Abstract
Maintaining adequate numbers of spermatogonial stem cells is required for the production of the millions of sperm required for male fertility. To date, however, the mechanisms that regulate the size of this pool in the adult are poorly defined. Glial cell line-derived neurotrophic factor (GDNF) is required for establishing this pool in the prepubertal animal, but its in vivo function in the normal adult testis has never been examined directly. We used a chemical-genetic approach to address this issue. We generated mice carrying a single amino acid mutation (V805A) in Ret, the kinase subunit of the GDNF receptor. This mutation does not affect normal GDNF signaling, but renders it susceptible to inhibition by the ATP competitive inhibitor, NA-PP1. When GDNF signaling was blocked in adults for 11 days, only a few cells remained that expressed the stem spermatogonial markers, Gfrα1 and Zbtb16 and testicular Ret mRNA content was reduced markedly. These decreases were associated with depletion of functional stem spermatogonia; some were lost when GDNF signaling was inhibited for only 2 days while others survived for up to 11 days. However, when signaling was restored, the remaining stem cells proliferated, initiating tissue restoration. In conclusion, these results provide the first direct proof that GDNF acutely regulates the numbers of spermatogonial stem cells in the normal adult testis. Additionally, these results demonstrate different sensitivities among subpopulations of these stem cells to inhibition of GDNF signaling.
Keywords: stem spermatogonia, GDNF, Sertoli cell, Ret, spermatogenesis
Introduction
Spermatogonial stem cells are the foundation of fertility of the adult male and preserving this foundation requires that when these cells replicate the formation of new stem cells is balanced with the generation of differentiating progeny 1. The mechanisms that control this balance prevent the pathological accumulation of stem cells or, alternatively, the depletion of the stem cell pool. There is considerable evidence that Sertoli cells are integral to these mechanisms because they secrete GDNF, which has been hypothesized to promote the self-renewal over the differentiation of replicating stem cells (reviewed in: 1). However, not all data support this hypothesis, and it has never been tested directly in vivo in the adult.
Evidence for a role of GDNF in regulating numbers of spermatogonial stem cells comes from both in vitro and in vivo studies. In long-term culture GDNF is required to maintain and expand stem spermatogonia, as measured by their ability to restore spermatogenesis when transplanted into a germ cell-deficient testis 2. However, those results could be obtained either if GDNF promoted self-renewing replication of the stem spermatogonia in vitro or if it acted primarily as a stem cell survival factor. If GDNF specifically promotes self-renewal of replicating stem spermatogonia, absence of GDNF should result in a rapid loss of some of these cells within one cell cycle, which is estimated to be 43-46 hours in duration 3-8. However, when cultures enriched in mouse stem spermatogonia were deprived of GDNF for 3 or 6 days, there was a significant increase in numbers of functional stem cells and this increase was identical to what was observed when cells were incubated with GDNF 9. Moreover, the number of spermatogonia that did not act as functional stem cells was substantially higher when GDNF was added to the cultures. These results are the opposite of what is predicted by the hypothesis that GDNF promotes self-renewal over differentiation. The current hypothesis is also challenged by the analysis of cell fate decisions by individual rat stem spermatogonia in culture 10. In these experiments, one stem cell could be observed to undergo self-renewing replication while another in the same culture microenvironment produced differentiating progeny, leading to the proposal that cell fate decisions were regulated by mechanisms intrinsic to the stem cells themselves.
In vivo studies using traditional transgenic overexpression or a gene knockout of GDNF have examined the consequences of altered GDNF expression from the time of birth or earlier, but have allowed neither an analysis of the effects of the loss of GDNF signaling specifically in the adult nor the consequences of restoration of this signaling. In vivo studies demonstrate that GDNF is essential for establishing the stem spermatogonial pool in the immature testis. Over expression of GDNF resulted in the formation of clusters of undifferentiated spermatogonia many of which died by apoptosis, resulting in an infertile adult with many tubules that contained only a rim of spermatogonia 11. Whether any were functional stem spermatogonia could not be determined, however, because they expressed the transgene and consequently recapitulated their abnormal phenotype when transplanted into a germ cell-deficient testis 12. Testes of GDNF null mice were depleted of almost all germ cells within 7 days and the few germ cells that were present did not replicate 13. Mice that were haploinsufficient for GDNF initiated the first wave of spermatogenesis but afterwards, many of their tubules experience a progressive loss of spermatogonia, spermatocytes and then spermatids, resulting in a deficiency in germ cells when the mice reached sexual maturity. While these results can be viewed as supporting the hypothesis that GDNF promotes self-renewing replication of stem spermatogonia, it should be noted that the first wave of spermatogenesis is initiated from gonocytes, and not from stem spermatogonia 14. Thus, an alternative explanation for these data is that the primary effect of a deficiency in GDNF at birth is the failure of the gonocytes to give rise to a stem spermatogonial pool of normal size and function.
Given the importance of the proper regulation of numbers of stem spermatogonia to male fertility throughout adulthood and the fact that our knowledge of the regulation of these cells by GDNF is limited by available experimental paradigms, we concluded that a new paradigm was needed that allowed one to study the effects of manipulating GDNF signaling to stem spermatogonia within the physiological context of the normal adult testis. Therefore, we developed a chemical-genetic approach that allows signaling from this growth factor to be reversibly inhibited in a highly specific manner 15-18. GDNF signaling is initiated when it binds to the ligand-binding subunit of its receptor, Gfrα1, which causes activation and autophosphorylation of Ret, the tyrosine kinase subunit of the receptor 19. The mice used for our studies carry a single amino acid mutation (V805A) in Ret, which has no effect on baseline Ret kinase activity. However, it substantially increases its affinity for the cell permeable ATP competitive inhibitor, NA-PP1 20. While Ret is also a subunit for receptors of other GDNF family members, knockouts of the ligand binding subunits for these other receptors have no effect on testis morphology or on male fertility 21-23. Therefore, inhibition of Ret kinase activity by NA-PP1 allows one to specifically evaluate the function of GDNF in the normal adult testis. Furthermore, when NA-PP1-treatment is terminated, GDNF signaling is restored.
