Our analysis using the Δ1 statistic test gave support to this, indicating a significant shift in diversification rate at the node corresponding to a southern African lineage (P = 0.015; Fig. 3).
Figure 3.
Bayesian maximum clade credibility tree of Encephalartos inferred from the combination of all four DNA regions included in this study. Outgroups are not shown (but see Figure S1). The red cross indicates the node of the southern African clade with highest diversification rate. The geographical pattern of species along the phylogeny is indicated as well as habitat preferences. The green “tick” symbol indicates the node where nuclear and plastid genes were incongruent. Dates on the scale axis are in million years. SA = South Africa; MZ = Mozambique.
Finally, we examined the patterns of clades accumulation from 2.66 MYA to the present day using the γ-statistics. We found a negative but still nonsignificant value (γ = −0.80, P = 0.21), suggesting that, in addition to the rate-constant diversification before ∼2.66 MYA, the diversification from ∼2.66 MYA onward was also constant. Overall, the diversification rate of the African cycad is constant before and after ∼2.66 MYA with a drastic increase around 2.66 MYA.
Discussion
Our estimate of absolute net diversification rate for the genus Encephalartos assuming no extinction (0.372 sp. Myr−1) is comparable with that of the angiosperms under similar assumption, but only for the angiosperms younger than 30 Myr (0.349 sp. Myr−1; Crisp and Cook 2011). However, this rate is greater than the mean rate found for all gymnosperms in general (0.166 sp. Myr−1; Crisp and Cook 2011). Conversely, under the assumption of high extinction rate, our net rate for Encephalartos (0.208 sp. Myr−1) is lower than that of the angiosperms (1.713 sp. Myr−1) and gymnosperms (0.721 sp. Myr−1; Crisp and Cook 2011). Also, our rate is far lower than those reported for other plant taxonomic groups especially angiosperms that underwent spectacular radiation (e.g., see Baldwin and Sanderson 1998; Richardson et al. 2001; Verboom et al. 2003; Klak et al. 2004; Kay et al. 2005; Hughes and Eastwood 2006; Garcίa-Maroto et al. 2009; Valente et al. 2010). This lower speed of diversification may arise due to a high background rate of extinction, lower rate of speciation, or the combination thereof (Hill and Brodribb 1999; Cantrill and Poole 2005; Ricklefs 2006; Mittelbach et al. 2007; Schemske 2009; Gorelick and Olson 2011). A recent study revealed that a high extinction rate in gymnosperms is more likely specifically in a recent past (7–5 MYA; Niklas 1997; Crepet and Niklas 2009); we propose here that this past extinction may also account for the low rate of diversification found in this study for Encephalartos.
Looking into the overall diversification over time, we found evidence for a rate-constant pattern (γ-statistics). Counterintuitively, the AIC statistics favored a Yule3rate model over both rate-constant models tested. Why these apparently conflicting results? From the origin to 2.66 MYA, the diversification rate was constant. The rate was also constant from 2.66 MYA to the present day. However, at 2.66 MYA, a significant rate shift occurred, but this rate decreased almost immediately to a lower rate that remained constant toward the present day. Because the acceleration and deceleration occurred almost immediately, both events might offset their respective effects on the overall patterns of speciation. As a result, the punctual rate shift observed does not influence significantly the overall diversification rate. However, it creates a pattern of rate heterogeneity across lineages, as indicated by the fit of a rate-variable model.
