Abstract
The physical and ecological ‘fingerprints’ of anthropogenic climate change over the past century are now well documented in many environments and taxa. We reviewed the evidence for phenotypic responses to recent climate change in fish. Changes in the timing of migration and reproduction, age at maturity, age at juvenile migration, growth, survival and fecundity were associated primarily with changes in temperature. Although these traits can evolve rapidly, only two studies attributed phenotypic changes formally to evolutionary mechanisms. The correlation-based methods most frequently employed point largely to ‘fine-grained’ population responses to environmental variability (i.e. rapid phenotypic changes relative to generation time), consistent with plastic mechanisms. Ultimately, many species will likely adapt to long-term warming trends overlaid on natural climate oscillations. Considering the strong plasticity in all traits studied, we recommend development and expanded use of methods capable of detecting evolutionary change, such as the long term study of selection coefficients and temporal shifts in reaction norms, and increased attention to forecasting adaptive change in response to the synergistic interactions of the multiple selection pressures likely to be associated with climate change.
Keywords: adaptation, climate change, evolutionary theory, fisheries management, life-history evolution, phenotypic plasticity
Introduction
Anthropogenic climate change1 is one of the most important threats to global biodiversity over the next century (Sala et al. 2000; Thomas et al. 2004; Lovejoy and Hannah 2005). Now that ‘Warming of the climate is unequivocal’ (IPCC 2013), how are biological systems reacting? A rich literature documents potential evolutionary and plastic responses to physical drivers in fish. However, predicting actual responses in natural populations remains a core challenge because observed responses typically fail to match predictions based on theory or laboratory experiments (Merilä et al. 2001). To clarify what patterns have been observed, we undertook a specific review of the literature that detected phenotypic responses to climate change in wild fish populations, with a particular interest in the extent to which these responses could be attributed to evolutionary or plastic processes.
To anticipate the phenotypic consequences of climate change in fish, we can draw on a vast literature (for reviews, see Brett 1956; Fry 1967; Brett 1995; Wootton 1998; Walters and Martell 2004; Rijnsdorp et al. 2009). This volume of work stems from the high economic and cultural value of fisheries globally, fish farming and hatcheries, the aquarium trade, and use of fish as model systems in developmental genetics and disease research. The concept of fisheries-induced evolution, initiated primarily in the late 1970s/early 1980s (Handford et al. 1977; Ricker 1981), generated numerous analyses to evaluate the magnitude and likelihood of this form of evolutionary change (reviewed by Dieckmann and Heino 2007; Hutchings and Fraser 2008). Over roughly the same period of time, the salmon aquaculture industry and the salmonid hatchery ‘industry’ generated numerous papers pertaining to selection responses, trait heritability, temperature effects on myriad characteristics and genetic differentiation (e.g. references cited by Mousseau and Roff 1987; Purdom 1993). Armed with significantly enhanced (relative to most other taxa) empirically and financially rewarding research opportunities, fish geneticists, population biologists, ecologists and evolutionary biologists have made considerable advances in our knowledge of adaptation, selection responses, and rates of evolutionary change in fishes (e.g. Carlson et al. 2004; Hendry and Stearns 2004; Barrett et al. 2011). However, the profoundly intertwined mechanisms of evolution and plasticity in most climate-sensitive traits presents a major challenge for detecting adaptation to climate change in natural populations.
Reaction norms and acclimation
Phenotypic responses (e.g. growth rate, timing of reproduction) to environmental conditions, especially temperature, are routinely plastic in nature, but genetic variability differentiates the plastic response among families within populations, among populations and between species (e.g. Haugen and Vøllestad 2000; Jensen et al. 2008; Baumann and Conover 2011; Hutchings 2011). Reaction norms, graphical representations of phenotypic change along an environmental gradient (Scheiner 1993; Schlichting and Pigliucci 1998; Hutchings et al. 2007), constitute a standard means of describing plasticity. Evidence of genetic differentiation, and possible adaptation, appear through differences in the shape, intercept and (or) slope of reaction norms (Lande 2009; Chevin et al. 2010).
Plasticity in stress tolerance usually takes the form of ‘acclimation’, in which a history of exposure to particular conditions changes an organism's response to a challenge (Angilletta 2009; Kassahn et al. 2009). Because climate change involves prolonged exposure to altered conditions, acclimation will presumably play a key role in effecting phenotypic changes (Stillman 2003; Hofmann and Todgham 2010). Experiments measuring stress tolerance typically expose all individuals to a common rearing environment, attempting to control for acclimation responses (Beitinger et al. 2000; Johansen and Jones 2011). Variation in the extent to which acclimation alters performance can, in some instances, reflect local adaptation, as evidenced by Antarctic fishes that experience unusually constant temperatures (Bilyk and DeVries 2011). However, the conditions necessary to trigger an acclimatory response differ among species, complicating full characterization of this response by experimental methods. For example, prolonged warm acclimation enhances high-temperature tolerance in killifish (Fundulus heteroclitus), but repeated heat shocks do not (Healy and Schulte 2012). In zebrafish (Danio rerio), developmental plasticity affects acclimation to temperature substantially later in life (Scott and Johnston 2012). Similarly, developmental conditions can affect reaction norms for growth in Atlantic cod, Gadus morhua (Hurst et al. 2012). Thus, short-term exposure to acclimatory conditions during later life stages might underestimate the full acclimation potential of some species. At the extreme, a full generation might be necessary to trigger acclimation responses, as shown in the tropical damselfish Acanthochromis polyacanthus (Donelson et al. 2012) and sheepshead minnows Cyprinodon variegatus (Salinas and Munch 2012).
Are these responses adaptive?
There is some evidence that genetic changes in phenotypically plastic responses to temperature can be adaptive. One notable example in freshwater fish pertains to Norwegian populations of grayling (Thymallus thymallus). Although they once shared a common ancestor, the populations have been reproductively isolated from one another and exposed to different environments for more than 15 generations. Based on the results of a common-garden experimental protocol, this timeframe was sufficient to allow for population differences to emerge in plastic responses of several early-life traits to temperature (Haugen and Vøllestad 2000). Populations in colder lakes developed a more cold-adapted reaction norm for growth, including better growth at cooler temperatures and more efficient conversion from yolk to body mass compared with populations from warmer lakes (Kavanagh et al. 2010). These responses show a signature of selection as opposed to genetic drift (Qst > Fst), and these patterns correlated with lake temperature rather than physical distance.
Arguments in favour of the hypothesis that population differences in thermal reaction norms represented adaptive responses to local environments were based on observations that the traits examined were closely linked to fitness and that survival was highest at the temperatures that they were most likely to experience in the wild (a similar approach was adopted by Hutchings et al. 2007 in their reaction-norm study in Atlantic cod). The hypothesis that genetic variation in plasticity represents adaptive responses to different thermal regimes in early life is also supported by the discovery of temperature-associated SNPs (single nucleotide polymorphisms) in Atlantic cod that appear to be under selection (Bradbury et al. 2010, 2013). A particularly interesting example of genomic thermal plasticity in fish was reported by Croisetière et al. (2010) in brook trout (Salvelinus fontinalis). They found that the way in which the expression of the MHC classIIβ gene changes with temperature is associated with the basepair length of an associated temperature-sensitive mini-satellite, which may suggest a genomic underpinning for thermal plasticity. Immune-relevant genes in general follow latitudinal clines that are correlated with temperature (Dionne et al. 2007; Tonteri et al. 2010).
Common-garden experiments have featured prominently in many studies of the adaptive significance of population differences in phenotypic responses to temperature (Franks et al. 2013). Particularly relevant examples in fish include those of countergradient variation, which occurs when genetic differences counteract environmental effects, reducing phenotypic variation between populations; it is expected when stabilizing selection favours similar phenotypes in different environments (Conover and Schultz 1995). In fishes, evidence that countergradient variation reflects adaptation to thermal environments exists for some species – for example, Atlantic silversides, Menidia menidia (Conover and Present 1990) and Atlantic cod (Marcil et al. 2006) but not necessarily others – for example, mummichog, Fundulus heteroclitus (Fangue et al. 2009).
Given the genetic variability in temperature responses documented in many fish species (Beitinger et al. 2000), coupled with persuasive evidence in support of the hypothesis that phenotypic responses to temperature can be adaptive, it seems highly probable that many fish possess sufficient additive genetic variability to respond adaptively to climate change, although our review reveals only limited evidence of this to date.
Primary physical impacts of climate change relevant for fishes
The two primary physical drivers of climate change in the ocean are rising ocean temperature and carbon dioxide absorption (Hoegh-Guldberg and Bruno 2010; Gruber 2011; Hale et al. 2011; Koehn et al. 2011; Doney et al. 2012; Gruber et al. 2012; Collins 2014; Reusch 2014). These drivers have clearly changed over the past century in response to rising greenhouse gas emissions (IPCC 2007; Blunden and Arndt 2013; NCADAC 2013). Effects of warming oceans cascade beyond temperature change alone to alter Arctic ice volume, sea level and hence coastal habitat quality and quantity, salinity, vertical stratification, weather (i.e. precipitation, storm intensity and wind; Francis and Vavrus 2012; Liu et al. 2012), ocean current circulation and hypoxia (i.e. low oxygen levels; IPCC 2007). Carbon dioxide absorption interacts with many of these thermally induced phenomena to increase exposure to corrosive and hypoxic water. Rates of change vary geographically and in some cases local processes reverse the global trends (e.g. more intense wind sheer can increase coastal upwelling of deep water, which reduces local temperatures because deep water is much cooler than surface water). Forecasted large-scale changes in ocean circulation patterns are uncertain, as are consequences for multidecadal oscillations in climate, as reflected by indices such as the North Atlantic Oscillation (NAO), the Pacific Decadal Oscillation (PDO) and El Niño-Southern Oscillation (ENSO).
In fresh water, the primary drivers of climate change include rising water temperature, altered hydrological regimes (i.e. the timing of flows of different magnitudes), thermal stratification, decreased dissolved oxygen and increased toxicity of pollutants (Ficke et al. 2007; Stoks et al. 2014; Urban 2014). Hydrological regimes are in transition in regionally specific ways, including shifts in the magnitude and timing of floods, increasingly intense droughts and heat waves (IPCC 2012). More frequent and more intense precipitation events have numerous consequences, including added run-off of pollutants and nutrients into the water, increasing sediment load and eutrophication. These inputs can reduce the quality of fish habitat and result in harmful algal blooms and hypoxic ‘dead zones’ (NCADAC 2013). Loss of water, due to increased water vapour in the air and competition with humans, will affect groundwater, aquifers and wetlands. Many cold-water fish are expected to move or contract their ranges to higher elevation (Wenger and Olden 2012), while warm-water invasive species expand their ranges (Rahel and Olden 2008; Al-Chokhachy et al. 2013). Key fish habitats, such as coral reefs, mangrove and kelp forests, will likely decline (Hoegh-Guldberg and Bruno 2010).
Natural climatic fluctuations
Although many recent environmental trends are consistent with anthropogenic climate change, these trends might not continue in a linear fashion. Short-term trends easily nest within longer climate cycles, and generally ecological studies encompass only parts of these cycles. Figure 1 shows the NAO and PDO indices for 100–150 years and the portions of these time series captured by the studies listed in Table 1. The trend lines fit to the shorter time series show a range of slopes from negative to positive, depending on which phase of the larger oscillation coincided with the study. Although local temperatures do not track the larger climate indices perfectly, there are clear signals of these oscillations across continents as well as the ocean (Mantua et al. 1997; Stenseth and Mysterud 2005). For example, more streams in the western United States have cooled than warmed since 1987 (Arismendi et al. 2012). Warming trends predominate when records stretch back to 1950 (Arismendi et al. 2012), consistent with PDO cycling (Fig. 1).
Figure 1.
