Abstract
Background
Probiotic treatment strategy based on the hygiene hypothesis, such as administration of ova from the non-pathogenic helminth, Trichuris suis, (TSO) has proven safe and effective in autoimmune inflammatory bowel disease.
Objective
To study the safety and effects of TSO in a second autoimmune disease, multiple sclerosis (MS), we conducted the phase 1 Helminth-induced Immunomodulatory Therapy (HINT 1) study.
Methods
Five subjects with newly diagnosed, treatment-naive relapsing–remitting multiple sclerosis (RRMS) were given 2500 TSO orally every 2 weeks for 3 months in a baseline versus treatment control exploratory trial.
Results
The mean number of new gadolinium-enhancing magnetic resonance imaging (MRI) lesions (n-Gd+) fell from 6.6 at baseline to 2.0 at the end of TSO administration, and 2 months after TSO was discontinued, the mean number of n-Gd+ rose to 5.8. No significant adverse effects were observed. In preliminary immunological investigations, increases in the serum level of the cytokines IL-4 and IL-10 were noted in four of the five subjects.
Conclusion
TSO was well tolerated in the first human study of this novel probiotic in RRMS, and favorable trends were observed in exploratory MRI and immunological assessments. Further investigations will be required to fully explore the safety, effects, and mechanism of action of this immunomodulatory treatment.
Keywords: cytokines, hygiene hypothesis, immunomodulation, multiple sclerosis, probiotic, Trichuris, whipworm
Introduction
The hygiene hypothesis is one of several explanations which have been advanced to account for the recent, marked increase of allergy and autoimmunity in developed countries.1–3 According to this hypothesis, a lack of evolutionarily normal infectious exposures in early life in areas with high levels of sanitation contributes to abnormal immune regulation in susceptible individuals. In this regard, Leibowitz and colleagues4 were the first to demonstrate that multiple sclerosis (MS) was more prevalent in areas of high sanitation, and subsequent epidemiological surveys have confirmed this association.5
Treatment paradigms based on the hygiene hypothesis include probiotic strategies, that is, the administration of non-pathogenic live microorganisms which, when given in adequate amounts, confer a health benefit on the host (World Health Organization definition).6 In this regard, many helminths are evolutionarily well adapted to their hosts and are known to reduce host inflammatory responses;7 in fact, every mammal living in a natural environment,8 including hominoids and, until recently, all humans, has been colonized with helminths.7
Experimentally, helminths have been successfully applied in animal models of allergic and autoimmune diseases, including colitis, arthritis, type 1 diabetes, and experimental autoimmune encephalomyelitis.2,9,10 In addition, beneficial immunomodulation by helminths in humans has been demonstrated in an observational study of relapsing–remitting multiple sclerosis (RRMS) patients with asymptomatic, community-acquired gastrointestinal infections.11,12 Safety and benefit have also been demonstrated in controlled clinical trials of Trichuris suis (porcine whipworm) ova (TSO) in inflammatory bowel disease (IBD), including ulcerative colitis13 and Crohn’s disease,14 although a clinical trial of TSO in allergic rhinitis did not demonstrate a change in symptom scores.15 Recently, a comprehensive cellular and molecular analysis of changes in the intestinal mucosa of a patient with IBD before and after colonization by the human whipworm, T. trichiura, demonstrated that active colitis was associated with T helper cells producing IL-17 and expression of proinflammatory genes such as IL-17 and IL-13RA2; during parasitic colonization, colitis remitted, and a decrease in IL-17-producing cells, a dramatic increase in IL-22-producing cells, and relative reduction in proinflammatory gene expression were observed.16
After ingestion of T. suis ova in the human (non-natural) host, immature larvae hatch in the proximal small bowel and migrate through the gastrointestinal lumen to the mucosa of the colon, where transient colonization is established for several weeks, followed by immune-mediated elimination.17,18 From these studies it has been demonstrated that T. suis is not a human pathogen; does not multiply within the host; has no systemic spread or invasion beyond the lumen and superficial mucosa of the colon; is not infectious for household contacts, since any excreted ova are not embryonated; does not entail the risk of transmitting human pathogens from human source materials; and may be produced in specific pathogen-free animals and purified according to good manufacturing standards (GMP) for investigational pharmaceuticals and biologics.19
Given the demonstrated benefit of helminth TSO administration in IBD, we sought to study the safety and promise of TSO in a second autoimmune disease, MS. We found that new active magnetic resonance imaging (MRI) lesions were decreased after TSO administration and that most of the subjects developed an anti-inflammatory response that was associated with increases in the serum levels of IL-4 and IL-10 cytokines. Together, our findings reveal a novel immunomodulatory approach for RRMS that warrants further evaluation.