Using this chemical-genetic approach we examined the hypotheses that GDNF was essential for maintenance of the pool of spermatogonial stem cells in the normal adult testis and that one function of this growth factor was to stimulate self-renewing replication of the stem cell pool. We test the prediction made by the second hypothesis that stem spermatogonia are lost if GDNF signaling is inhibited for two days, the approximate length of their cell cycle. We demonstrate that treatment of Ret (V805A) mice with NA-PP1 for two days causes loss of some stem spermatogonia, while others persist for up to 11 days. Furthermore, our data suggest that once GDNF signaling is restored, the remaining stem cells not only proliferate, but they also move within the basal compartment of the epithelium, insuring that empty stem cell niches are reseeded with the cells that ultimately give rise to the millions of sperm, which are required for male fertility 24.
Materials and Methods
Animals
Mice carrying a mutation (V805A) in the ATP binding site of Ret were generated as previously described 25. The frt-flanked neomycin resistance cassette in the targeting construct (Supplemental Fig. S1) was removed by crossing these mice with B6; SJL-Tg(ACTFLPe) 9205 DG M/J mice (Jackson Laboratories, Bar Harbor, ME). Mice that were homozygous for the Ret (V805A) mutation were identified by PCR analysis of genomic DNA using primers that crossed the 5’ LoxP site of the targeting construct:
Ret F (36580): CCTTGGGCCTGCTGAGCACGGG
RET R (36858): GGAGGCAGGAAGGCCTGTGC
PCR conditions were: 4 minutes at 95°C followed by 35 cycles of: 30 sec at 95°C, 45 sec for 57°C, 45 sec at 72°C, followed by a 7 min incubation at 72°C. Mice were 70-100 days of age at the start of the experiment and the Johns Hopkins University Institutional Animal Care and Use Committee approved their use.
Testing the efficacy of the different ATP competitive inhibitors of Ret (V805A)
Full-length cDNAs for wild type Ret and Ret (V805A) were cloned into the pRK5 vector (BD Biosciences; San Diego, CA) and transfected into confluent HEK 293 cells using lipofectamine (Invitrogen, Carlsbad, CA). After three hours, medium with serum was added along with NA-PP1 or a related inhibitor. Cells were lysed 16 hrs. later, fractioned by SDS-PAGE, blotted onto nylon and incubated with Anti- Ret (Y1062) (Santa Cruz Biotechnology, Santa Cruz, CA) and the ECL detection system (GE Healthcare, Piscataway, NJ). Some blots were re-probed for total Ret (Santa Cruz Biotechnology).
Synthesis and administration of NA-PP1
NA-PP1 and related compounds were synthesized as previously described 20. To convert NA-PP1 into an HCl salt, 400 mg of NA-PP1 was dissolved in 40 ml of methanol plus 5 ml of 1.25M HCl in methanol. NA-PP1 was dissolved by heating and stirring, the salt was dried using a rotovap, dissolved in ethanol (62.5 mg/ml) and stored under nitrogen at -20°C. One part of NA-PP1 in ethanol was diluted into 9 parts in saline:cremophor EL (7:2) and 62.5 mg NA-PP1/kg body weight was injected subdermally between the scapulae. Vehicle-treated control mice were injected with 100 microliters of ethanol:saline:cremophor EL per 10 grams body weight.
Analysis of tissues
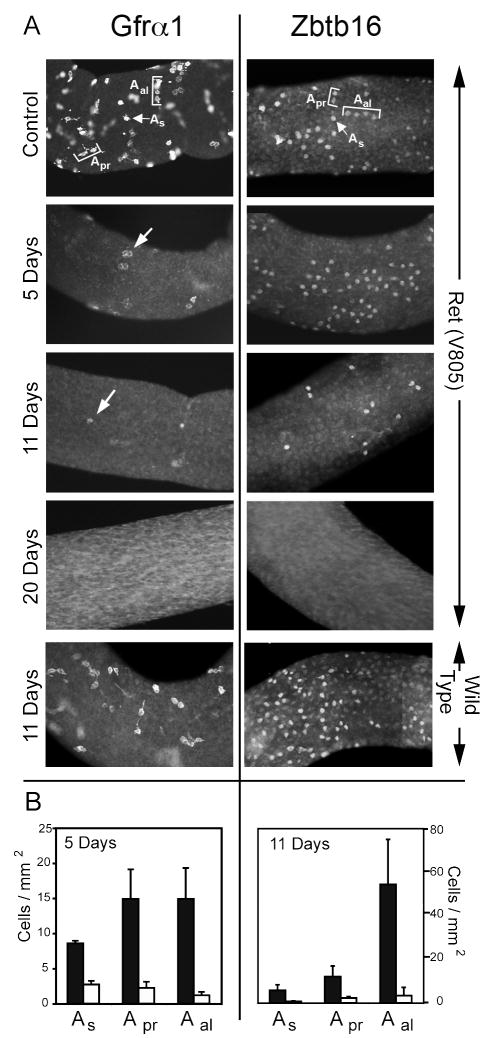
Seminiferous tubules were manually isolated in PBS and fixed for 2 hours in 4% paraformaldehyde. After extensive washing, tubules were processed for detection of the stem spermatogonial markers, Zbtb16 (previously called PLZF) and Gfrα1. To detect Zbtb16, tubules were dehydrated through graded methanol, incubated for two hours in methanol:DMSO:3%H2O2 (4:1:1) and rehydrated through a graded series of methanol to PBS. Tubules were next incubated for two hours in PBS-MT (2 grams nonfat dried milk, 0.5ml Triton X-100 per 100 ml PBS) and then incubated overnight at 4°C with shaking in 4 μg/ml goat anti-PLZF (R&D Systems, Minneapolis, MN) in PBS-MT. Tubules were washed at room temperature twice for 15 minutes, 5 times for one hour in PBS-MT and then incubated overnight at 4°C in 4 μg/ml Alexa fluor-488 rabbit anti-goat IgG (Invitrogen, Carlsbad, CA) in PBS-MT. Following washing, tubules were mounted in Vectashield (Vector Laboratories, Burlingame, CA). To detect Gfrα1, tubules were incubated for 2 hours in PBS-AT (1 gram BSA, 0.1gram Triton X-100 per 100 ml PBS) and then overnight at 4°C in 2 μg/ml goat anti-Gfrα1(R&D systems) diluted in PBS-AT. Tubules were washed six times for 15 minutes in PBS-AT and then incubated overnight at 4°C in 4 μg/ml Alexa fluor-488 rabbit anti-goat IgG diluted in PBS-AT. Following washing, tubules were mounted in Vectashield. Digital images were captured with a Nikon Eclipse Microscope equipped with a cooled CCD camera (QImaging, Surrey, BC, CA) and imported into iVision (Biovision Technologies, Exton, PA). From these images, we evaluated the numbers and distribution of Zbtb16+ and Gfrα1+ spermatogonia. We classified these cells as A single (As) spermatogonia, a subpopulation of which are the functional stem cells, as differentiating progeny of As cells, the A paired (Apr) spermatogonia, or as A aligned (Aal) spermatogonia, which form chains of 4-16 cells 1 (Fig 1). A change in the ratio of Apr or Aal spermatogonia to As cells would indicate increased differentiation of replicating stem cells.