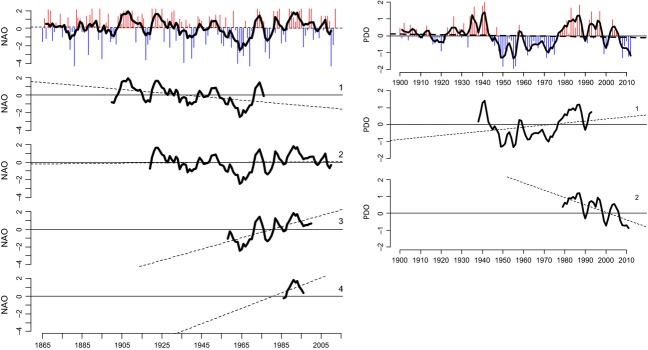
a Left: The North Atlantic Oscillation (NAO) station-based seasonal index for winter (includes December – February) since 1865 (Hurrell 1995): annual index (vertical lines) are shown in the top panel, and 5 year running mean (black line) is shown in all panels. The portion of the NAO time series in each lower panel show the years included in a selection of studies in Table 1: (i) Sundby and Nakken (2008); (ii) Rogers et al. (2011); (iii) Beaugrand et al. (2003); (iv) Kjesbu et al. (1998). Dotted lines are linear regression lines fit to the period of data shown. Right: The PDO index since 1900: annual index (vertical lines) are shown in the top panel, and 3 year running mean (black line) is shown in all panels. The portion of the PDO time series in each lower panel show the years included in selected studies from Table 1: (i) Quinn and Adams (1996); (ii) Kovach et al. (2012). Dotted lines are linear regression lines fit to the period of data shown. Note that local temperatures do not necessarily follow the PDO, but may have additional trend upon them, as in the Kovach study.
Table 1.
Environmental drivers and temporal coverage of the studies of phenotypic change
| Species | Location | Years | # Years | Reference | Trait | Driver |
|---|---|---|---|---|---|---|
| Atlantic salmon (S. salar) | Norway | 1991–2005 | 14 | Otero et al. (2012) | Age at maturity | SST |
| Atlantic salmon (S. salar) | Scotland | 1975–2010 | 35 | Todd et al. (2012) | Age at maturity | Stream T |
| Atlantic salmon (S. salar) | River Imsa, Norway | 1976–2001 | 25 | Juanes et al. (2004) | Age at maturity | NAO |
| Cod (G. morhua) | North Atlantic | 1943–1999 | 56 | Ottersen et al. (2006) | Age at maturity | SST |
| Sockeye salmon (O. nerka) | Fraser River, Canada | 1952–1993 | 42 | Cox & Hinch, (1997) | Age at maturity | SST |
| Atlantic salmon (S. salar) | Scotland | 1975–2010 | 35 | Todd et al. (2012) | Age at smolting | Stream T |
| Atlantic salmon (S. salar) | 31 stocks N. Am & Eur | 1989–2009 | 20 | Russell et al. (2012) | Age at smolting | |
| Eurasian ruffe (G. cernuus) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Appearance | Air T, NAO |
| European perch (Perca fluviatilis) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Appearance | Air T, NAO |
| Cod (G. morhua) | Arcto-Norwegian region | 1900–1976 | 76 | Sundby and Nakken (2008) | Fecundity | SST |
| Cod (G. morhua) | Barents Sea | 1986–1996 | 10 | Kjesbu et al. (1998) | Fecundity | SST-capelin |
| Atlantic salmon (S. salar) | Scotland & Canada | 1964–1993 | 29 | Friedland et al. (2005) | Growth | SST |
| Atlantic salmon (S. salar) | NE Atlantic | 1992–2006 | 14 | Todd et al. (2008) | Growth | SST |
| Cod (G. morhua) | Gulf of Alaska | 2006–2008 | 3 | Hurst et al. (2012) | Growth | SST |
| Herring (Clupea harengus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Growth | NAO |
| Plaice (Pleuronectes platessa) | North Sea | 1970–2004 | 34 | Teal et al. (2008) | Growth | SST |
| Smelt (Osmerus eperlanus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Growth | NAO |
| Sole (Solea solea) | North Sea | 1970–2004 | 34 | Teal et al. (2008) | Growth | SST |
| Sprat (Sprattus sprattus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Growth | NAO |
| Whiting (Merlangius merlangus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Growth | NAO |
| Cod (G. morhua) | Barents Sea | 1958–2000 | 42 | Beaugrand et al. (2003) | Juvenile survival | SST–copepods |
| American shad (Alosa sapidissima) | Columbia River, US | 1938–1993 | 55 | Quinn and Adams (1996) | Migration timing (A) | Stream T |
| Atlantic salmon (S. salar) | NE US & SE Canada | 1978–1999 | 21 | Juanes et al. (2004) | Migration timing (A) | Stream T |
| Atlantic salmon (S. salar) | Dalälven River | 1960–2002 | 42 | Dahl et al. (2004) | Migration timing (A) | SST & stream T |
| Atlantic salmon (S. salar) | Asturian Rivers, Spain | 1956–2006 | 50 | Valiente et al. (2011) | Migration timing (A) | Air T & NAO |
| Brown Trout (S. trutta) | Dalälven River | 1960–2002 | 42 | Dahl et al. (2004) | Migration timing (A) | SST & stream T |
| Cutthroat trout (O. clarkii clarkii) | Auke Creek, Alaska | 1970–2010 | 40 | Kovach et al. (2013) | Migration timing (A) | Stream T |
| Dolly Varden char (S. malma) | Auke Creek, Alaska | 1970–2010 | 40 | Kovach et al. (2013) | Migration timing (A) | Stream T |
| Flounder (Platichthys flesus) | UK | 1953–1965 | 13 | Sims et al. (2004) | Migration timing (A) | SST, NAO |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | 1979–2011 | 32 | Kovach et al. (2012) | Migration timing (A) | Stream T |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | 1972–2005 | 33 | Taylor (2008) | Migration timing (A) | Stream T |
| Sockeye salmon (O. nerka) | Columbia River, US | 1949–1993 | 44 | Quinn and Adams (1996) | Migration timing (A) | Stream T |
| Sockeye salmon (O. nerka) | Columbia River, US | 1949–2005 | 56 | Crozier et al. (2011) | Migration timing (A) | Stream T & flow |
| Atlantic salmon (S. salar) | Northern Ireland | 1978–2008 | 30 | Kennedy and Crozier (2010) | Migration timing (J) | Stream T |
| Atlantic salmon (S. salar) | 62 stocks N. Am & Eur | Variable | Russell et al. (2012) | Migration timing (J) | ||
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | 1972–2005 | 33 | Taylor (2008) | Migration timing (J) | Stream T |
| Coho salmon (O. kisutch) | Auke Creek, Alaska | 1970–2010 | 40 | Kovach et al. (2013) | Migration timing (J,A) | Str T, flow & SST |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | 1970–2010 | 40 | Kovach et al. (2013) | Migration timing (J,A) | Stream T |
| Sockeye salmon (O. nerka) | Auke Creek, Alaska | 1970–2010 | 40 | Kovach et al. (2013) | Migration timing (J,A) | Stream T & flow |
| Bass (Dicentrarchus labrax) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Cod (G. morhua) | Norway | 1919–2010 | 91 | Rogers et al. (2011) | Size | SST |
| Dab (Limanda limanda) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Flounder (Platichthys flesus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Plaice (Pleuronectes platessa) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Smelt (Osmerus eperlanus) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Sockeye salmon (O. nerka) | SW Alaska | 1962–2002 | 40 | Schindler et al. (2005) | Size | Ice out |
| Sole (Solea solea) | Thames estuary, UK | 1977–1992 | 16 | Attrill and Power (2002) | Size | NAO |
| Bream (Abramis brama) | Estonia | 1951–1990 | 39 | Noges and Jarvet (2005) | Spawn timing | Stream T |
| Burbot (Lota lota) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| Eurasian dace (Leuciscus cephalus) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| Eurasian ruffe (G. cernua) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| European perch (Perca fluviatilis) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| Northern pike (Esox lucius) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| Roach (Rutilus rutilus) | Estonia | 1951–1990 | 39 | Noges and Jarvet (2005) | Spawn timing | Stream T |
| Roach (Rutilus rutilus) | Lake Geneva, France | 1983–2000 | 18 | Gillet and Quétin (2006) | Spawn timing | Lake T |
| Smelt (Osmerus eperlanus) | Estonia | 1951–1998 | 47 | Ahas and Aasa (2006) | Spawn timing | Air T, NAO |
| Walleye (Sander vitreus) | 12 populations, Minnesota, US | Variable | Schneider et al. (2010) | Spawn timing | Ice out |
Species genera: S. Salmo, O. Oncorhynchus, G. cernua: Gymnocephalus, G. morhua: Gadus. Trait: A, adult, J, juvenile. Driver: T, temperature, SST, sea surface temperature.
If the variability of the NAO or PDO increases, subperiod trends will be steeper and persist longer. Although the impact of anthropogenic climate change on the variability in these oscillations is uncertain (NCADAC 2013), some authors have argued that changes are already apparent. First, a long-term linear trend in global temperature overlays the oscillations (Fig. 1 in Klyashtorin et al. 2009). When this trend is removed, the ∼60-year cycle is quite apparent (Fig. 2 in Klyashtorin et al. 2009). The impact of the trend is most pronounced at the peaks of the oscillation but could be detected at any point if the natural cycle is accounted for. Second, the intensity of the periodicity has increased during the last millennium, reaching a peak at the end of the twentieth century (Klyashtorin et al. 2009). Climate change might be affecting the range (peak to trough) of these cycles (Goodkin et al. 2008).
Figure 2.
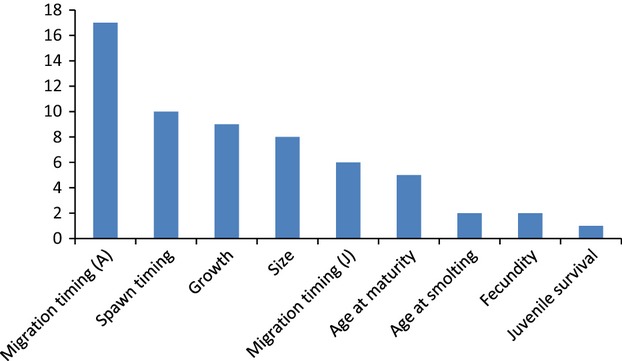
Frequency distribution of traits showing shifts that correlate with environmental drivers in recent decades. Migration timing is broken into adult migrations (A), which are usually spawning migrations (includes “appearance” in Table 1) or seawater to freshwater migrations, and juvenile (J) or freshwater to saltwater migrations.
Proximate drivers and traits likely to respond to climate change
Ecological impacts of these physical changes will vary by species and location. Physiologically, rising temperatures have some nearly universal effects in fish, such as increasing metabolic rates (Fry 1967). However, the ecological and evolutionary consequences of rising temperature depend on many factors, including population-specific proximity to lethal limits or growth optima (Pörtner and Peck 2010; Somero 2010), interspecific dynamics (Finstad et al. 2011) and disease impacts (Marcos-Lopez et al. 2010). Behavioural responses can reduce expression of physiological responses through thermoregulation (Angilletta 2009), or increase them because of competing pressures such as disease infection (Landis et al. 2012). Multiple stressors might act additively or synergistically (e.g. with compounding effects) and thus need to be considered in any examination of phenotypic shifts. Nonetheless, most research has focused on single factors.
Direct stress (temperature, hypoxia, pCO2, diseases)
The most straightforward proximate driver of global change in fish is direct physiological stress due to various factors − such as lowered pH, lowered oxygen levels, rising temperature − which leads secondarily to increased disease prevalence and morbidity in many cases (Rijnsdorp et al. 2009). Organisms integrate multiple stressors physiologically, accumulating factors that limit oxygen metabolism and aerobic scope (Pörtner et al. 2001; Pörtner and Farrell 2008; Anttila et al. 2013). Tolerance of these conditions has a strong genetic basis and shows high levels of local adaptation (Côté et al. 2012; Donelson and Munday 2012; Madeira et al. 2012; Munday et al. 2012), and thus, these traits clearly evolve by natural selection. Although most studies have focused on heat tolerance, because many fish communities face prolonged winter periods in temperate and high latitude areas, specialized adaptations for tolerating winter will also face a changing selection regime (Pörtner and Peck 2010; Shuter et al. 2012). Many other traits show temperature sensitivity and might threaten population viability, such as sex determination, sexual abnormalities and fertility (Strussmann et al. 2010; Pankhurst and Munday 2011). However, the use of latent genetic variation in local adaptations, such as development time in rainbow trout (Oncorhynchus mykiss), suggests evolution in key traits could occur quickly (Miller et al. 2012).