Alternative hypotheses underlying the rate shift include meta-community dynamics (McPeek 2008) and acceleration of molecular evolution (Bromham 2003). However, adaptive radiation with the development of key innovations (Klak et al. 2004; Ree 2005) perhaps driven by environmental change (Lovette and Bermingham 1999) has for long been the emerging explanation (see reviews in Gavrilets and Losos 2009; Glor 2010), and this was found in diverse biological systems including lizards (Harmon et al. 2003), birds (Weir 2006; Phillimore and Price 2008), fish (Rüber and Zardoya 2005), and plants (Davies et al. 2004). Indeed, the punctual explosive radiation that we found coincides with the environmental shift from the Pliocene epoch (5.3–2.6 MYA) where climate was cooler, drier (compared with the immediately preceding Miocene), and seasonal to a new climatic regime in the Pleistocene (2.6 MYA–11.700 MYA) characterized by repeated glaciation events worldwide. During that period of global glaciation events, the African climate shifted to more arid conditions (deMenocal 1995), especially in subtropical Africa (deMenocal 2004). The increased aridification may have promoted the radiation of species capable of surviving the changing environment through a development of key innovations. The occurrence of subterranean stems in the southern African clade that underwent the punctual explosive radiation (Fig. 3) may be regarded, to some extent, as key innovation developed to adapt to high temperature and general aridity that prevailed during the Pliocene–Pleistocene transition in southern Africa. Members of this clade occur predominantly at cool, high elevations in gorges and rocks, but also in fire-prone ecosystems (savannas, grasslands) and forests occasionally exposed to fire (Vorster 2004). This adds to the ongoing controversy surrounding the role of the Quaternary climatic change as a driver of speciation; evidence of Quaternary speciation has been reported in some analyses (e.g., Weir and Schluter 2007; Janssens et al. 2009; Valente et al. 2010; Mullen et al. 2011; Nagalingum et al. 2011), but not in others (e.g., Hoorn et al. 2010; Wesselingh et al. 2010).
However, there are a number of evidences that discount these alternative hypotheses including explosive radiation as the strong forces shaping the observed antisigmoidal pattern. First, typical explosive radiation results in an increase without plateau (Turgeon et al. 2005; McKenna & Farrell, 2006). Second, the fact that six different genera of cycads diverge almost simultaneously across the globe (Nagalingum et al. 2011) underscore the development of innovative characters as underlying the synchronous diversification globally. Third, the phylogeny of the African cycads is characterized by “phylogenetic fuses,” indicated by long branches from the origin (∼9 MYA) toward the tips (the last 1 MYA) where the largest radiation of the genus occurs (Fig. 3). Such phylogenetic fuses were also reported, for example, by Nagalingum et al. (2011) for all cycads in general and could be either the result of low diversification or mass extinction. Combining simulations and empirical data, Crisp and Cook (2009) demonstrated convincingly that the pattern of antisigmoidal curve with a plateau is driven by mass extinctions. Indeed, gymnosperms in general experienced a mass extinction between 7–5 Myr (Niklas 1997; Crepet and Niklas 2009), a timeframe preceding roughly the period of large radiation of the African cycads (see Fig. 3). As such, the observed explosive radiation may be the result of a rapid recolonization of niches that have been emptied during the mass extinction events.
Overall, the diversification of the African cycads is constant through time. However, the mass extinction that occurred between 7 and 5 Myr (Niklas 1997; Crepet and Niklas 2009) may have created empty niches that have been refilled during the transition Pliocene–Pleistocene, leading to the accelerated radiation observed. The standing diversity of cycads on African continent may therefore have been shaped by the Quaternary climatic change.
Acknowledgments
We thank the Government of Canada through Genome Canada and the Ontario Genomics Institute (2008-OGI-ICI-03) and the University of Johannesburg for financial support. Two anonymous reviewers comment on an earlier version of this manuscript.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Bayesian maximum clade credibility chronogram inferred from the combined plastid + nuclear data (rbcLa + matK + trnH-psbA + nrITS) with 95% highest posterior density for node ages (blue boxes along branches).
Table S1. List of samples included in this study and their GenBank/EBI accession numbers.
Table S2. Summary statistics of TRACER analysis.