Figure 1.
Inhibition of GDNF signaling results in the sequential loss of Gfrα1+ and then Zbtb16+ As, Apr and Aal spermatogonia. Panel A shows representative whole mounts of seminiferous tubules of Ret (V805A) mice that were treated daily with 63.5 mg/kg of NA-PP1 for 5,11 or 20 days or of Ret (V805A) mice that were treated for 20 days with vehicle (Control) (N=4-6 mice/group). Also shown are tubules of wild-type mice that were treated for 11 days with NA-PP1 (n=4/group). Representative Gfrα1+ or Zbtb16+ As, Apr and Aal spermatogonia are identified on the control tubules. The fluorescence that is out of focus in the control tubule immunostained for Gfrα1 originates from cells on the other side. There were only a few faintly stained GFRα1+ cells on tubules of Ret (V805A) mice following 5 or 11 days of treatment with NA-PP1 (see arrows). The density of Zbtb16+ spermatogonia was normal when GDNF signaling was inhibited for 5 day but was decreased after 11 days. Gfrα1+ and Zbtb16+ cells were absent when GDNF signaling was inhibited for 20 days. Panel B quantifies the effect of injecting Ret (V805A) mice with NA-PP1 (white bar) or vehicle (black bar) on the densities of As, Apr and Aal spermatogonia that expressed Gfrα1+ (n=3) or Zbtb16+ (n=4). Analysis of Gfrα1± and Zbtb16+ cells was conducted after 5 and 11 days of treatment, respectively. Data are expressed as mean + SEM.
To evaluate the relationship between duration of inhibited GDNF signaling and loss of functional stem cells, we took advantage of the fact that this loss is followed by depletion of increasing mature spermatogenic cells. Testes were fixed in 5% glutaraldehyde in cacodylate buffer, postfixed in osmium tetroxide, embedded in Epon 812, and 1 micron thick sections stained with Toluidine blue. Four to six different testis cross sections are evaluated and a minimum of 300 tubules per testis were examined for the presence or absence of spermatogonia, spermatocytes and/or spermatids and for their stage of the cycle of the seminiferous epithelium. (Each stage is defined by the presence of differentiated spermatogonia, spermatocytes and spermatids at specific phases of development. These cell mature synchronously, and consequently the epithelium progresses from one stage to the next (see Supplemental Fig S2).) This analysis identified the stages during which a given spermatogenic cell type was lost. Since the duration of each stage is defined the number of days over which the stem cells are lost is reflected in the number of days over which a more mature germ cell type is lost 26, 27.
Kidneys and livers were emersion fixed in Bouins fixative, embedded in paraffin and 5 micron sections stained with hematoxylin and eosin.
Measurement of transcript levels
RNA was isolated using RNAeasy kits (Quigen, Valencia CA), cDNA was synthesized using Superscript III (Invitrogen) and transcripts encoding Zbtb16, Gfrα1, Ret and 18S rRNA were quantified using TaqMan primers (Life Technologies Corp, Carlsbad, CA). Standard curves for each assay were generated from cloned, sequence-verified cDNA standards and the amount of each transcript was normalized to the amount of 18S rRNA in each sample.
Statistical analysis
Cell counts were analyzed using a nested ANOVA and other data were analyzed by ANOVA. Statistical analysis used StatView (SAS Institute Inc, Cary, NC). Differences were defined as significant at p≤0.05.
Results
Characterization of Ret (V805A) mice and their general response to NA-PP1
The first step in generating a mouse allowing reversible inhibition of GDNF signaling was to engineer the Ret kinase domain such that an otherwise inert small molecule could act as a high affinity inhibitor of kinase activity. We selected the mutation (V805A) by comparing the sequence of RET to other kinases that have been targeted in this manner (Supplemental Fig. S3). Transient transfection analysis demonstrated that this mutation did not affect baseline kinase activity, but made RET susceptible to inhibition by NA-PP1 (Supplemental Fig. S4). We then generated mice that were homozygous for this mutation and verified the ability of NA-PP1 to inhibit Ret by injecting it into pregnant mice from embryonic day 9 until birth. Homozygous pups born to these mothers died by P2 due to hypoplastic kidneys, thereby recapitulating the Ret knockout phenotype 28. However, treating adult male Ret (V805A) mice or wild-type mice for 30 days with NA-PP1 had no effect on body weight, or on kidney and liver histology (Supplemental Fig. S5 & S6). Additionally, testes weights of wild-type and Ret (V805A) mice were unaffected when treated with NA-PP1 for 30 and 20 days, respectively (Supplemental Fig. S5). Thus, NA-PP1 is an effective in vivo inhibitor of Ret function and has no demonstrable toxicity or off-target effects in adults.
Inhibition of GDNF signaling in the adult causes loss of the expression of the stem spermatogonial markers, Gfrα1, Zbtb16 and Ret
Initially, to test the hypothesis that GDNF is required by spermatogonial stem cells in the normal adult testis, we treated adult male Ret (V805A) mice for 5,11 or 20 days with NA-PP1 and examined Gfrα1+ and Zbtb16+ spermatogonia. Figure 1A presents results that are characteristic of four to six mice per experimental group. When GDNF signaling was inhibited for 5 days, there were fewer Gfrα1+ spermatogonia on the tubules and the amount of protein per cell as measured by signal intensity was reduced as well. Very few Gfrα1+ cells were detected after 11 days and none after 20 days. In contrast, numbers of Zbtb16+ spermatogonia appeared unchanged at 5 days, but their numbers were reduced after 11 days and they were absent after 20 days. In contrast, NA-PP1 had no effect on wild-type mice (Fig. 1A). (The faintly staining Zbtb16+ cells on tubules of wild-type mice are normally detected at stages X to II of the cycle (Fig. S7)).