Selection for disease tolerance will likely intensify because warmer environments exhibit a general increase in the diversity of diseases, increased population growth rates of most microorganisms (Macnab and Barber 2012) and increased vulnerability of coldwater fishes. Furthermore, ongoing human activity tends to spread pathogens (Harvell et al. 1999; Marcos-Lopez et al. 2010). Historically, fish have adapted to high disease loads in warmer environments by enhancing the diversity of Major Histocompatibility Complex (MHC) genes (Dionne et al. 2007; Bowden 2008; Marcos-Lopez et al. 2010) and local adaptation to specific diseases (Beacham and Evelyn 1992; Bartholomew 1998).
Impacts of ocean acidification on fish are less well understood than those associated with temperature (Kroeker et al. 2010; Denman et al. 2011; but see examples of phytoplankton evolution in Reusch 2014). High pCO2 affects fish physiology directly, through developmental exposure (Franke and Clemmesen 2011; Frommel et al. 2012), olfaction (Munday et al. 2009; Dixson et al. 2010), and a variety of behaviours such as settlement and the avoidance of predators (Munday et al. 2012). Fish sensitivity also includes vulnerability to habitat loss (Gruber 2011; Gruber et al. 2012) and prey availability because of difficulties for skeleton- or shell-forming organisms under lower calcium-carbonate saturation states (Orr et al. 2005; Heath et al. 2012). Marine viruses interact with ocean biogeochemical cycles and fish dynamics in ways that are currently unpredictable but may profoundly influence ocean ecosystems (Danovaro et al. 2011).
Food-web dynamics affect behaviour, growth and survival
Although direct physical stressors are clearly limiting on some level, the primary mechanism cited in the literature by which climate impacts fish population dynamics involves the food web. Physical oceanographic, hydrological and limnological drivers determine the geographical distribution, total abundance, species composition and physiological condition of phytoplankton, zooplankton and plants (Collins 2014; Reusch 2014), which then alter fish growth and survival through trophic interactions (e.g. Schindler et al. 2005). Traits in fishes influenced by individual growth are wide ranging, including (but not limited to) age at maturity, size at maturity, brood number (fecundity), offspring (egg) size, timing of developmental stage (e.g. migration, metamorphosis), habitat type, choice of prey, vulnerability to predators and many more (Roff 1986, 2002; Jobling 1994; Wootton 1998; Hutchings 2002). Differences between species or populations in thermal reaction norms for growth can lead to competitive exclusion by other species with a better adapted reaction norm for a given environment. For example, Arctic char (Salvelinus alpinus) are more energetically efficient under cold temperatures or under ice but can be competitively excluded by brown trout (Salmo trutta) under warmer or more nutrient-rich conditions (Finstad et al. 2011; Helland et al. 2011). Fish can respond to changes in prey availability and energetic quality by modifying their distribution at broad spatial scales in the ocean (Perry et al. 2005; Sorte et al. 2010; Pinsky et al. 2013), vertically in the water column in lakes or the ocean (Dulvy et al. 2008; Pinsky et al. 2013), within stream networks (Comte and Grenouillet 2013), or by selecting different prey (Volkov 2012).
Other drivers of selection
Importantly, the effects of climate change on organisms will rarely act in isolation of other selection pressures. Many anthropogenic impacts drive contemporary evolution (Kinnison and Hendry 2001; Reznick and Ghalambor 2001; Stockwell et al. 2003; Hendry et al. 2008) and affect many of the same traits as climate change. Changes in age at maturity, demography and density (abundance), caused by fisheries, for example, feed back into the rate of response to climate change that we might expect because of the influence that factors such as effective population size, genetic variance and generation time have on rates of evolution (Hutchings and Fraser 2008). Changes in species composition can also generate rapid evolution (e.g. Reznick and Bryga 1987; Reznick et al. 1990; Walsh and Reznick 2011), and climate change is causing large-scale redistribution of fish communities simultaneously with human-mediated species movements (Perry et al. 2005; Dulvy et al. 2008; Comte and Grenouillet 2013).
Evidence for potential evolutionary responses in key traits
The most compelling evidence for the potential of evolutionary responses to anthropogenic climate change originates from cases of contemporary evolution in particularly relevant traits, especially through allochronic adaptation (i.e. changes over time) in introduced species. For example, sockeye salmon (Oncorhynchus nerka) introduced into Lake Washington in the 1930s and 1940s have diverged in developmental rates and survival at different temperatures (Hendry et al. 1998). Recent evolution in thermal tolerance has occurred in fish exposed to thermal effluents, such as mosquitofish tested after 30 years of exposure to abnormally warm water (Meffe et al. 1995). Artificial selection can induce much faster evolution, such as enhanced cold tolerance in three-spined sticklebacks (Gasterosteus aculeatus) within just three generations (Barrett et al. 2011), and improved heat tolerance in rainbow trout within 15 generations (Ineno et al. 2005). Spawn timing can also respond quickly to hatchery selection: a 2-week advance followed just four generations of selection in coho salmon (Oncorhynchus kistutch) (Neira et al. 2006).
Numerous other relevant traits have also evolved rapidly when exposed to a new environment (Reznick and Ghalambor 2001). For example, Chinook salmon (Oncorhynchus tshawytscha) from a single-source population in the Sacramento Valley in California, US, introduced to New Zealand, diverged quickly from their ancestral phenotypes in many traits: size at age and age at maturity (Kinnison et al. 2011), freshwater growth rates and migration timing (Quinn et al. 2001), egg size and number (Kinnison et al. 1998).
Evidence for climate-induced phenotypic change in fish
For this special issue, we undertook a specific literature review of phenotypic responses to climate change in wild fish populations to clarify whether impacts of climate change are evident and what is known about the mechanisms behind these responses. We assessed the methods of inference for genetic or plastic mechanisms in each paper, using the categories identified by Merilä and Hendry (this volume). They identified six methods for inferring genetic change (quantitative genetic animal models, common-garden studies, model predictions, experimental evolution, space-for-time substitution, and molecular genetics) and five methods for inferring plastic change (quantitative genetic animal models, common-garden studies, experimental evolution, fine-grained population responses and individual plasticity in nature). They further asked whether the response was adaptive and how the causal driver was inferred.
To identify papers that described a phenotypic change in a natural population and provided evidence that climate drove the phenotypic change, we searched for literature in the Web of Science in which the words climate' or ‘climatic change’ (‘climat* change’), and ‘adaptation’, ‘plasticity’ or ‘phenotypic change’, appeared in the publication title or topic area. Because these searches primarily identified papers addressing human adaptation to climate change or existing variation among populations, we refined our search by combining (‘climat* change’) with phenotypic traits we expected to be sensitive to climate (‘spawning’ or ‘spawn timing’, ‘migration timing’ or ‘migrat*’, ‘emergence timing’, ‘age at maturity’, ‘egg size’, ‘development time’ or ‘development rate’). Finally, we combined ‘adaptation’ with common names of fishes that have been well studied (stickleback, perch, bass, char, smelt, herring, pollock, cod and salmon). Although this is not an exhaustive list of all possible search combinations, it encompasses a broad cross section of the available literature.
Most of the papers that initially appeared relevant instead described (i) existing heterogeneity among populations (e.g. along spatial climatic gradients) that presumably reflects adaptation to different environments, (ii) experimental exposure to conditions predicted with climate change, such as elevated temperature or lower pH or (iii) changes in abundance or recruitment and thus did not satisfy our criteria. Because our results might appear to be biased towards salmonids, we made a concerted effort to obtain evidence of phenotypic change in other fish taxa by thoroughly checking major reviews of fish responses to climate change in both freshwater and marine environments (e.g. Roessig et al. 2004; Ficke et al. 2007; Graham and Harrod 2009; Staudinger et al. 2012; Griffiths 2013), and taxonomically broader reviews of evolutionary responses to climate change (e.g. Hendry and Kinnison 1999; Kinnison and Hendry 2001; Stockwell et al. 2003; Parmesan 2006; Carroll et al. 2007; IPCC 2007). In sum, we believe that our results reflect broader patterns in the literature on observed, natural fish responses to climate change.
We found 30 papers that generally fit our criteria. These papers examined 11 traits (Fig. 2) in 26 species (Table 1). Attrill and Power (2002) examined six additional species (Trisopterus luscus, Trisopterus minutus, Pomatoschistus spp., Anguilla anguilla, Agonus cataphractus, and Syngnathus rostellatus) in which they found significant correlations between the NAO and abundance but not growth. Most reports of phenotypic change described shifts in reproductive phenology (N = 17 adult migration timing and N = 10 spawn timing; Table 1). Changes in growth and juvenile size (N = 17), age at maturity (N = 5), age at seaward migration/smolting (N = 2) and fecundity (N = 2) were also reported. The distribution of studied taxa was biased towards salmon (especially Atlantic salmon [Salmo salar], but also Pacific salmon [Oncorhynchus spp.]) and Atlantic cod. All studies attributed phenotypic change to temperature variation, either through water temperature measurements directly, or air temperature and the NAO, ice break-up dates, temperature-driven changes in prey abundance or stream flow (Fig. 3). Geographically, the studies included North America and Europe, with most marine reports being from the North Atlantic (Table 1).
Figure 3.
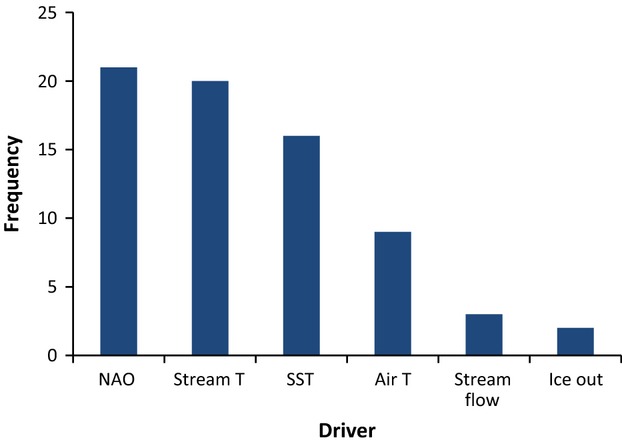
Frequency distribution of environmental drivers correlated with phenotypic change. NAO, North Atlantic Oscillation, T, temperature, SST, sea surface temperature; ice out is the day when a lake is free of all ice. We grouped counts by the species within a reference.
Evolutionary mechanisms postulated or demonstrated
Only one paper (Kovach et al. 2012) utilized molecular genetic data to document a shift in genotype frequencies associated with a shift in phenotypes (Table 2). Pink salmon (O. gorbuscha) in Auke Creek, Alaska, historically maintained a bimodal distribution in migration timing, with the early and late migrants about 3 weeks apart. A putatively neutral genetic marker was experimentally inserted into late-migrants in 1979; marker frequencies were stable from 1981 to 1989 and clearly differentiated early and late migrants. The two segments of the run had distinct morphological traits and maturation schedules, and genetic data showed little gene flow between them. Kovach et al. (2012) tracked the frequency of this late-migrant marker compared with other markers from 1983 to 2011. The late-migrant marker decreased rapidly in the late 1980s or early 1990s, and the proportion of the run exhibiting the late-migration phenotype has remained very low since then. Incidentally, this was near the peak of a PDO cycle (Fig. 1).
Table 2.