References
- Álvaréz-Yepiz JC, Dovciak M, Búrquez A. Persistence of a rare ancient cycad: effects of environment and demography. Biol. Conserv. 2011;144:122–130. [Google Scholar]
- Baldwin BG,, Sanderson MJ. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc. Natl Acad. Sci. USA. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough TG,, Vogler AP. Recent diversification rates in North American tiger beetles estimated from a dated mtDNA phylogenetic tree. Mol. Biol. Evol. 2002;19:1706–1716. doi: 10.1093/oxfordjournals.molbev.a003993. [DOI] [PubMed] [Google Scholar]
- Bortolussi N, Durand E, Blum MGB, François O. Aptreeshape: statistical analysis of phylogenetic tree shape. Bioinformatics. 2006;22:363–364. doi: 10.1093/bioinformatics/bti798. [DOI] [PubMed] [Google Scholar]
- Bromham L. Molecular clocks and explosive radiations. J. Mol. Evol. 2003;57:S13–S20. doi: 10.1007/s00239-003-0002-7. [DOI] [PubMed] [Google Scholar]
- Burleigh JG, Barbazuk WB, Davis JM, Morse AM, Soltis PS. Exploring diversification and genome size evolution in extant gymnosperms through phylogenetic synthesis. J. Bot. 2012;2012:1–6. [Google Scholar]
- Cantrill DJ,, Poole I. Taxonomic turnover and abundance in Cretaceous to Tertiary wood floras of Antarctica: implications for changes in forest ecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;215:205–219. [Google Scholar]
- Crepet WL,, Niklas KJ. Darwin's second “abominable mystery”: why are there so many angiosperm species? Am. J. Bot. 2009;96:366–381. doi: 10.3732/ajb.0800126. [DOI] [PubMed] [Google Scholar]
- Crisp MD, Cook LG. Explosive radiation or cryptic mass extinction? Interpreting signatures in molecular phylogenies. Evolution. 2009;63:2257–2265. doi: 10.1111/j.1558-5646.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- Crisp MD,, Cook LG. Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperm. New Phytol. 2011;192:997–1009. doi: 10.1111/j.1469-8137.2011.03862.x. [DOI] [PubMed] [Google Scholar]
- Davies TJ, Savolainen V, Chase MW, Moat J, Barraclough TG. Environmental energy and evolutionary rates in flowering plants. Proc. R. Soc. Lond. B Biol. Sci. 2004;271:2195–2200. doi: 10.1098/rspb.2004.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalle R, Brower AVZ. Process partitions, congruence and the independence of characters: inferring relationships among closely-related Hawaiian Drosophila from multiple gene regions. Syst. Biol. 1997;46:751–764. doi: 10.1093/sysbio/46.4.751. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcίa-Maroto F, Mańas-Fernández A, Garrido-Cárdenas JA, López-Alonso D, Guil-Guerrero JL, Guzmán B, et al. Δ6-Desaturase sequence evidence for explosive Pliocene radiations within the adaptive radiation of Macaronesian Echium (Boraginaceae) Mol. Phylogenet. Evol. 2009;52:563–574. doi: 10.1016/j.ympev.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Losos JB. Adaptive radiation: Contrasting theory with data. Science. 2009;323:732–737. doi: 10.1126/science.1157966. [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Vose A. Dynamic patterns of adaptive radiation. Proc. Natl Acad. Sci. USA. 2005;102:18040–18045. doi: 10.1073/pnas.0506330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glor RE. Phylogenetic insights on adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 2010;41:251–270. [Google Scholar]
- Golding JS,, Hurter PJH. A Red List account of Africa's cycads and implications of considering life-history and threats. Biodivers. Conserv. 2003;12:507–528. [Google Scholar]
- Gorelick R, Olson K. Is lack of cycad (Cycadales) diversity a result of a lack of polyploidy? Bot. J. Linn. Soc. 2011;165:156–167. [Google Scholar]