To quantify these results, we enumerated Gfrα1+ or Zbtb16+ As, Apr and Aal spermatogonia on tubules from Ret (V805A) mice treated for 5 or 11 days with NA-PP1 or with vehicle. After 5 days, densities of Gfrα1+ As, Apr and Aal spermatogonia were significantly reduced to 32%, 15% and 7% of vehicle-treated controls, respectively (Fig. 1B). After 11 days, the densities of Zbtb16+ As, Apr and Aal spermatogonia were significantly reduced to 12%, 21% and 6% of control, respectively (Fig. 1B).
To extend this analysis, we measured the levels of transcripts encoding Ret, Gfrα1 and Zbtb16 (Fig. 2). The testicular levels of Ret mRNA and Gfrα1 mRNA were similar in control mice, but after GDNF signaling was inhibited, there was a more rapid, logarithmic decline of expression of Ret mRNA; at 20 days Ret and Gfrα1 mRNA levels were 9% and 34% of control, respectively. Additionally, the expression of Zbtb16 mRNA decreased linearly to 33% of control by 20 days. Taken together, the data in figures 1 and 2 demonstrate that in the normal adult testis, inhibition of GDNF signaling leads to the rapid loss of expression of three different markers of spermatogonial stem cells. Furthermore, these data suggest that in vivo, the Ret gene is a direct target of the signal transduction cascade emanating from the kinase that it encodes.
Figure 2.
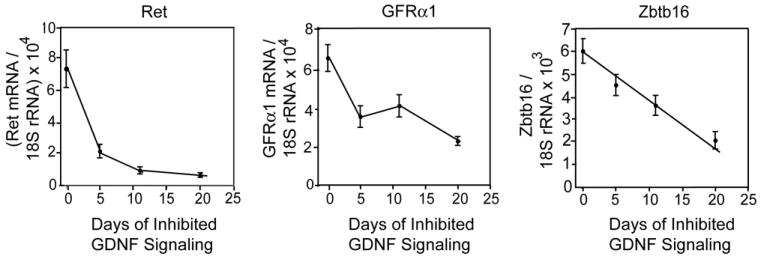
Inhibition of GDNF signaling results in a progressive loss of Ret, Gfrα1 and Zbtb16 mRNAs from the testis. Data (mean + SEM; n=5-6/group) are expressed as the numbers of molecules of each transcript divided by numbers molecules of 18S rRNA in the same sample. Control Ret (V805A) mice (0 days inhibited GDNF signaling) were injected with vehicle for 5 or 11 days.
Inhibition of GDNF signaling for 30 days causes loss of all functional stem spermatogonia from a normal adult testis
While figure 2 shows that levels of transcripts encoding Ret, Gfrα1 and Zbtb16 were greatly reduced after 20 days, these transcripts were still detectable, raising the issue of whether inhibition of GDNF signaling caused stem and other undifferentiated spermatogonia to be lost or whether this inhibition only reduced the expression of the stem cell markers to levels that were not detectable by immunocytochemistry. Therefore, to test for the loss of functional stem cells, we injected adult Ret (V805A) and wild-type mice with NA-PP1 for 30 days and then collected tissues immediately or after an additional 35 days (n=5 mice/group). We treated mice for 30 days because extrapolation of the data in figure 2 predicted that this period of time was required for the complete disappearance of transcripts encoding Ret, Gfrα1 and Zbtb16. Testes were collected 35 days after treatment because this is the time required for completion of spermatogenesis in the mouse 26. Thus, an animal without any stem and other As, Apr and Aal spermatogonia would have no spermatogenic cells 35 day later. Consistent with this prediction, testes of NA-PP1-treated Ret (V805A) mice weighed 55.4 + 4.4 mg while testes of vehicle-treated mice weighed 217 +9.4 mg (mean + SEM) Figure 3 shows the testicular histology of wild type and Ret (V805A) mice that had been injected for 30 days with NA-PP1 and testes collected 35 days later. Examination of at least 300 tubules from each of 5 treated wild type mice demonstrated that 97± 2.5% (mean + SEM) of the tubules contained a full complement of spermatogenic cells (Fig. 3A&B). By contrast, none of tubules of the five Ret (V805A) mice contained a single spermatogenic cell. Thus, spermatogonial stem cells were lost during the 30 days of treatment of Ret (V805A) mice with NA-PP1 (Fig. 3C&D).
Figure 3.
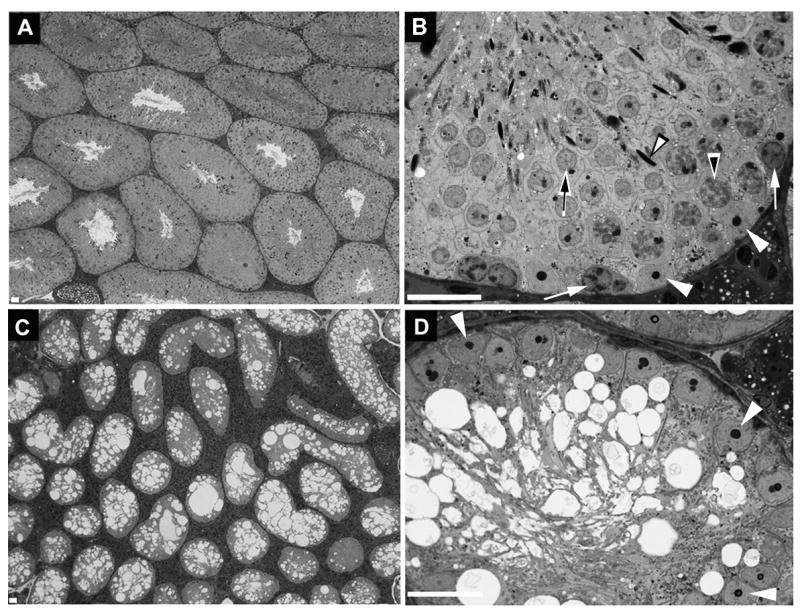
Inhibition of GDNF signaling for 30 days results in the loss of all spermatogonial stem cells from the mature testis of Ret (V805A) mice. This figure shows the testicular histology of wild-type (A,B) and Ret (V805A) (C,D) mice treated for 30 days with NA-PP1 and testes collected 35 days thereafter. The data are representative of all 5 mice in each group. In Panels B and D white arrowheads point to Sertoli cell nuclei. In panel B white arrows point to spermatogonia, the black-on-white arrowhead points to a nucleus of a pachytene spermatocyte, the black-on-white arrow points to a round spermatid and the white-on-black arrowhead points to a nucleus of an elongate spermatid. Bar = 20 microns.