List of studies of phenotypic trends in response to climate
| Species | Location | Trait | Genetic | Plastic | Adaptive | Causality | Reference |
|---|---|---|---|---|---|---|---|
| American shad (Alosa sapidissima) | Columbia River, US | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Quinn and Adams (1996) |
| Atlantic salmon (S. salar) | Norway | Age at maturity | – | Yes (1) | – | Yes (1, 2) | Otero et al. (2012) |
| Atlantic salmon (S. salar) | Norway | Age at maturity | – | Yes (1) | – | Yes (1, 2) | Jonsson & Jonsson (2004) |
| Atlantic salmon (S. salar) | Scotland | Age at maturity | – | Yes (1) | – | Yes (1, 2) | Todd et al. (2012) |
| Atlantic salmon (S. salar) | 31 stocks N. Am & Eur | Age at smolting | – | – | – | Yes (1, 2) | Russell et al. (2012) |
| Atlantic salmon (S. alar) | Scotland | Age at smolting | – | Yes (1) | – | Yes (1, 2) | Todd et al. (2012); |
| Atlantic salmon (S. salar) | Scotland & Canada | Growth | – | Yes (1) | – | Yes (1, 2) | Friedland et al. (2005) |
| Atlantic salmon (S. salar) | NE Atlantic | Growth | – | Yes (1) | – | Yes (1, 2) | Todd et al. (2008) |
| Atlantic salmon (S. salar) | Dalälven River | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Dahl et al. (2004) |
| Atlantic salmon (S. salar) | Asturian Rivers, Spain | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Valiente et al. (2011) |
| Atlantic salmon (S. salar) | NE US & SE Canada | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Juanes et al. (2004) |
| Atlantic salmon (S. salar) | Northern Ireland | Migration timing (J) | – | Yes (1) | – | Yes (1, 2) | Kennedy and Crozier (2010) |
| Atlantic salmon (S. salar) | 62 stocks N. Am & Eur | Migration timing (J) | – | – | – | Yes (1, 2) | Russell et al. (2012) |
| Bass (Dicentrarchus labrax) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Bream (Abramis brama) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Bream (Abramis brama) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Noges and Jarvet (2005) |
| Brown Trout (S. trutta) | Dalalven River | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Dahl et al. (2004) |
| Cod (G. morhua) | North Atlantic | Age at maturity | – | Yes (1) | – | Yes (1, 2) | Ottersen et al. (2006) |
| Cod (G. morhua) | Barents Sea | Fecundity | – | Yes (1) | – | Yes (1, 2) | Kjesbu et al. (1998) |
| Cod (G. morhua) | Arcto-Norwegian region | Fecundity | – | Yes (1) | – | Yes (1, 2) | Sundby and Nakken (2008) |
| Cod (G. morhua) | Gulf of Alaska | Growth | – | Yes (1) | – | Yes (1, 2) | Hurst et al. (2012) |
| Cod (G. morhua) | Barents Sea | Juvenile survival | – | Yes (1) | – | Yes (1, 2) | Beaugrand et al. (2003) |
| Cod (G. morhua) | Norway | Size | – | Yes (1) | – | Yes (1, 2) | Rogers et al. (2011) |
| Coho salmon (O. kisutch) | Auke Creek, Alaska | Migration timing (J,A) | – | Yes (1) | – | Yes (1, 2) | Kovach et al. (2013) |
| Cutthroat trout (O. clarkii clarkii) | Auke Creek, Alaska | Migration timing (FW to S) | – | Yes (1) | – | Yes (1, 2) | Kovach et al. (2013) |
| Dab (Limanda Limanda) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Dolly Varden char (S. malma) | Auke Creek, Alaska | Migration timing (FW to S) | – | Yes (1) | – | Yes (1, 2) | Kovach et al. (2013) |
| Eurasian dace (Leuciscus cephalus) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Eurasian ruffe (G. cernua) | Estonia | Appearance | – | No (1) | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Eurasian ruffe (G. cernua) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| European perch (Perca fluviatilis) | Estonia | Appearance | – | No (1) | – | Yes (1, 2) | Ahas and Aasa (2006) |
| European perch (Perca fluviatilis) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Flounder (Platichthys flesus) | UK | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Sims et al. (2004) |
| Flounder (Platichthys flesus) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Herring (Clupea harengus) | Thames estuary, UK | Growth | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Northern pike (Esox lucius) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | Migration timing (A) | Yes (1) | – | – | Yes (1, 2) | Kovach et al. (2012) |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | Migration timing (A) | – | Yes (1) | – | Yes (1, 2) | Taylor (2008) |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | Migration timing (J) | – | Yes (1) | – | Yes (1, 2) | Taylor (2008) |
| Pink salmon (O. gorbuscha) | Auke Creek, Alaska | Migration timing (J,A) | – | Yes (1) | – | Yes (1, 2) | Kovach et al. (2013) |
| Plaice (Pleuronectes platessa) | North Sea | Growth | – | Yes (1,2) | – | Yes (1, 2) | Teal et al. (2008) |
| Plaice (Pleuronectes platessa) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Roach (Rutilus rutilus) | Lake Geneva, France | Ovary devpmt, spawn timing | – | Yes (1) | – | Yes (1, 2) | Gillet and Quétin (2006) |
| Roach (Rutilus rutilus) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Noges and Jarvet (2005) |
| Smelt (Osmerus eperlanus) | Thames estuary, UK | Growth | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Smelt (Osmerus eperlanus) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Smelt (Osmerus eperlanus) | Estonia | Spawn timing | – | – | – | Yes (1, 2) | Ahas and Aasa (2006) |
| Sockeye salmon (O. nerka) | Fraser River, Canada | Age at maturity | – | Yes (1) | – | Yes (1, 2) | Cox & Hinch (1997) |
| Sockeye salmon (O. nerka) | Columbia River, US | Migration timing (A) | Yes (2) | Yes (1) | Yes (1) | Yes (1, 2) | Crozier et al. (2011) |
| Sockeye salmon (O. nerka) | Columbia River, US | Migration timing (A) | – | No (1) | – | Yes (1, 2) | Quinn and Adams (1996) |
| Sockeye salmon (O. nerka) | Auke Creek, Alaska | Migration timing (J,A) | – | Yes (1) | – | Yes (1, 2) | Kovach et al. (2013) |
| Sockeye salmon (O. nerka) | SW Alaska | Size | – | Yes (1) | – | Yes (1, 2) | Schindler et al. (2005) |
| Sole (Solea solea) | North Sea | Growth | – | Yes (1,2) | – | Yes (1, 2) | Teal et al. (2008) |
| Sole (Solea solea) | Thames estuary, UK | Size | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Sprat (Sprattus sprattus) | Thames estuary, UK | Growth | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
| Walleye (Sander vitreus) | Minnesota, US | Spawn timing | – | Yes (1) | – | Yes (1, 2) | Schneider et al. (2010) |
| Whiting (Merlangius merlangus) | Thames estuary, UK | Growth | – | Yes (1) | – | Yes (1, 2) | Attrill and Power (2002) |
The columns identify whether a genetic or plastic basis for the trait was identified (Yes, No, or – = not tested explicitly), and by what method (Genetic: 1 = Molecular genetic methods, 2 = Comparison to model predictions; Plastic: 1 = Fine-grained population response, 2 = Experimental studies). If the study tested whether the response was adaptive, it is indicated in the next column (1 = phenotypic selection estimates). All studies attributed causality to environmental factors through regression analysis and reference to other work (Causality = Yes, 1 = Common sense or existing knowledge, 2 = phenotype-environment correlations). Species genera: S. Salmo, O. Oncorhynchus, G. cernua: Gymnocephalus, G. morhua: Gadus. Trait: A, adult, J, juvenile, FW to S, freshwater to saltwater migration.
Rapid changes occurred in the late-migrant locus, but not numerous microsatellite loci, indicating that natural selection caused the shift rather than genetic drift. However, the phenotypic target of selection is not entirely clear. High temperatures occurred during the years of rapid allele frequency change, and the early-migrating phenotype appears to have adaptations to warm temperature at multiple life stages (Fukushima and Smoker 1997; Smoker et al. 1998). Kovach et al. (2012) noted that the loss of the late-migrating phenotype was sudden and apparently due to selection against them, but that the more gradual trend in the median migration timing was consistent with other plastic drivers of migration date. Historically, marine survival was lower in the early-migrating fish, suggesting that the shift in adult migration timing might have negative consequences at other life stages. However, the adaptiveness of ongoing phenotypic change requires further testing.
Several other studies have explored climatic drivers of selection on sockeye salmon, which appears to be more likely to respond evolutionarily to certain pressures than other species. Quinn and Adams (1996) contrasted the responses of sockeye salmon with American shad (Alosa sapidissima) migration timing through a shared river basin, the Columbia River. They predicted shad would employ plasticity to respond to river conditions because of the high predictability and short-time interval between adult migration timing and larval emergence, which is presumably the target of selection. Consistent with this hypothesis, they documented a very fast shift in migration timing in shad and high interannual correlation with temperature (a fine-grained population response). However, this response was faster than the cue they had postulated as the driver (river temperature), suggesting they might not have identified the full cue for the response. Quinn and Adams (1996) postulated that, unlike shad, sockeye would rely more on a genetically determined migration date because of their relatively long larval incubation time and, hence, a lack of correlation between adult and juvenile environmental conditions. Consistent with this hypothesis, they found that sockeye lagged behind the rate of temperature change and responded much more slowly than shad. Thus, the mode of inference was through phenotypic-environment correlations, and no genetic analyses or direct tests of the adaptive nature of the response were conducted. Nonetheless, extensive corollary evidence (Naughton et al. 2005; Keefer et al. 2008) that high migration temperatures reduce survival in Columbia River sockeye salmon supports the hypothesis that elevated river temperature is the primary driver of the response.
Crozier et al. (2011) followed up Quinn and Adams' (1996) paper with a more specific model of selection pressure on sockeye salmon. Crozier et al. (2011) used a functional relationship between river temperature and survival, based on individually-tracked migrating cohorts, to estimate the annual selection pressure experienced by the population. They calculated a selection differential for each year since 1949 by reconstructing fish exposure using daily migration counts and temperature measurements at dams. Building on a method pioneered by Swain et al. (2007) study of fisheries-induced evolution in cod, they then used this annual selection differential to predict the shift in mean return migration timing of offspring. They allowed plastic drivers of migration timing, including river flow, a direct (within-year) effect of temperature, and oceanic factors such as the PDO and the North Pacific Gyre Oscillation (NPGO, Di Lorenzo et al. 2008) to modify the mean expected migration timing as plastic effects. Through model selection in a state-space modelling framework, they found very strong support for including the selection differential as a predictor of migration timing; none of the alternative plastic drivers tested could explain the observed shift in migration timing nearly as well.
The model results indicated that plasticity for migration timing in this sockeye population is largely a function of river flow and that the intercept for the norm of reaction has shifted by 3–6 days over 60 years (15 generations). Thus, this approach utilized both inferential evidence for genetic change (i.e. comparing model predictions with observed phenotypic change) and inferential evidence for plastic change (i.e. a fine-grained population response). The analysis of phenotypic selection estimates supports the hypothesis that the change was adaptive, and phenotype-environment correlations and comparison of alternative drivers point specifically to climate as the selective force. The advantage of this approach over strictly genetic methods is the strong link between the purported driver and target of selection, and response of the population.
One final sockeye study (Carlson and Quinn 2007) empirically estimated selection differentials over a decade and linked the selection directly to environmental conditions (lake level). They demonstrated strong links between climate and selection on a phenotypic trait (body size). Although the environmental trends during that study were too short to demonstrate a persistent response to climate change, Carlson and Quinn (2007) presented a compelling argument that evolution in this trait is likely to occur under climate scenarios of declining July precipitation and warming lake temperature. Other methods, such as the Price equation (Boutin and Lane, this special issue, and Price 1970, 1972; Coulson et al. 2010), cannot be applied to fish populations at this point. Although animal models are beginning to be used in fish populations (Neira et al. 2006; Serbezov et al. 2010; Debes et al. 2013), they are typically only practical under hatchery conditions or in highly constricted populations where nearly all the fish can be handled.
Plastic responses to recent climate variability or change
The remaining papers documented correlations between phenological change and environmental drivers. A strong statistical relationship at an annual time step is consistent with a plastic response rather than an evolutionary response (Merilä and Hendry 2014, this volume). Of course, correlations alone do not establish a causal link with the driver because many environmental and other factors are correlated with each other. However, independent studies demonstrating plasticity in the trait as a function of temperature, or very high resolution responsiveness, are compelling in some cases.