- Harmon LJ, Schulte JA, Larson A, Losos JB. Tempo and model of evolutionary radiation in iguanian lizards. Science. 2003;301:961–964. doi: 10.1126/science.1084786. [DOI] [PubMed] [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. Geiger: investigating evolutionary radiations. Bioinformatics. 2008;24:129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- Harvey PH, May RM, Nee S. Phylogenies without fossils. Evolution. 1994;48:523–529. doi: 10.1111/j.1558-5646.1994.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Hill RS, Brodribb TJ. Southern conifers in time and space. Aust. J. Bot. 1999;47:639–696. [Google Scholar]
- Hill KD,, Stevenson DW. World List of Cycads. Sydney: Royal Botanic Gardens; 2004. [Google Scholar]
- Hoorn C, Wesselingh FP, ter Steege H. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- Hughes C, Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA. 2006;103:339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens SB, Knox EB, Huysmans S, Smets EF, Merckx VSFT. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: Result of a global climate change. Mol. Phylogenet. Evol. 2009;52:806–824. doi: 10.1016/j.ympev.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kadereit JW, Greibler EM, Comes HP. Quaternary diversification in European alpine plants: pattern and process. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:265–274. doi: 10.1098/rstb.2003.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KM, Reeves PA, Olmstead RG, Schemske DW. Rapid speciation and the evolution of hummingbird pollination in neotropical Costus subgenus Costus (Costaceae): evidence from nrDNA ITS and ETS sequences. Am. J. Bot. 2005;92:1899–1910. doi: 10.3732/ajb.92.11.1899. [DOI] [PubMed] [Google Scholar]
- Keppel G, Hodgskiss PD, Plunkett GM. Cycads in the insular South-west Pacific: dispersal or vicariance? J. Biogeogr. 2008;35:1004–1015. [Google Scholar]
- Klak C, Reeves G, Hedderson T. Unmatched tempo of evolution in southern African semi-desert ice plants. Nature. 2004;427:63–65. doi: 10.1038/nature02243. [DOI] [PubMed] [Google Scholar]
- Kozak KH, Weisrock DW, Larson A. Rapid lineage accumulation in a non-adaptive radiation: phylogenetic analysis of diversification rates in eastern North American woodland salamanders (Plethodontidae: Plethodon. Proc. R. Soc. Lond. B Biol. Sci. 2006;273:539–546. doi: 10.1098/rspb.2005.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao ZH, Chen M, Guo L, Gong YF, Tang F, Sun XF, et al. Rapid isolation of high-quality total RNA from Taxus and Ginkgo. Prep. Biochem. Biotechnol. 2004;34:209–214. doi: 10.1081/PB-200026790. [DOI] [PubMed] [Google Scholar]
- Linder HP, Eldenäs P, Briggs BG. Contrasting patterns of radiation in African and Australian Restionaceae. Evolution. 2003;57:2688–2702. doi: 10.1111/j.0014-3820.2003.tb01513.x. [DOI] [PubMed] [Google Scholar]
- Lovette IJ, Bermingham E. Explosive speciation in the New World Dendroica warblers. Proc. R. Soc. Lond. B. 1999;266:1629–1636. [Google Scholar]
- Machordom A, Macpherson E. Rapid radiation and cryptic speciation in squat lobsters of the genus Munida (Crustacea, Decapoda) and related genera in the southwest Pacific: molecular and morphological evidence. Mol. Phylogenet. Evol. 2004;33:259–279. doi: 10.1016/j.ympev.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Magallon S,, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- McKenna DD,, Farrell BD. Tropical forests are both evolutionary cradles and museums of leaf beetle diversity. Proc. Natl. Acad. Sci. USA. 2006;103:10947–10951. doi: 10.1073/pnas.0602712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin S, Vajda V. Ancient wollemi pines resurgent. Am. Sci. 2005;9:540–547. [Google Scholar]
- McPeek MA. The ecological dynamics of clade diversification and community assembly. Am. Nat. 2008;172:E270–E284. doi: 10.1086/593137. [DOI] [PubMed] [Google Scholar]