Does inhibition of GDNF signaling cause a synchronous loss of spermatogonial stem cells or are these cells lost over an extended period of time? We answered this question by examining testes from mice that were injected for 30 days with NA-PP1 and samples collected 24 hours later. As discussed in Materials and Methods, because loss of stem cells leads subsequently to loss of more mature spermatogenic cells, the time over which the stem cells are lost is reflected in the time over which a more mature germ cell type is lost. This loss required a substantial period of time for it was common to find two apposing tubules at stages VII or VIII where one tubule contained pachytene spermatocytes, round spermatids and elongate spermatids while the other contained only the later two cell types (Supplemental Fig. S8). Quantitative analysis showed that at stages I through V, only 9% of the tubules contained pachytene spermatocytes while at stage IX, 96% of the tubules contained these cells (Fig. 4). As approximately 4.1 days are required for a tubule to progress from stage III (half way between I and V) to stage IX (Supplemental Fig S7), we conclude that during the 30 days that the Ret (V805A) mice were treated with NA-PP1, 87% of the stem spermatogonia were lost over a period of 4.1 days.
Figure 4.
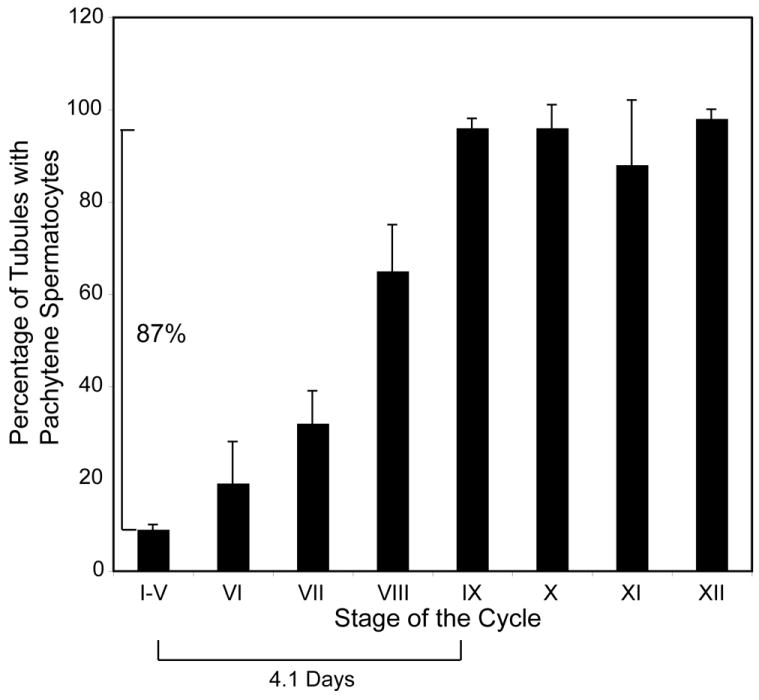
When GDNF signaling is inhibited, 87% of stem spermatogonia are lost over a period of 4.1 days. Ret V805A mice were treated for 30 days with NA-PP1, testes were collected 1 day after the last injection and then processed for light microscopy. The stage of the cycle of each tubule in a testis cross section was identified and scored as containing no pachytene spermatocytes or as containing at least 1 pachytene spermatocyte. Data are presented as the percentage of tubules at each stage containing at least 1 pachytene spermatocyte (mean ± SEM; n=4 animals). At stages XI and XII, diplotene or secondary spermatocytes were scored rather then pachytene spermatocytes. Since the loss of stem spermatogonia eventually leads to the loss of pachytene spermatocytes, and since the duration of each stage of the cycle is known (Fig S7) the period of time over which 87% of the pachytene spermatocytes are lost provides an estimate of the period of time over which a similar percentage of stem spermatogonial are lost.
A small percentage of stem spermatogonia remain after inhibition of GDNF signaling for 11 days
To gain a better understanding how rapidly loss of GDNF signaling causes loss of stem spermatogonia, we also treated Ret (V805A) mice and wild-type mice for 11 days with NA-PP1, which reduces Ret mRNA levels in testes of Ret (V805A) mice to 10% of control (Fig. 2). Thirty-five days later, testes of all wild-type mice were morphologically normal (Fig. 5A,B). Testes of Ret (V805A) collected 24 hours after the last injection with NA-PP1 were also morphologically normal, through analysis of tubule whole-mounts revealed very few Gfrα1+ cells all of which were As spermatogonia (Fig. 5C-D). In contrast, 35 days later, 97% of the seminiferous tubules of Ret (V805A) mice contained either no spermatogenic cells or only elongate spermatids (Supplemental Fig. S9). However, 3% of the tubules exhibited active spermatogenesis and this correlated with the presence of scattered but dense clusters of Gfrα1+ spermatogonia (Fig. 5E,F). The numbers of these clusters (0.24 ± 0.14 clusters/mm2; mean ±SEM) was similar to the number of the individual Gfrα1+ As spermatogonia on tubules that were examined 24 hours after the last of the 11 injections of NA-PP1 (0.46±0.14 cells/mm2). We conclude that inhibition of GDNF signaling for 11 days caused loss of approximately 97% of the stem spermatogonia. However, when GDNF signaling was restored, the remaining undifferentiated spermatogonia acted as stem cells, proliferated and began to rebuild the tissue.
Figure 5.