Migration/spawn timing
Several papers found that migration timing in juvenile salmon has advanced at a rate similar to that of water temperature (Kennedy and Crozier 2010; Russell et al. 2012; Todd et al. 2012). The relatively short time frame (<35 years), combined with independent evidence that temperature is a strong proximate cue for smolt migration, suggests that these responses are mostly plastic. Each of these papers expressed concern that the response was maladaptive. Kennedy and Crozier (2010) showed a correlation between migration timing and marine mortality and concluded that the trend towards earlier migration has increased marine mortality because of a mismatch between river and ocean temperatures. Russell et al. (2012) and Todd et al. (2012) argued that the smaller size of younger smolts lowered their marine survival and hence could be maladaptive. However, they did not formally test for adaptiveness. Juanes et al. (2004) found that long-term trends in migration timing of Atlantic salmon are consistent with a temperature response. Most of this shift occurred almost immediately upon transplantation from a more northern stock, and thus probably represents a plastic response. They argued that the change is adaptive based on a space-for-time comparison of trends in migration timing across a latitudinal gradient associated with temperature in many populations. Ongoing changes in many populations could have an evolutionary component, although this has not been explicitly tested. Schneider et al. (2010) examined time series of ice-out and walleye (Sander vitreus) spawning across numerous lakes in Minnesota, USA. They found a strong correlation between ice-out and spawn timing independent from the long-term trend, indicating a probable plastic cause.
Age at maturation
Recent work indicates that over recent decades, some Norwegian Atlantic salmon have bred at progressively older ages (1991–2005; Otero et al. 2012). The authors presented a thorough discussion of the possible mechanisms of this shift, although there is no direct evidence of a causal link between driving factors and the phenotypic change. Nonetheless, age at maturation is known to have both genetic and plastic components (Hutchings 2002). The prevailing mechanism is thought to be a threshold body size or growth rate at particular times of year, such that individuals mature only if they exceed the threshold. This threshold is genetically determined and varies among populations (Piché et al. 2008), but whether the threshold is reached in a given year depends on environmentally determined growth conditions. Thus, the immediate trend appears to be a plastic response to growth conditions.
Growth/survival
The majority of papers in our review documented annual variation in growth or survival that correlated with temperature variation, which indicates a plastic response. Attrill and Power (2002) documented strong interannual correlations between juvenile growth rates across a wide spectrum of marine fishes that use the Thames River estuary as a nursery. This appears to be a plastic response because detrended data showed even stronger statistical relationships, indicating annual response times, which is much too short to reflect evolutionary processes. Furthermore, they suggested use of the estuary is a facultative response to growing conditions, and found similar relationships between population abundance and individual body sizes. This is inconsistent with a rapid evolutionary response, which would entail strong selection and depress population sizes, at least temporarily. Thus, the primary basis for inference is the fine-grained population response and mechanistic reasoning. Similarly, sole (Solea solea) and plaice (Pleuronectes platessa) growth rates (Teal et al. 2008), sockeye juvenile growth (Schindler et al. 2005), cod size (Rogers et al. 2011) and survival (Beaugrand et al. 2003) and fecundity (Kjesbu and Witthames 2007) are well explained by plastic, reaction-norm responses to prey quality and quantity and seasonal timing. There is no indication that these represent evolutionary responses to climate change.
Plastic or evolutionary mechanisms
The remaining papers in our review documented coarser-grained correlations between temperature and phenotypic change, or no phenotypic change at all despite environmental change. Weak correlations could reflect either a plastic or an evolutionary response, or be coincidental. They might also reflect difficulty in identifying or procuring data on the most direct environmental driver (e.g., Pinsky et al. 2013). Nonetheless, Ahas and Aasa (2006) found that spring spawning periods have advanced significantly in three freshwater fish species (pike, Esox lucius, ruffe, Gymnocephalus cernua and bream, Abramis brama) and that migration timing has advanced in one species (smelt, Osmerus eperlanus) from 1951 to 1999, concurrently with a trend towards warmer springs. However, most of the monitored fish species exhibited no significant trends. They postulated that earlier snow melt and reduced spring flooding might have driven the observed shifts, partly because March and April weather showed the strongest correlations. One of the papers included in our review (Noges and Jarvet 2005) reported changes in spawning date over 40 years in two Estonian fish (bream and roach, Rutilus rutilus). They found that the former species had advanced its breeding date by 10 days, apparently tracking water temperature, but that roach spawn timing had remained constant. Roach now encounter water that is 3°C warmer during spawning than in the 1950s. This apparent lack of thermal plasticity might expose this species to selection and present opportunities for an evolutionary response over a longer time frame.
In summary, the majority of papers in our review documented relatively fine-grained population responses to temperature or snowmelt/ice-out over relatively short time frames. Although a very quick response was also documented from a single strong selection event in an extreme year (Kovach et al. 2012), as a general pattern, it provides strongest support for plastic responses to metrics of climate change. Most of these studies detrended time series prior to analysis, or otherwise removed the raw trend to isolate stochastic variation in the environmental factor as a driver of phenotypic variation. However, statistical sophistication varied. In two species (pink and sockeye salmon), the authors reported either genetic data (Kovach et al. 2012) or a pattern of phenotypic change consistent with an evolutionary response, based on a model of selection pressure (Crozier et al. 2011) or direct estimates of selection (Carlson and Quinn 2007). Most of these trends were considered adaptive for some life history stages, but all authors expressed concern that the responses might be maladaptive for subsequent life stages. None of these hypotheses regarding adaptiveness was explicitly tested. All studies linked the observed phenotypic changes to the environmental driver by phenotype-environment correlation, with or without detrending.
Discussion
We found that despite significant long-term trends in influential environmental factors, such as ocean and freshwater temperature, and despite abundant evidence that rapid evolution in climate-sensitive traits is possible, studies of natural adaptation to climate change in fishes are rare. Most studies reported correlations between temperature and population responses at annual time steps, which are consistent with plastic responses to environmental conditions: growth, fecundity, survival, migration and reproductive phenology are all changing in concert with environmental change. Given the high level of plasticity in these traits, detecting shifts in reaction norms would require additional methods.
Whether these changes are adaptive in the context of a warming climate remains an open question. The adaptive significance of observed shifts is complicated by the existence of multiple selective pressures acting on multiple life stages. The general use of stage-specific measurements and proxies for fitness rather than lifetime fitness make detecting adaptiveness difficult. For example, although a shift in gene frequencies might reflect adaptation, the trait being measured might not be the target of selection and thus not in itself appear adaptive. For example, Kovach et al. (2012) suggested that earlier migrants had more warm-adapted phenotypes in other life stages, which increased their fitness over late migrants. If this is the case, we might expect a reversal of the trend towards earlier migration in this population because the trend towards earlier migration timing itself exposed the fish to higher temperatures. In fact, changes in emigration and spawn timing often appeared to produce a phenological mismatch. For example, in Scotland, advanced smolt emigration from rivers in response to rising stream temperature correlated with reduced marine survival (Kennedy and Crozier 2010). But the lifetime costs and benefits of these shifts are not known.
It is clear that sufficiently high selection intensities can yield measurable selection responses in few generations in fishes. Crucial traits such as heat tolerance (Ineno et al. 2008), thermal reaction norms for growth (Kavanagh et al. 2010) and spawn timing (Neira et al. 2006) can evolve rapidly. Thus, evolution in response to climate change is certainly possible, and indeed likely, in fish. Furthermore, many studies spanned multiple generations, with the median study duration at 34 years, and the maximum at 91 years (Fig. 4). So why did so few studies document it? We propose three possible explanations.
Figure 4.
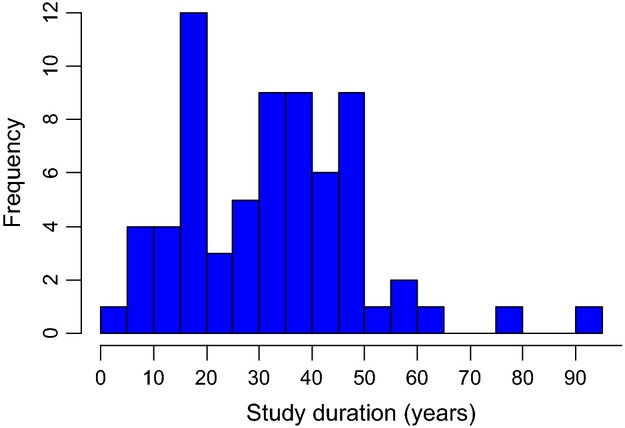
Frequency distribution of the number of years analyzed in each study.
First, the correlation-based methods generally employed are incapable of detecting the subtle shifts in reaction norms that are expected over the few generations spanned in most studies. One approach that might hold promise as a means of detecting evolutionary responses to climate change is the estimation of selection differentials over long time series (Swain et al. 2007; Crozier et al. 2011). Another might involve within-population studies of temporal shifts in the shapes of univariate or bivariate norms of reaction (Hutchings 2011). One means of detecting the latter has involved the use of probabilistic maturation reaction norms (PMRNs), albeit primarily for studies of fisheries-induced evolution (Dieckmann and Heino 2007). However, experimental studies have cautioned that temporal shifts in PMRNs can be influenced by nongenetic factors and might not always be indicative of evolution (Uusi-Heikkilä et al. 2011; Hurst et al. 2012; Díaz Pauli and Heino 2013). Research on genetic change in reaction norms could be usefully accompanied by experimental studies of selection responses by reaction norms induced by key metrics of climate change, such as temperature.
Second, natural climate variability and ‘regime shifts’ often dominated the temperature signals during study periods, rather than directional climate change (e.g. Kjesbu et al. 1998; Sundby and Nakken 2008; Rogers et al. 2011; Hurst et al. 2012). In addition to simple annual variation in climate, reversals in temperature and hence selection from decadal climate cycles might slow evolutionary responses to long-term warming and cause phenotypic trends in the opposite direction from that expected due to anthropogenic climate change (Fig. 1; Chevin 2013). These cycles can also amplify temperature trends in short-term records, but these trends do not necessarily reflect anthropogenic climate change alone. Decadal climate oscillations, such as the PDO and the NAO, cycle at 50–70 year periods. To capture evolution over these time frames is beyond the scope of most studies. In an oscillating climate, directional warming trends might impose strong selection primarily during extreme years, which will occur most often near peaks of natural decadal cycles. Kovach et al. (2012) demonstrated the potential long-term consequences of such a strong selection event quite eloquently. However, few studies will catch these exceptional events. The full evolutionary implications of natural climate cycles were not considered in any of the studies in our review.
Third, multiple selection pressures (e.g. fishing, competition from stocked fish) have the potential to overwhelm our ability to detect responses of fish to climate change. In the future, compounding threats from multiple stressors, such as hypoxia (Moran et al. 2010; Healy and Schulte 2012), high concentrations of pCO2 (Enzor et al. 2013) or contaminants (Terzi and Verep 2012) can lower thermal tolerance, thus increasing the likelihood that many more populations will surpass critical thermal thresholds. However, the correlation between hypoxia and temperature tolerance in a more general stress response (Anttila et al. 2013) suggests that evolution might also proceed faster than independent selection on uncorrelated traits (Etterson and Shaw 2001).
In general, the strongest evolutionary responses to climate change will likely occur in species with short generation times, subject to high or consistent selection pressure, especially near peaks of natural cycles, and in traits controlled relatively simply or with genetic variation present in existing populations. Of course adaptation, while allowing genotypes to fare better than they would have otherwise, need not translate into increased probability of persistence (Kopp and Matuszewski 2014). Many species in highly variable environments, such as the intertidal zone (Somero 2010; Tomanek 2010; Madeira et al. 2012), and many coral reef fishes are currently at or very near their thermal maxima, such that a slight increase in temperature can potentially impose strong selection (Munday et al. 2008, 2012), which might then drive populations extinct before they can adapt. However, if individual fitness tends to be higher at warm temperatures (i.e. ‘hotter is better’), then quantitative genetic models show that species in warmer environments might face lower extinction rates than those in cooler environments (Walters et al. 2012). Evolution is expected to be slower in Antarctic fishes, because they have lost functionality in relevant gene and gene regulatory areas, compared with simpler, single amino acid replacements, that would be necessary for other fish (Somero 2010; Tomanek 2010). Nonetheless, given abundant evidence that many traits in fish can respond rapidly to changes in environmentally driven selection pressures and that these traits are strongly plastic, we recommend exploration of more methods suitable for detecting temporal changes in reaction norms in fish.