- McPeek MA,, Brown JM. Building a regional species pool: diversification of the Enallagma damselflies in eastern North American waters. Ecology. 2000;81:904–920. [Google Scholar]
- deMenocal PB. Plio-Pleistocene African climate. Science. 1995;270:53–59. doi: 10.1126/science.270.5233.53. [DOI] [PubMed] [Google Scholar]
- deMenocal PB. African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet. Sci. Lett. 2004;220:3–24. [Google Scholar]
- Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Moore BR, Chan KMA, Donoghue MJ. Detecting diversification rate variation in supertrees. In: Bininda-Emonds ORP, editor. Phylogenetic Supertrees. Combining information to reveal the tree of life. Dodrecht, Netherlands: Kluwer Academic; 2004. pp. 487–533. [Google Scholar]
- Morrison CL, Rios R, Duffy JE. Phylogenetic evidence for an ancient rapid radiation of Caribbean sponge-dwelling snapping shrimps (Synalpheus) Mol. Phylogenet. Evol. 2004;30:563–581. doi: 10.1016/S1055-7903(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Mullen SP, Savage WK, Wahlberg N, Willmott KR. Rapid diversification and not clade age explains high diversity in neotropical Adelpha butterflies. Proc. Biol. Sci. 2011;278:1777–1785. doi: 10.1098/rspb.2010.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. Recent synchronous radiation of a living fossil. Science. 2011;334:796–799. doi: 10.1126/science.1209926. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. The evolutionary biology of plants. Chicago, IL: Univ. of Chicago Press; 1997. [Google Scholar]
- Nylander JAA. Sweden: Evolutionary Biology Centre, Uppsala Univ; 2004. Modeltest v2. Program Distributed by the Author. [Google Scholar]
- Phillimore AB,, Price TD. Density-dependent cladogenesis in birds. PLoS Biol. 2008;6:e71. doi: 10.1371/journal.pbio.0060071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus RG,, Harvey PH. Testing macro-evolutionary models using incomplete molecular phylogenies. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:2267–2272. doi: 10.1098/rspb.2000.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing; 2011. ISBN 3-900051-07-0, Available at: http://www.rproject.org/ [Google Scholar]
- Rabosky DL. LASER: A maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evol. Bioinform. Online. 2007;2:273–276. [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Lovette IJ. Explosive evolutionary radiations: increasing extinction or decreasing speciation through time? Evolution. 2008;62:1866–1875. doi: 10.1111/j.1558-5646.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- Rambaut A,, Drummond AJ. 2007. TreeAnnotator (version 1.5.4). Available at http://beast.bio.ed.ac.uk.
- Ree RH. Detecting the historical signature of key innovations using stochastic models of character evolution and cladogenesis. Evolution. 2005;59:257–265. [PubMed] [Google Scholar]
- Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. Rapid diversification of a species-rich genus of Neotropical rainforest trees. Science. 2001;293:2242–2245. doi: 10.1126/science.1061421. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Community diversity: relative roles of local and regional processes. Science. 1987;235:167–171. doi: 10.1126/science.235.4785.167. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Speciation and diversity: the integration of local and regional processes. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland, MA: Sinauer; 1989. pp. 599–622. [Google Scholar]
- Ricklefs RE. Global variations in the diversification rate of passerine birds. Ecology. 2006;87:2468–2478. doi: 10.1890/0012-9658(2006)87[2468:gvitdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Schluter D. Species diversity in ecological communities. Chicago, IL: Univ. of Chicago Press; 1993. [Google Scholar]