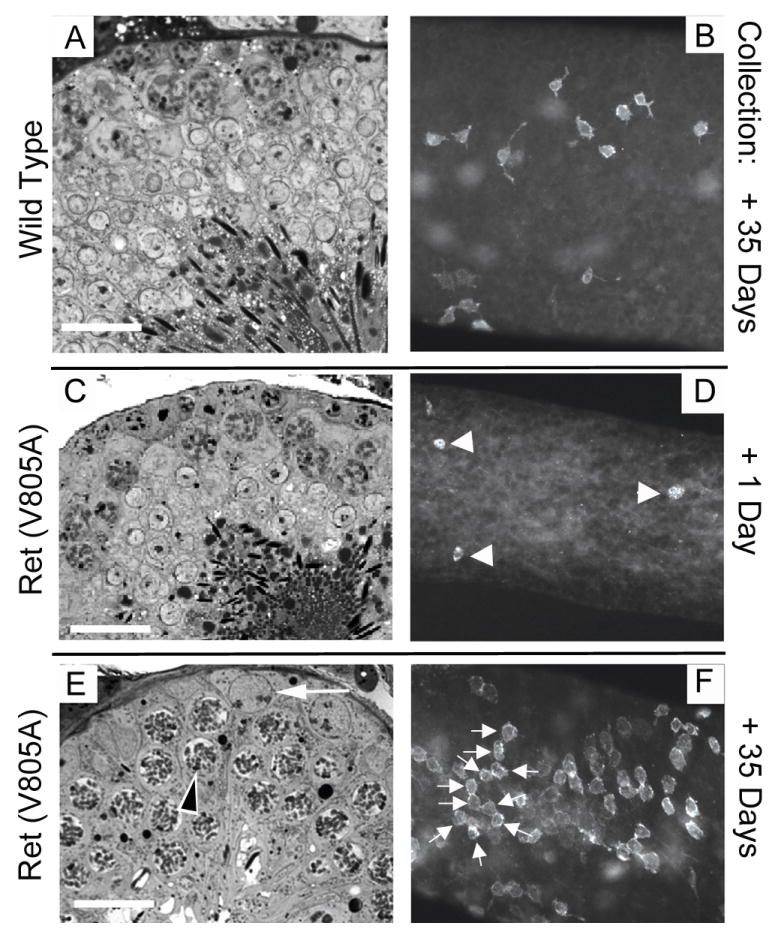
A small percentage of stem spermatogonia persist when GDNF signaling is inhibited for 11 days, and they begin to rebuild the stem cell pool when signaling is restored. Ret (V805A) and wild type mice were treated or 11 days with NA-PP1 and testes were collected for analysis either 1 or 35 days after the last injection (n=4 mice/group). Panels A, C and E are one micron thick cross sections of seminiferous tubules and panels B, D and F are whole mounts of tubules immunostained for Gfrα1. Panels A through C are representative of all tubules in their respective treatment groups. Arrowheads in D point to three of the few weakly stained GFRα1+ As spermatogonia that persisted when GDNF signaling was inhibited for 11 days. Panel E is representative of about 3% of the tubule cross sections of each testis of Ret (V805A) mice that were examined 35 days after the last injection of NA-PP1. The black on white arrowhead points to a spermatocyte and the white arrow points to a spermatogonium. The rest of the tubules in this treatment group contained either no spermatogenic cells or only elongate spermatids (See Supplemental Fig. S9). Panel F shows one of the dense patches of Gfrα1+ Aal spermatogonia, which were dispersed along the tubules of Ret (V805A) mice that were examined 35 days after treatment. In this patch, the arrows point to 10 cells in a chain. Bar = 20 microns.
Some stem spermatogonia are lost after inhibition of GDNF signaling for 2 days
As noted in the Introduction, it has been hypothesized that GDNF promotes self-renewal over differentiation of replicating spermatogonial stem cells. This hypothesis predicts that treatment of Ret (V805A) mice with NA-PP1 for two days, the approximate duration of the one stem cell cycle, would cause significant stem cell loss. Therefore, we injected Ret (V805A) mice for 2 days with NA-PP1 or with vehicle and examined the testes 44 days later (n=4/group). Testes of all control mice were normal, but there was considerable heterogeneity in the histology of tubules of NA-PP1-treated mice (Fig. 6). While 25.6% of the tubules of treated mice were morphologically normal, 9% of the tubules contained Sertoli cells and only a few elongate spermatids, indicating that these tubules lacked stem spermatogonia. The remaining tubules were missing one or two generations of germ cells though almost all contained spermatogonia and/or preleptotene spermatocytes. To assess the numbers of undifferentiated spermatogonia at the end of the experiment, we assayed both the densities of Gfrα1+ spermatogonia and the cross sectional areas of the seminiferous tubules (Supplemental Fig. S10). While the densities of undifferentiated spermatogonia were similar in treated and control mice, the cross sectional areas of tubules of controls were 40% larger than tubules of treated mice. Since the decrease in the cross sectional area of the tubule is proportional to the decrease in its total surface area, the testes of treated mice contained significantly fewer Gfrα1+ spermatogonia than controls.
Figure 6.
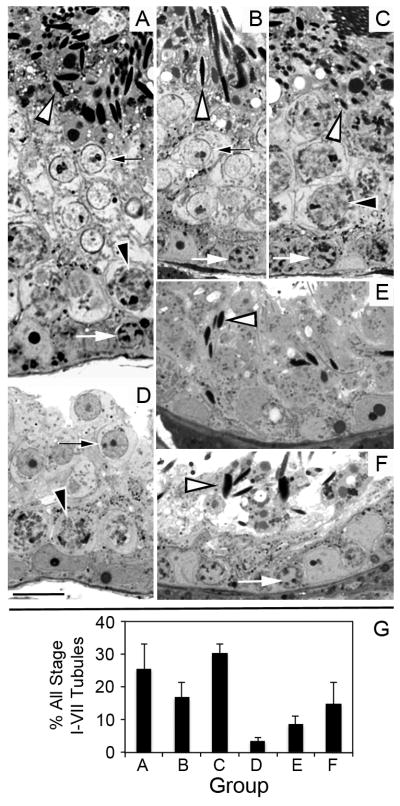
Some stem spermatogonia are lost when GDNF signaling is inhibited for only 2 days, resulting in a diversity of seminiferous tubule morphologies 44 days later. Panel A shows a normal stage VII seminiferous tubule containing all spermatogenic cell types; B shows a stage VII tubule lacking pachytene spermatocytes; C shows a stage VII tubule lacking round spermatids; D shows a stage I-VII tubule lacking elongate spermatids E shows a seminiferous tubule that contains only Sertoli cells and elongate spermatids; F shows a stage VII seminiferous tubule lacking both round spermatids and pachytene spermatocytes. White arrows point to spermatogonia or preleptotene spermatocytes. Black-on-white arrowheads point to pachytene spermatocytes. Black-on-white arrows point to round spermatids. White-on-black arrowheads point to nuclei of elongate spermatids. G. The mean percentage + SEM of stage I-VII seminiferous tubules exhibiting each of these morphologies. Testes from 4 separate animals were analyzed. Images in panels A-F are all at the same magnification and the bar in panel D equals 20 microns.