Acknowledgments
We thank Andrew Hendry and Juha Merilä for initiating this project and providing very helpful editorial guidance, as well as two anonymous reviewers. JAH was supported by funding awarded by the Natural Sciences and Engineering Research Council (Canada).
Footnotes
We use the term anthropogenic climate change sensu the United Nations Framework Convention on Climate Change as ‘a change in climate due to human activity that alters the composition of the global atmosphere and which is in addition to natural climate variability observed over comparable time periods’.
Literature cited
- Ahas R, Aasa A. The effects of climate change on the phenology of selected Estonian plant, bird and fish populations. International Journal of Biometeorology. 2006;51:17–26. doi: 10.1007/s00484-006-0041-z. [DOI] [PubMed] [Google Scholar]
- Al-Chokhachy R, Alder J, Hostetler S, Gresswell R, Shepard B. Thermal controls of yellowstone cutthroat trout and invasive fishes under climate change. Global Change Biology. 2013;19:3069–3081. doi: 10.1111/gcb.12262. [DOI] [PubMed] [Google Scholar]
- Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press; 2009. [Google Scholar]
- Anttila K, Dhillon RS, Boulding EG, Farrell AP, Glebe BD, Elliott JAK, Wolters WR, et al. Variation in temperature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. Journal of Experimental Biology. 2013;216:1183–1190. doi: 10.1242/jeb.080556. [DOI] [PubMed] [Google Scholar]
- Arismendi I, Johnson SL, Dunham JB, Haggerty R, Hockman-Wert D. The paradox of cooling streams in a warming world: regional climate trends do not parallel variable local trends in stream temperature in the Pacific continental United States. Geophysical Research Letters. 2012;39:L10401. [Google Scholar]
- Attrill MJ, Power M. Climatic influence on a marine fish assemblage. Nature. 2002;417:275–278. doi: 10.1038/417275a. [DOI] [PubMed] [Google Scholar]
- Barrett RDH, Paccard A, Healy TM, Bergek S, Schulte PM, Schluter D, Rogers SM. Rapid evolution of cold tolerance in stickleback. Proceedings of the Royal Society B-Biological Sciences. 2011;278:233–238. doi: 10.1098/rspb.2010.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew JL. Host resistance to infection by the myxosporean parasite Ceratomyxa shasta: a review. Journal of Aquatic Animal Health. 1998;10:112–120. [Google Scholar]
- Baumann H, Conover DO. Adaptation to climate change: contrasting patterns of thermal-reaction-norm evolution in Pacific versus Atlantic silversides. Proceedings of the Royal Society B-Biological Sciences. 2011;278:2265–2273. doi: 10.1098/rspb.2010.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham TD, Evelyn TPT. Population and genetic variation in resistance of Chinook salmon to vibriosis, furunculosis, and bacterial kidney disease. Journal of Aquatic Animal Health. 1992;4:153–167. [Google Scholar]
- Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426:661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes. 2000;58:237–275. [Google Scholar]
- Bilyk KT, DeVries AL. Heat tolerance and its plasticity in Antarctic fishes. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2011;158:382–390. doi: 10.1016/j.cbpa.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Blunden J, Arndt DS. State of the climate in 2012. Bulletin of the American Meteorological Society. 2013;94:S1–S238. [Google Scholar]
- Bowden TJ. Modulation of the immune system of fish by their environment. Fish & Shellfish Immunology. 2008;25:373–383. doi: 10.1016/j.fsi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Bradbury I, Hubert S, Higgins B, Borza T, Bowman S, Paterson I, Snelgrove P, et al. Parallel adaptive evolution of Atlantic cod on both sides of the Atlantic Ocean in response to temperature. Proceedings of the Royal Society B-Biological Sciences. 2010;277:3725–3734. doi: 10.1098/rspb.2010.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury I, Hubert S, Higgins B, Bowman S, Borza T, Paterson I, Snelgrove P, et al. Genomic islands of divergence and their consequences for the resolution of spatial structure in an exploited marine fish. Evolutionary Applications. 2013;6:450–461. doi: 10.1111/eva.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett JR. Some principles of the thermal requirements of fishes. Quarterly Review of Biology. 1956;31:75–87. [Google Scholar]
- Brett JR. Energetics. In: Groot C, Margolis L, Clarke WC, editors. Physiological Ecology of Pacific Salmon. Vancouver: UBC Press; 1995. pp. 4–68. [Google Scholar]
- Carlson SM, Quinn TP. Ten years of varying lake level and selection on size-at-maturity in sockeye salmon. Ecology. 2007;88:2620–2629. doi: 10.1890/06-1171.1. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Hendry AP, Letcher BH. Natural selection acting on body size, growth rate and compensatory growth: an empirical test in a wild trout population. Evolutionary Ecology Research. 2004;6:955–973. [Google Scholar]
- Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Functional Ecology. 2007;21:387–393. [Google Scholar]
- Chevin LM. Genetic constrains on adaptation to a changing environment. Evolution. 2013;67:708–721. doi: 10.1111/j.1558-5646.2012.01809.x. [DOI] [PubMed] [Google Scholar]
- Chevin LM, Lande R, Mace GM. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Rost B, Rynearson TA. Evolutionary potential ofmarine phytoplankton under ocean acidification. Evolutionary Applications. 2014;7:140–155. doi: 10.1111/eva.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte L, Grenouillet G. Do stream fish track climate change? Assessing distribution shifts in recent decades. Ecography. 2013;36:1236–1246. [Google Scholar]
- Conover DO, Present TMC. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia. 1990;83:316–324. doi: 10.1007/BF00317554. [DOI] [PubMed] [Google Scholar]
- Conover DO, Schultz ET. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends in Ecology & Evolution. 1995;10:248–252. doi: 10.1016/S0169-5347(00)89081-3. [DOI] [PubMed] [Google Scholar]
- Côté J, Roussel JM, Cam S, Bal G, Evanno G. Population differences in response to hypoxic stress in Atlantic salmon. Journal of Evolutionary Biology. 2012;25:2596–2606. doi: 10.1111/jeb.12007. [DOI] [PubMed] [Google Scholar]
- Coulson T, Tuljapurkar S, Childs DZ. Using evolutionary demography to link life history theory, quantitative genetics and population ecology. Journal of Animal Ecology. 2010;79:1226–1240. doi: 10.1111/j.1365-2656.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SP, Hinch SG. Changes in size at maturity of Fraser River sockeye salmon (Oncorhynchus nerka) (1952–1993) and associations with temperature. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1159–1165. [Google Scholar]
- Croisetière S, Bernatchez L, Belhumeur P. Temperature and length-dependent modulation of the MH class IIβ gene expression in brook charr (Salvelinus fontinalis) by a cis-acting minisatellite. Molecular Immunology. 2010;47:1817–1829. doi: 10.1016/j.molimm.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Scheuerell MD, Zabel RW. Using time series analysis to characterize evolutionary and plastic responses to environmental change: a case study of a shift toward earlier migration date in sockeye salmon. American Naturalist. 2011;178:755–773. doi: 10.1086/662669. [DOI] [PubMed] [Google Scholar]
- Dahl J, Dannewitz J, Karlsson L, Petersson E, Lof A, Ragnarsson B. The timing of spawning migration: implications of environmental variation, life history, and sex. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2004;82:1864–1870. [Google Scholar]
- Danovaro R, Corinaldesi C, Dell'Anno A, Fuhrman JA, Middelburg JJ, Noble RT, Suttle CA. Marine viruses and global climate change. Fems Microbiology Reviews. 2011;35:993–1034. doi: 10.1111/j.1574-6976.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- Debes PV, McBride MC, Fraser DJ, Hutchings JA. Multigenerational hybridisation and its consequences for maternal effects in Atlantic salmon. Heredity. 2013;111:238–247. doi: 10.1038/hdy.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman K, Christian JR, Steiner N, Portner HO, Nojiri Y. Potential impacts of future ocean acidification on marine ecosystems and fisheries: current knowledge and recommendations for future research. Ices Journal of Marine Science. 2011;68:1019–1029. [Google Scholar]
- Di Lorenzo E, Schneider N, Cobb KM, Chhak K, Franks PJS, Miller AJ, McWilliams JC, et al. North Pacific Gyre Oscillation links ocean climate and ecosystem change. Geophysical Research Letters. 2008;35:L08607. NPGO Index available at http://eros.eas.gatech.edu/npgo/ [Google Scholar]
- Díaz Pauli B, Heino M. The importance of social dimension and maturation stage for the probabilistic maturation reaction norm in Poecilia reticulata. Journal of Evolutionary Biology. 2013;26:2184–2196. doi: 10.1111/jeb.12215. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths, and limitations. Marine Ecology-Progress Series. 2007;335:253–269. [Google Scholar]
- Dionne M, Miller KM, Dodson JJ, Caron F, Bernatchez L. Clinal variation in mhc diversity with temperature: evidence for the role of host-pathogen interaction on local adaptation in Atlantic salmon. Evolution. 2007;61:2154–2164. doi: 10.1111/j.1558-5646.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- Dixson DL, Munday PL, Jones GP. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecology Letters. 2010;13:68–75. doi: 10.1111/j.1461-0248.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- Donelson JM, Munday PL. Thermal sensitivity does not determine acclimation capacity for a tropical reef fish. Journal of Animal Ecology. 2012;81:1126–1131. doi: 10.1111/j.1365-2656.2012.01982.x. [DOI] [PubMed] [Google Scholar]
- Donelson JM, Munday PL, McCormick MI, Pitcher CR. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Climate Change. 2012;2:30–32. [Google Scholar]
- Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, et al. Climate change impacts on marine ecosystems. Annual Review of Marine Science. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- Dulvy NK, Rogers SI, Jennings S, Stelzenmuller V, Dye SR, Skjoldal HR. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. Journal of Applied Ecology. 2008;45:1029–1039. [Google Scholar]
- Enzor LA, Zippay ML, Place SP. High latitude fish in a high CO2 world: synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology. 2013;164:154–161. doi: 10.1016/j.cbpa.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Etterson JR, Shaw RG. Constraint to adaptive evolution in response to global warming. Science. 2001;294:151–154. doi: 10.1126/science.1063656. [DOI] [PubMed] [Google Scholar]
- Fangue NA, Podrabsky JE, Crawshaw LI, Schulte PM. Countergradient variation in temperature preference in populations of killifish Fundulus heteroclitus. Physiological and Biochemical Zoology. 2009;82:776–786. doi: 10.1086/606030. [DOI] [PubMed] [Google Scholar]
- Ficke AD, Myrick CA, Hansen LJ. Potential impacts of global climate change on freshwater fisheries. Reviews in Fish Biology and Fisheries. 2007;17:581–613. [Google Scholar]
- Finstad AG, Forseth T, Jonsson B, Bellier E, Hesthagen T, Jensen AJ, Hessen DO, et al. Competitive exclusion along climate gradients: energy efficiency influences the distribution of two salmonid fishes. Global Change Biology. 2011;17:1703–1711. [Google Scholar]
- Francis JA, Vavrus SJ. Evidence linking Arctic amplification to extreme weather in mid-latitudes. Geophysical Research Letters. 2012;39:L06801. [Google Scholar]
- Franke A, Clemmesen C. Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.) Biogeosciences. 2011;8:3697–3707. [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Applications. 2013;7:123–139. doi: 10.1111/eva.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland KD, Chaput G, MacLean JC. The emerging role of climate in post-smolt growth of Atlantic salmon. Ices Journal of Marine Science. 2005;62:1338–1349. [Google Scholar]
- Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, et al. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nature Climate Change. 2012;2:42–46. [Google Scholar]
- Fry REJ. Responses of vertebrate poikilotherms to temperature. In: Rose AH, editor. Thermobiology. New york: Academic Press; 1967. pp. 375–409. [Google Scholar]
- Fukushima M, Smoker WW. Determinants of stream life, spawning efficiency, and spawning habitat in pink salmon in the Auke Lake system, Alaska. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:96–104. [Google Scholar]
- Goodkin NF, Hughen KA, Doney SC, Curry WB. Increased multidecadal variability of the North Atlantic Oscillation since 1781. Nature Geoscience. 2008;1:844–848. [Google Scholar]
- Gillet C, Quétin P. Effect of temperature changes on the reproductive cycle of roach in Lake Geneva from 1983 to 2001. Journal of Fish Biology. 2006;69:518–534. [Google Scholar]
- Graham CT, Harrod C. Implications of climate change for the fishes of the British Isles. Journal of Fish Biology. 2009;74:1143–1205. doi: 10.1111/j.1095-8649.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RB. 2013. Oceans and Marine Resources in a Changing Climate. Techical Input to the 2013 National Climate Assessment U.S. Global Change Research Program.