- Rousseau P. Johannesburg, South Africa: Univ. of Johannesburg; 2012. A molecular systematic study of the African endemic cycads; p. 176. [M.Sc. thesis] [Google Scholar]
- Rüber L,, Zardoya R. Rapid cladogenesis in marine fishes revisited. Evolution. 2005;59:1119–1127. [PubMed] [Google Scholar]
- Schemske DW. Speciation and patterns of diversity. In: Butlin R, Bridle J, Schluter D, editors. Biotic interactions and speciation in the tropics. Cambridge: Cambridge Univ. Press; 2009. pp. 219–239. [Google Scholar]
- Schluter D. The Ecology of adaptive radiation. Oxford, U.K: Oxford Univ. Press; 2000. [Google Scholar]
- Shaw AJ, Cox CJ, Goffinet B, Buck WR, Boles SB. Phylogenetic evidence of a rapid radiation of pleurocarpous mosses (Bryophyta) Evolution. 2003;57:2226–2241. doi: 10.1111/j.0014-3820.2003.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Simpson G. The major features of evolution. New York: Columbia Univ. Press; 1953. p. 434. [Google Scholar]
- Sorenson MD,, Franzosa EA. TreeRot, version 3. Boston, MA: Boston Univ; 2007. [Google Scholar]
- Stoks R,, McPeek MA. A tale of two diversifications: reciprocal habitat shifts to fill ecological space along the pond permanence gradient. Am. Nat. 2006;168:S50–S72. doi: 10.1086/509045. [DOI] [PubMed] [Google Scholar]
- Turgeon J, Stoks R, Thum RA, Brown JM, McPeek MA. Simultaneous quaternary radiations of three damselfly clades across the Holarctic. Am. Nat. 2005;165:E78–E107. doi: 10.1086/428682. [DOI] [PubMed] [Google Scholar]
- Valente LM, Savolainen V, Vargas P. Unparalleled rates of species diversification in Europe. Proc. R. Soc. Lond. B Biol. Sci. 2010;277:1489–1496. doi: 10.1098/rspb.2009.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bank FH, Vorster P, Wink M, Treutlein P, Brand J, Van der Bank M, et al. Allozyme and DNA sequence comparisons of nine species of Encephalartos (Zamiaceae) Biochem. Syst. Ecol. 2001;29:241–266. doi: 10.1016/s0305-1978(00)00064-8. [DOI] [PubMed] [Google Scholar]
- Verboom GA, Linder HP, Stock WD, Baum D. Phylogenetics of the grass genus Ehrharta: evidence for radiation in the summer-arid zone of the South African cape. Evolution. 2003;57:1008–1021. doi: 10.1111/j.0014-3820.2003.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Vorster P. Classification concepts in Encephalartos. In: Walters T, Osborne R, editors. Cycad Classification: Concepts and recommendations. Wallingford, U.K. and Cambridge, MA: CABI Publishing; 2004. pp. 69–83. [Google Scholar]
- Weir J. Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution. 2006;60:842–855. [PubMed] [Google Scholar]
- Weir JT,, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1574–1576. doi: 10.1126/science.1135590. [DOI] [PubMed] [Google Scholar]
- Wesselingh FP, Hoorn C, Kroonenberg SB, Antonelli A, Lundberg JG, Vonhof HB. On the origin of Amazonian landscapes and biodiversity, a synthesis. In: Hoorn C, Wesselingh F, et al., editors. Amazonia, landscape and species evolution. Oxford: Wiley-Blackwell; 2010. pp. 421–431. [Google Scholar]
- Williams ST, Reid DG. Speciation and diversity on tropical rocky shores: a global phylogeny of snails of the genus Echinolittorina. Evolution. 2004;58:2227–2251. doi: 10.1111/j.0014-3820.2004.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Xiang Q-Y, Manchester SR, Thoma DT, Zhang W, Fan C. Phylogeny, biogeography, and molecular dating of cornelian cherries (Cornus, Cornaceae): tracking tertiary plant migration. Evolution. 2005;59:1685–1700. [PubMed] [Google Scholar]
- Xiao LQ, Moller M, Zhu H. High nrDNA ITS polymorphism in the ancient extant seed plant Cycas, incomplete concerted evolution and the origin of pseudogenes. Mol. Phylogenet. Evol. 2010;55:168–177. doi: 10.1016/j.ympev.2009.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.