Discussion
A unique in vivo approach to the study of adult stem cells
This is the first report of the use of a chemical-genetic approach to study the regulation of any adult stem cell by a growth factor. There are three advantages to this experimental strategy that are generally relevant to stem cell biology. The first is that the study starts with a completely normal pool of stem cells. The second is that it allows one to analyze the in vivo response of stem cells to acute changes in signaling from a specific growth factor. The matching of the mutation in the ATP binding site of the kinase subunit of the receptor with the structure of the ATP competitive inhibitor insures that inhibition is both efficient and specific. The other currently available approach to inactivating receptor signaling in the adult, inducible Cre-mediated recombination, requires a substantially longer period of time. Cre recombinase expression or translocation into the nucleus must be followed by excision of the targeted gene, turnover of the encoded transcript and then turnover of the receptor subunit itself. The third advantage of the chemical-genetic approach is that the loss of signaling is reversible. These advantages are evident in our results.
Previous studies have demonstrated that in vivo GDNF is required for formation of the stem spermatogonial pool during pubertal maturation of the testis. Ours is the first to prove that this growth factor is required for the maintenance of these stem cells in the normal adult testis. And in contrast to the report that in vitro stem spermatogonial numbers increased when cultured in the absence of GDNF for 3 or 6 days 9, our data show that in vivo inhibition of GDNF signaling for only two days causes stem cell loss. However, many stem spermatogonia persisted even when GDNF signaling was inhibited for up to 11 days, suggesting that other factors intrinsic or extrinsic to these cells modulate their response to GDNF. We also observed that inhibition of GDNF signaling caused a more rapid loss GFRα1+ cells than of Zbtb16+ cells. Since in a mature testis almost all GFRα1+ cells also express Zbtb16, the sequential loss of GFRα1 expression and then of Zbtb16 expression suggests that GDNF suppresses cell differentiation 29. However, our data do not reveal an increase in the ratio of Apr or Aal spermatogonia to As spermatogonia, as is predicted by the hypothesis that GDNF promotes self-renewal of replicating stem and other As spermatogonia over their differentiation 1. The experimental paradigm described herein should allow one to determine if in a normal mature testis GDNF suppresses differentiation of both replicating and nonreplicating stem and other As spermatogonia or whether its primary in vivo function is to inhibit their apoptosis.
The potential explanation of why there are different sensitivities among subpopulations of stem spermatogonia to inhibition of GDNF signaling
We estimate that upon inhibition of GDNF signaling, most stem spermatogonia are lost in a 4.1-day window that occurs between 2 and 11 days of treatment. However, some stem cells are lost by 2 days and others persist for at least 11 days. While different types of adult stem cells in a tissue have been shown to respond differently to the same growth factor, our data are the first to show significantly different sensitivities of subpopulations of a single stem cell type to loss of growth factor signaling 30. A potential explanation for these different sensitivies comes from our report that in rats, Sertoli cell concentrations of both GDNF mRNA and protein are greatest at stages XII-III (equivalent to stages X-III in the mouse) 31. Therefore, loss of GDNF signaling may have a preferential affect on stem cells in an environment of elevated GDNF concentration. This proposal predicts that inhibition of GDNF signaling would primarily affect stem cells in stage X-II tubules. Since these stages constitute 3.7 days of the 8.6 day cycle of the mouse seminiferous epithelium, 4.9 days would be required for all stem spermatogonia to be affected. However, if as hypothesized, loss of GDNF signaling primarily affects replicating cells, the long duration of the cell cycle of stem and other undifferentiated spermatogonia and the fact that these cells do not replicate synchronously may also contribute to the variation we noted 1. Additionally, a small subpopulation of stem spermatogonia are long cycling cells, and it has been posited that such cells reenter the cell cycle in response to tissue damage 32-34. In our experiments, the immediate damage is the depletion of most of the stem spermatogonia as well as their immediate progeny. We hypothesize that in Ret (V805A) mice these long cycling cells remain in the seminiferous epithelium for at least 11 days of treatment with NA-PP1 and that these cells are lost if they reenter the cell cycle when GDNF signaling is still blocked.
A potential explanation for the diverse morphologies of seminiferous tubules that occur after GDNF signaling is inhibited for 2 days
Our data demonstrate that in vivo even a brief inhibition of GDNF signaling results in loss of some stem spermatogonia, for forty-four days later 8.7% of the tubules are devoid of all but a few elongate spermatids. In addition, loss of stem or other undifferentiated spermatogonia results in the absence of one or two generations of spermatogenic cells from all but 26% of the other seminiferous tubules. We can conservatively estimate when this loss happens because Oakberg and Clermont 26, 27 defined the amount of time required for completion of each phase of spermatogenesis and because we have established that during 11 days of treatment with NA-PP1, 97% of stem spermatogonia are lost from testes of Ret (V805A) mice (see Fig. 5 and Supplemental Fig. S9). Thus, in tubules missing preleptotene spermatocytes, pachytene spermatocytes or round spermatids, stem and other undifferentiated spermatogonia were lost no earlier than 26 days, 17 days or 9.2 days, respectively, after the start of the experiment. This raises the question of why this loss occurred after treatment with NA-PP1 ceased. We propose that such losses result from the fact that undifferentiated spermatogonia move within the basal compartment of a seminiferous tubule 14, 35. When numbers of these cells are depleted some regions of this basal compartment will be empty until they are refilled by an influx of new cells. This refilling will lead to the production of new type A1 spermatogonia and then to more advanced spermatogenic cell-types. At the same time, until the numbers of stem and other undifferentiated spermatogonia are restored by the replication of the remaining cells, this migration will deplete undifferentiated spermatogonia in another part of the tubule. As a consequence, in specific regions of a tubule there will be temporary pauses in the production of differentiated spermatogenic cells, causing the diverse morphologies we observed 44 days after the last injection of NA-PP1. However, the movement of these cells also insures that after a loss of some stem and other undifferentiated spermatogonia the remaining stem cells are able to repopulate empty niches within the basal compartment and thereby rebuild the entire seminiferous epithelium and consequently male fertility.