- Gruber N. Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2011;369:1980–1996. doi: 10.1098/rsta.2011.0003. [DOI] [PubMed] [Google Scholar]
- Gruber N, Hauri C, Lachkar Z, Loher D, Frolicher TL, Plattner GK. Rapid progression of ocean acidification in the California Current System. Science. 2012;337:220–223. doi: 10.1126/science.1216773. [DOI] [PubMed] [Google Scholar]
- Hale R, Calosi P, McNeill L, Mieszkowska N, Widdicombe S. Predicted levels of future ocean acidification and temperature rise could alter community structure and biodiversity in marine benthic communities. Oikos. 2011;120:661–674. [Google Scholar]
- Handford P, Bell G, Reimchen T. A gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, et al. Review: marine ecology - emerging marine diseases - climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Haugen TO, Vøllestad LA. Population differences in early life-history traits in grayling. Journal of Evolutionary Biology. 2000;13:897–905. [Google Scholar]
- Healy TM, Schulte PM. Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 2012;182:49–62. doi: 10.1007/s00360-011-0595-x. [DOI] [PubMed] [Google Scholar]
- Heath MR, Neat FC, Pinnegar JK, Reid DG, Sims DW, Wright PJ. Review of climate change impacts on marine fish and shellfish around the UK and Ireland. Aquatic Conservation-Marine and Freshwater Ecosystems. 2012;22:337–367. [Google Scholar]
- Helland IP, Finstad AG, Forseth T, Hesthagen T, Ugedal O. Ice-cover effects on competitive interactions between two fish species. Journal of Animal Ecology. 2011;80:539–547. doi: 10.1111/j.1365-2656.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Stearns SC. Evolution Illuminated: Salmon and their Relatives. Oxford: Oxford University Press; 2004. [Google Scholar]
- Hendry AP, Hensleigh JE, Reisenbichler RR. Incubation temperature, developmental biology, and the divergence of sockeye salmon (Oncorhynchus nerka) within Lake Washington. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1387–1394. [Google Scholar]
- Hendry AP, Farrugia TJ, Kinnison MT. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology. 2008;17:20–29. doi: 10.1111/j.1365-294X.2007.03428.x. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world's marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Todgham AE. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Annual Review of Physiology. 2010;72:127–145. doi: 10.1146/annurev-physiol-021909-135900. [DOI] [PubMed] [Google Scholar]
- Hurst TP, Munch SB, Lavelle KA. Thermal reaction norms for growth vary among cohorts of Pacific cod (Gadus macrocephalus. Marine Biology. 2012;159:2173–2183. [Google Scholar]
- Hutchings JA. Life histories of fish. In: Hart PJB, Reynolds JD, editors. Handbook of Fish Biology and Fisheries. Fish Biology. Oxford: Blackwell Science; 2002. pp. 149–174. [Google Scholar]
- Hutchings JA. Old wine in new bottles: reaction norms in salmonid fishes. Heredity. 2011;106:421–437. doi: 10.1038/hdy.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Swain DP, Rowe S, Eddington JD, Puvanendran V, Brown JA. Genetic variation in life-history reaction norms in a marine fish. Proceedings of the Royal Society, Series B: Biological Sciences. 2007;274:1693–1699. doi: 10.1098/rspb.2007.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrell J. 1995. NAO Index Data provided by the Climate Analysis Section, NCAR, Boulder, USA. http://climatedataguide.ucar.edu/guidance/hurrell-north-atlantic-oscillation-nao-index-station-based (accessed on March 2013)
- Ineno T, Tsuchida S, Kanda M, Watabe S. Thermal tolerance of a rainbow trout Oncorhynchus mykiss strain selected by high-temperature breeding. Fisheries Science. 2005;71:767–775. [Google Scholar]
- Ineno T, Endo M, Watabe S. Differences in self-feeding activity between thermally selected and normal strains of rainbow trout Oncorhynchus mykiss at high temperatures. Fisheries Science. 2008;74:372–379. [Google Scholar]
- IPCC. 2007. Climate change 2007: the physical science basis: intergovernmental panel on climate change fourth assessment report. http://www.ipcc.ch/ (accessed on March 2013)
- IPCC. Managing the risks of extreme events and disasters to advance climate change adaptation. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, et al., editors. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. UK and New York, NY, USA: Cambridge University Press, Cambridge; 2012. p. 582. [Google Scholar]
- IPCC. 2013. Climate change 2013: the physical science basis: intergovernmental panel on climate change fourth assessment report. http://www.climatechange2013.org/ (accessed Sept 2013)
- Jensen LF, Hansen MM, Pertoldi C, Holdensgaard G, Mensberg KLD, Loeschcke V. Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proceedings of the Royal Society B-Biological Sciences. 2008;275:2859–2868. doi: 10.1098/rspb.2008.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling M. Fish Bioenergetics. New York: Chapman and Hall; 1994. [Google Scholar]
- Johansen JL, Jones GP. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Global Change Biology. 2011;17:2971–2979. [Google Scholar]
- Jonsson N, Jonsson B. Size and age of maturity of Atlantic salmon correlate with the North Atlantic Oscillation Index (NAOI) Journal of Fish Biology. 2004;64:241–247. [Google Scholar]
- Juanes F, Gephard S, Beland K. Long-term changes in migration timing of adult Atlantic salmon (Salmo salar) at the southern edge of the species distribution. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:2392–2400. [Google Scholar]
- Kassahn KS, Crozier RH, Portner HO, Caley MJ. Animal performance and stress: responses and tolerance limits at different levels of biological organisation. Biological Reviews. 2009;84:277–292. doi: 10.1111/j.1469-185X.2008.00073.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh KD, Haugen TO, Gregersen F, Jernvall J, Vollestad LA. Contemporary temperature-driven divergence in a Nordic freshwater fish under conditions commonly thought to hinder adaptation. Bmc Evolutionary Biology. 2010;10:350. doi: 10.1186/1471-2148-10-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer ML, Peery CA, Heinrich MJ. Temperature-mediated en route migration mortality and travel rates of endangered Snake River sockeye salmon. Ecology of Freshwater Fish. 2008;17:136–145. [Google Scholar]
- Kennedy RJ, Crozier WW. Evidence of changing migratory patterns of wild Atlantic salmon Salmo salar smolts in the River Bush, Northern Ireland, and possible associations with climate change. Journal of Fish Biology. 2010;76:1786–1805. doi: 10.1111/j.1095-8649.2010.02617.x. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Hendry AP. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica. 2001;112–113:145–164. [PubMed] [Google Scholar]
- Kinnison MT, Unwin MJ, Hershberger WK, Quinn TP. Egg size, fecundity, and development rate of two introduced New Zealand Chinook salmon (Oncorhynchus tshawytscha) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1946–1953. [Google Scholar]
- Kinnison MT, Quinn TP, Unwin MJ. Correlated contemporary evolution of life history traits in New Zealand Chinook salmon, Oncorhynchus tshawytscha. Heredity. 2011;106:448–459. doi: 10.1038/hdy.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjesbu OS, Witthames PR. Evolutionary pressure on reproductive strategies in flatfish and groundfish: relevant concepts and methodological advancements. Journal of Sea Research. 2007;58:23–34. [Google Scholar]
- Kjesbu OS, Witthames PR, Solemdal P, Walker MG. Temporal variations in the fecundity of Arcto-Norwegian cod (Gadus morhua) in response to natural changes in food and temperature. Journal of Sea Research. 1998;40:303–321. [Google Scholar]
- Klyashtorin L, Borisov V, Lyubushin A. Cyclic changes of climate and major commercial stocks of the Barents Sea. Marine Biology Research. 2009;5:4–17. [Google Scholar]
- Koehn JD, Hobday AJ, Pratchett MS, Gillanders BM. Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation. Marine and Freshwater Research. 2011;62:1148–1164. [Google Scholar]
- Kopp M, Matuszewski S. Rapid evolution of quantitative traits: theoretical perspectives. Evolutionary Applications. 2014;7:169–191. doi: 10.1111/eva.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach RP, Gharrett AJ, Tallmon DA. Genetic change for earlier migration timing in a pink salmon population. Proceedings of the Royal Society B-Biological Sciences. 2012;279:3870–3878. doi: 10.1098/rspb.2012.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach RP, Joyce JE, Echave JD, Lindberg MS, Tallmon DA. Earlier migration timing, decreasing phenotypic variation, and biocomplexity in multiple salmonid species. Plos One. 2013;8 doi: 10.1371/journal.pone.0053807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim RN, Singh GG. Review and Synthesis: meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters. 2010;141:9–1434. [Google Scholar]
- Lande R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. Journal of Evolutionary Biology. 2009;22:1435–1446. doi: 10.1111/j.1420-9101.2009.01754.x. [DOI] [PubMed] [Google Scholar]
- Landis SH, Sundin J, Rosenqvist G, Roth O. Behavioral adjustments of a pipefish to bacterial Vibrio challenge. Behavioral Ecology and Sociobiology. 2012;66:1399–1405. [Google Scholar]
- Liu JP, Curry JA, Wang HJ, Song MR, Horton RM. Impact of declining Arctic sea ice on winter snowfall (vol 109, pg 4074, 2012) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6781–6783. doi: 10.1073/pnas.1114910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy TE, Hannah L. Climate Change and Biodiversity. New Haven: CT, Yale University Press; 2005. [Google Scholar]
- Macnab V, Barber I. Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Global Change Biology. 2012;18:1540–1548. [Google Scholar]
- Madeira D, Narciso L, Cabral HN, Vinagre C. Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. Journal of Sea Research. 2012;70:32–41. [Google Scholar]
- Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC. A Pacific interdecadal climate oscillation with impacts on salmon production. Bulletin of the American Meteorological Society. 1997;78:1069–1079. [Google Scholar]
- Marcil J, Swain DP, Hutchings JA. Countergradient variation in body shape of a widespread marine fish, the Atlantic cod (Gadus morhua. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2006;273:217–223. doi: 10.1098/rspb.2005.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos-Lopez M, Gale P, Oidtmann BC, Peeler EJ. Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transboundary and Emerging Diseases. 2010;57:293–304. doi: 10.1111/j.1865-1682.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- Meffe GK, Weeks SC, Mulvey M, Kandl KL. Genetic differences in thermal tolerance of eastern mosquitofish (Gambusia holbrooki Poeciliidae) from ambient and thermal ponds. Canadian Journal of Fisheries and Aquatic Sciences. 1995;52:2704–2711. [Google Scholar]
- Merilä J, Hendry AP. Climate change, adaptation and phenotypic plasticity: the problem and the evidence. Evolutionary Applications. 2014;7:1–14. doi: 10.1111/eva.12137. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilä J, Sheldon BC, Kruuk LEB. Explaining stasis: microevolutionary studies in natural populations. Genetica. 2001;112–113:199–222. [PubMed] [Google Scholar]
- Miller M, Brunelli J, Wheeler P, Liu S, Rexroad C, Palti Y, Doe C, et al. A conserved haplotype controls parallel adaptation in geographically distant salmonid populations. Molecular Ecology. 2012;21:237–249. doi: 10.1111/j.1365-294X.2011.05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R, Harvey I, Moss B, Feuchtmayr H, Hatton K, Heyes T, Atkinson D. Influence of simulated climate change and eutrophication on three-spined stickleback populations: a large scale mesocosm experiment. Freshwater Biology. 2010;55:315–325. [Google Scholar]
- Mousseau TA, Roff DA. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Munday PL, Jones GP, Pratchett MS, Williams AJ. Climate change and the future for coral reef fishes. Fish and Fisheries. 2008;9:261–285. [Google Scholar]
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1848–1852. doi: 10.1073/pnas.0809996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, McCormick MI, Nilsson GE. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? Journal of Experimental Biology. 2012;215:3865–3873. doi: 10.1242/jeb.074765. [DOI] [PubMed] [Google Scholar]