Summary
We have described a unique chemical-genetic approach to study the consequence of the reversible inhibition of GDNF signaling to stem spermatogonia in a normal adult testis. Using this approach, we have demonstrated that inhibiting GDNF signaling causes a sequential loss by undifferentiated spermatogonia of two stem cell markers, Gfrα1 and Zbtb16, and a logarithmic decrease in testis Ret mRNA levels. These decreases are associated with the loss of functional stem cells. Some stem cells are lost when GDNF signaling is inhibited for only 2 days, while others persisted for up to 11 days. We hypothesize that the stem cells are most sensitive to loss of GDNF signaling when they are present at stages of maximal GDNF concentration. The different sensitivities among subpopulations of stem spermatogonia to loss of GDNF signaling can therefore be partially explained by the fact that 8.6 days are required for a segment of tubule to progress through one entire cycle. However, once GDNF signaling is reestablished, the remaining stem cells both replicate and migrate within the basal compartment of the seminiferous epithelium, thereby refilling empty niches. In conclusion, these data provide the first direct support for the hypothesis that in a normal mature testis GDNF promotes self-renewal over differentiation of replicating stem spermatogonia.
Supplementary Material
Acknowledgments
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54055740 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility research. This research also was supported by the National Institute of Neurological Disorders and Stroke (K08 NS052624) and National Institute of Biomedical Imaging and Bioengineering (RO1 EB001987). We thank Barry Zirkin for critiquing many versions of the manuscript and Kyle Orwig for sharing the method for immunostaining intact seminiferous tubules for Zbtb16. We thank Dirk de Rooij for his estimates of the duration of the cell cycle of stem spermatogonia.
Footnotes
Author contributions: J. Savitt: Developed and characterized the mouse model used for these studies, provided funding, manuscript writing D. Singh and L-c Chen: Collection of data and data analysis and interpretation; C. Zhang: Provision of study material, manuscript writing J. Folmer : Collection of data; K.M. Shokat: Conception and design, financial support; manuscript writing; W. Wright; Conception and design, collection of data, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 2.Kubota H, Avarbock M, Brinster R. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monesi V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J Cell Biol. 1962;14:1–18. doi: 10.1083/jcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu J, Shima H, Nakagawa H. Glial cell line-derived neurotropic factor stimulates sertoli cell proliferation in the early postnatal period of rat testis development. Endocrinology. 1999;140:3416–3421. doi: 10.1210/endo.140.8.6922. [DOI] [PubMed] [Google Scholar]
- 5.Huckins C. The spermatogonial stem cell population in adult rats II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet. 1971;4:313–334. doi: 10.1111/j.1365-2184.1971.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 6.Fabrikant JI. Cell cycle of spermatogonia in mouse testis. Invest Radiol. 1979;14:189–191. doi: 10.1097/00004424-197903000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Lok D, de Rooij DG. Spermatogonial multiplication in the Chinese hamster. III. Labelling indices of undifferentiated spermatogonia throughout the cycle of the seminiferous epithelium. Cell Tissue Kinet. 1983;16 [PubMed] [Google Scholar]
- 8.Lok D, Jansen MT, de Rooij DG. Spermatogonial multiplication in the Chinese hamster. II. Cell cycle properties of undifferentiated spermatogonia. Cell Tissue Kinet. 1983;16:19–29. [PubMed] [Google Scholar]
- 9.Ebata KT, Yeh JR, Zhang X, et al. Soluble growth factors stimulate spermatogonial stem cell divisions that maintain a stem cell pool and produce progenitors in vitro. Experimental cell research. 2011;317:1319–1329. doi: 10.1016/j.yexcr.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Luby-Phelps K, Bugde A, et al. Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells. J Cell Biol. 2009;187:513–524. doi: 10.1083/jcb.200907047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 12.Creemers LB, Meng X, den Ouden K, et al. Transplantation of Germ Cells from Glial Cell Line-Derived Neurotrophic Factor-Overexpressing Mice to Host Testes Depleted of Endogenous Spermatogenesis by Fractionated Irradiation. Biol Reprod. 2002;66:1579–1584. doi: 10.1095/biolreprod66.6.1579. [DOI] [PubMed] [Google Scholar]
- 13.Naughton CK, Jain S, Strickland AM, et al. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Sukeno M, Nakagawa T, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Lilley BN, Zhang C, et al. A chemical-genetic strategy reveals distinct temporal requirements for SAD-1 kinase in neuronal polarization and synapse formation. Neural Dev. 2008;3:23. doi: 10.1186/1749-8104-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Ye H, Kuruvilla R, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Salomon D, Bonshtien A, Sessa G. A chemical-genetic approach for functional analysis of plant protein kinases. Plant Signal Behav. 2009;4:645–647. doi: 10.4161/psb.4.7.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- 19.Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 20.Bishop AC, Shokat KM. Acquisition of inhibitor-sensitive protein kinases through protein design. Pharmacol Ther. 1999;82:337–346. doi: 10.1016/s0163-7258(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 21.Rossi J, Luukko K, Poteryaev D, et al. Retarded Growth and Deficits in the Enteric and Parasympathetic Nervous System in Mice Lacking GFRa2, a Functional Neurturin Receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishino J, Mochida K, Ohfuji Y, et al. GFRa3, a Component of the Artemin Receptor, Is Required for Migration and Survival of the Superior Cervical Ganglion. Neuron. 1999;23:725–736. doi: 10.1016/s0896-6273(01)80031-3. [DOI] [PubMed] [Google Scholar]
- 23.Lindfors PH, Lindahl M, Rossi J, et al. Ablation of Persephin Receptor Glial Cell Line-Derived Neurotrophic Factor Family Receptor 4 Impairs Thyroid Calcitonin Production in Young Mice. Endocrinology. 2006;147:2237–2244. doi: 10.1210/en.2005-1620. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 25.Luo W, Wickramasinghe SR, Savitt JM, et al. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;7:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 27.Clermont Y, Trott M. Duration of the cycle of the seminiferous epithelium in the mouse and hamster determined by means of 3H-thymidine and radioautography. Fertil Steril. 1969;20:805–817. doi: 10.1016/s0015-0282(16)37153-9. [DOI] [PubMed] [Google Scholar]
- 28.Schuchardt A, D’Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 29.Grisanti L, Falciatori I, Grasso M, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- 30.Challen GA, Boles NC, Chambers SM, et al. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston DS, Wright WW, Dicandeloro P, et al. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc Natl Acad Sci U S A. 2008;105:8315–8320. doi: 10.1073/pnas.0709854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huckins C. The spermatogonial stem cell population in adjult rats. Evidence for a long-cycling population. Cell Tissue Kinet. 1971;4:335–349. doi: 10.1111/j.1365-2184.1971.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 33.Tokuda M, Kadokawa Y, Kurahashi H, et al. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T, Sharma M, Nabeshima YI, et al. Functional Hierarchy and Reversibility Within the Murine Spermatogenic Stem Cell Compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.