- Naughton GP, Caudill CC, Keefer ML, Bjornn TC, Stuehrenberg LC, Peery CA. Late-season mortality during migration of radio-tagged adult sockeye salmon (Oncorhynchus nerka) in the Columbia River. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:30–47. [Google Scholar]
- NCADAC. 2013. National climate assessment and development advisory committee. Third national climate assessment draft report: U.S. global change research program http://www.globalchange.gov/publications/reports (accessed on Jun 2013)
- Neira R, Diaz NF, Gall GAE, Gallardo JA, Lhorente JP, Alert A. Genetic improvement in coho salmon (Oncorhynchus kisutch). II: selection response for early spawning date. Aquaculture. 2006;257:1–8. [Google Scholar]
- Noges P, Jarvet A. Climate driven changes in the spawning of roach (Rutilus rutilus (L.)) and bream (Abramis brama (L.)) in the Estonian part of the Narva River basin. Boreal Environment Research. 2005;10:45–55. [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- Otero J, Jensen AJ, L'Abee-Lund JH, Stenseth NC, Storvik GO, Vollestad LA. Contemporary ocean warming and freshwater conditions are related to later sea age at maturity in Atlantic salmon spawning in Norwegian rivers. Ecology and Evolution. 2012;2:2192–2203. doi: 10.1002/ece3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen G, Hjermann DO, Stenseth NC. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fisheries Oceanography. 2006;15:230–243. [Google Scholar]
- Pankhurst NW, Munday PL. Effects of climate change on fish reproduction and early life history stages. Marine and Freshwater Research. 2011;62:1015–1026. [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics. 2006;37:637–669. [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- Piché J, Hutchings JA, Blanchard W. Genetic variation in threshold reaction norms for alternative reproductive tactics in male Atlantic salmon, Salmo salar. Proceedings of the Royal Society B: Biological Sciences. 2008;275:1571–1575. doi: 10.1098/rspb.2008.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. Marine taxa track local climate velocities. Science. 2013;341:1239–1242. doi: 10.1126/science.1239352. [DOI] [PubMed] [Google Scholar]
- Pörtner H, Farrell A. Physiology and climate change. Science of the Total Environment. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. Journal of Fish Biology. 2010;77:1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Berdal B, Blust R, Brix O, Colosimo A, Giuliani B, De Wachter A, et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus) Continental Shelf Research. 2001;21:1975–1997. [Google Scholar]
- Price GR. Selection and covariance. Nature. 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- Price GR. Extension of covariance selection mathematics. Annals of Human Genetics. 1972;35:485–490. doi: 10.1111/j.1469-1809.1957.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Purdom CE. Genetics and Fish Breeding. New York: Chapman and Hall; 1993. [Google Scholar]
- Quinn TP, Adams DJ. Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology. 1996;77:1151–1162. [Google Scholar]
- Quinn TP, Kinnison MT, Unwin MJ. Evolution of Chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica. 2001;112–113:493–513. [PubMed] [Google Scholar]
- Rahel FJ, Olden JD. Assessing the effects of climate change on aquatic invasive species. Conservation Biology. 2008;22:521–533. doi: 10.1111/j.1523-1739.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- Reusch T. Climate change in the oceans: evolutionary vs. phenotypically plastic responses. Evolutionary Applications. 2014;7:104–122. doi: 10.1111/eva.12109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick DN, Bryga H. Life-history evolution in guppies (Poecilia reticulata). 1. Phenotypic and genetic changes in an introduction experiment. Evolution. 1987;41:1370–1385. doi: 10.1111/j.1558-5646.1987.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. [PubMed] [Google Scholar]
- Reznick DA, Bryga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. [Google Scholar]
- Ricker WE. Changes in the average size and average age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38.12:1636–1656. [Google Scholar]
- Rijnsdorp AD, Peck MA, Engelhard GH, Möllmann C, Pinnegar JK. Resolving the effect of climate change on fish populations. ICES Journal of Marine Science. 2009;66:1570–1583. [Google Scholar]
- Roessig JM, Woodley CM, Cech JJ, Hansen LJ. Effects of global climate change on marine and estuarine fishes and fisheries. Reviews in Fish Biology and Fisheries. 2004;14:251–275. [Google Scholar]
- Roff DA. Predicting body size with life-history models. BioScience. 1986;36:316–323. [Google Scholar]
- Roff D. Comparing G matrices: A MANOVA approach. Evolution. 2002;56:1286–1291. [PubMed] [Google Scholar]
- Rogers LA, Stige LC, Olsen EM, Knutsen H, Chan KS, Stenseth NC. Climate and population density drive changes in cod body size throughout a century on the Norwegian coast. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1961–1966. doi: 10.1073/pnas.1010314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IC, Aprahamian MW, Barry J, Davidson IC, Fiske P, Ibbotson AT, Kennedy RJ, et al. The influence of the freshwater environment and the biological characteristics of Atlantic salmon smolts on their subsequent marine survival. Ices Journal of Marine Science. 2012;69:1563–1573. [Google Scholar]
- Sala OE, Armesto FS, III, Chapin JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Salinas S, Munch SB. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecology Letters. 2012;15:159–163. doi: 10.1111/j.1461-0248.2011.01721.x. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics. 1993;24:35–68. [Google Scholar]
- Schindler DE, Rogers DE, Scheuerell MD, Abrey CA. Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology. 2005;86:198–209. [Google Scholar]
- Schlichting CD, Pigliucci M. Phenotypic Evolution: A Reaction Norm Perspective. MA, Sinauer Assoc: Sunderland; 1998. [Google Scholar]
- Schneider KN, Newman RM, Card V, Weisberg S, Pereira DL. Timing of walleye spawning as an indicator of climate change. Transactions of the American Fisheries Society. 2010;139:1198–1210. [Google Scholar]
- Scott GR, Johnston IA. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14247–14252. doi: 10.1073/pnas.1205012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbezov D, Bernatchez L, Olsen EM, Vøllestad LA. Quantitative genetic parameters for wild stream-living brown trout: heritability and parental effects. Journal of Evolutionary Biology. 2010;23:1631–1641. doi: 10.1111/j.1420-9101.2010.02028.x. [DOI] [PubMed] [Google Scholar]
- Shuter BJ, Finstad AG, Helland IP, Zweimuller I, Holker F. The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change. Aquatic Sciences. 2012;74:637–657. [Google Scholar]
- Sims DW, Wearmouth VJ, Genner MJ, Southward AJ, Hawkins SJ. Low-temperature-driven early spawning migration of a temperate marine fish. Journal of Animal Ecology. 2004;73:333–341. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS. Genetic variation of return date in a population of pink salmon: a consequence of fluctuating environment and dispersive selection? Alaska Fishery Research Bulletin. 1998;5:46–54. [Google Scholar]
- Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. Journal of Experimental Biology. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Sorte CJB, Williams SL, Carlton JT. Marine range shifts and species introductions: comparative spread rates and community impacts. Global Ecology and Biogeography. 2010;19:303–316. [Google Scholar]
- Staudinger MD, Grimm NB, Staudt A, Carter SL, Chapin FS, III, Kareiva P, Ruckelshaus M, et al. 2012. Impacts of climate change on biodiversity, ecosystems, and ecosystem services: technical input to the 2013 national climate assessment, cooperative report to the 2013 national climate assessment. http://assessment.globalchange.gov (accessed on Jun 2013)
- Stenseth NC, Mysterud A. Weather packages: finding the right scale and composition of climate in ecology. Journal of Animal Ecology. 2005;74:1195–1198. [Google Scholar]
- Stillman JH. Acclimation capacity underlies the susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. [DOI] [PubMed] [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- Stoks R, Geerts A, De Meester L. Evolutionary and plastic responses of freshwater invertebrates to climate change: realized patterns and future potential. Evolutionary Applications. 2014;7:42–55. doi: 10.1111/eva.12108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strussmann CA, Conover DO, Somoza GM, Miranda LA. Implications of climate change for the reproductive capacity and survival of New World silversides (family Atherinopsidae) Journal of Fish Biology. 2010;77:1818–1834. doi: 10.1111/j.1095-8649.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- Sundby S, Nakken O. Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. Ices Journal of Marine Science. 2008;65:953–962. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SG. Climate warming causes phenological shift in Pink Salmon, Oncorhynchus gorbuscha, behavior at Auke Creek, Alaska. Global Change Biology. 2008;14:229–235. [Google Scholar]
- Teal LR, Rijnsdorp JJ, de Leeuw HW, van der Veer AD. Effects of climate change on growth of 0-group sole and plaice. Marine Ecology Progress Series. 2008;358:219–230. [Google Scholar]
- Terzi E, Verep B. Effects of water hardness and temperature on the acute toxicity of mercuric chloride on rainbow trout (Oncorhynchus mykiss) Toxicology and Industrial Health. 2012;28:499–504. doi: 10.1177/0748233711416943. [DOI] [PubMed] [Google Scholar]
- Thomas C, Cameron A, Green R, Bakkenes M, Beaumont L, Collingham Y, Erasmus B, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Todd CD, Hughes SL, Marshall CT, MacLean JC, Lonergan ME, Biuw EM. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Global Change Biology. 2008;14:958–970. [Google Scholar]
- Todd CD, Friedland KD, MacLean JC, Whyte BD, Russell IC, Lonergan ME, Morrissey MB. Phenological and phenotypic changes in Atlantic salmon populations in response to a changing climate. Ices Journal of Marine Science. 2012;69:1686–1698. [Google Scholar]
- Tomanek L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. Journal of Experimental Biology. 2010;213:971–979. doi: 10.1242/jeb.038034. [DOI] [PubMed] [Google Scholar]
- Tonteri A, Vasemagi A, Lumme J, Primmer CR. Beyond MHC: signals of elevated selection pressure on Atlantic salmon (Salmo salar) immune-relevant loci. Molecular Ecology. 2010;19:1273–1282. doi: 10.1111/j.1365-294X.2010.04573.x. [DOI] [PubMed] [Google Scholar]
- Urban J. Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evolutionary Applications. 2014;7:88–103. doi: 10.1111/eva.12114. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Heikkilä S, Kuparinen A, Wolter C, Meinelt T, O'Toole A, Arlinghaus R. Experimental assessment of probabilistic maturation reaction norm: condition matters. Proceedings of the Royal Society B: Biological Sciences. 2011;278:709–717. doi: 10.1098/rspb.2010.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente AG, Juanes F, Garcia-Vazquez E. Increasing regional temperatures associated with delays in Atlantic salmon sea-run timing at the southern edge of the European distribution. Transactions of the American Fisheries Society. 2011;140:367–373. [Google Scholar]
- Volkov AF. Is the mass emergence of Themisto libellula in the northern Bering Sea an invasion or a bloom? Russian Journal of Marine Biology. 2012;38:465–473. [Google Scholar]
- Walsh MR, Reznick DN. Experimentally induced life-history evolution in a killifish in resonse the introduction of guppies. Evolution. 2011;65:1021–1036. doi: 10.1111/j.1558-5646.2010.01188.x. [DOI] [PubMed] [Google Scholar]
- Walters CJ, Martell SJD. Fisheries Ecology and Management. New Jersey, Princeton University Press: Princeton; 2004. [Google Scholar]
- Walters RJ, Blanckenhorn WU, Berger D. Forecasting extinction risk of ectotherms under climate warming: an evolutionary perspective. Functional Ecology. 2012;26:1324–1338. [Google Scholar]
- Wenger SJ, Olden JD. Assessing transferability of ecological models: an underappreciated aspect of statistical validation. Methods in Ecology and Evolution. 2012;3:260–267. [Google Scholar]
- Wootton RJ. Ecology of Teleost Fishes. New York: Chapman & Hall; 1998. [Google Scholar]