Subjects and methods
Study design
During review of the proposed study design of phase 1 Helminth-induced Immunomodulatory Therapy (HINT 1) by the National Multiple Sclerosis Society Committee on Clinical Trials, it was suggested, in view of the novel live organism treatment and the unknown response of MS subjects to TSO, that the first investigation should be a brief pilot study designed primarily to evaluate safety and tolerability of this agent in a small group of RRMS subjects.
A baseline versus treatment design was adopted, consistent with established guidelines for pilot studies of new agents in MS in which MRI outcomes are used to determine initial safety and promise;20 statistically, a baseline versus treatment design is most efficient in exploratory studies, since each subject serves as his or her own control during the experimental intervention.21 The investigation was approved (# H-2007-0390) by the University of Wisconsin Health Sciences Institutional Review Board; in addition, an independent data and safety board (DSMB) consisting of faculty from neurology, infectious diseases, gastroenterology, immunology, and neuroradiology not otherwise involved with the study was established. The HINT 1 trial was registered at ClinicalTrials.gov (# NCT00645749), and the study was assigned Investigational New Drug number BB-IND 1385 by the US Food and Drug Administration (FDA).
Subjects
Participants consisted of five subjects who met the 2005 McDonald Committee criteria22 for RRMS and who declined standard disease-modifying treatment. All subjects were newly diagnosed, were treatment naïve, and had at least one active (gadolinium-enhancing) brain MRI lesion at study entry (Table 1). Subjects underwent 1 month of pre-treatment screening, 3 months of TSO treatment consisting of 2500 ova orally every 2 weeks, and 2 months of post-treatment observation. The dosing schedule for TSO was based on prior clinical trials,13–15,18 which in turn were based on experimental studies of T. suis administration in animals23,24 and humans.17 For each subject, study day 1 was designated as the date of first TSO dose administration. Healthy normal blood donor control subjects were recruited from relatives and friends of clinic patients, matching MS subjects as closely as possible demographically. Written informed consent was obtained from all subjects in the study.
Table 1.
Demographics of HINT 1 subjectsa at study entry
| Subject Number | Age (years) | Sex | Symptom duration//RRMS diagnosis (years)b | Relapses in last year | CSFc | EDSS | Prior corticosteroids | Enhancing MRI lesionsd |
|---|---|---|---|---|---|---|---|---|
| 001 | 37 | M | 4.2 / 0.5 | 1 (spinal) | L+ | 2.5 | None | 2 |
| 002 | 26 | F | 3.5 / 1.5 | 1 (brainstem) | NT | 0 | None | 15 |
| 003 | 42 | F | 3.5 / 1.0 | 1 (spinal) | H+ | 0 | None | 11 |
| 004 | 31 | F | 0.5 / 0.25 | 1 (optic nerve) | L+ | 0 | 6 months prior | 4 |
| 005 | 24 | F | 0.6 / 0.25 | 2 (spinal, spinal) | L+ | 3.0 | 3 months prior | 1 |
All subjects met the 2005 McDonald Committee Criteria for RRMS.19 Subjects 001, 002, 003, and 005 had two or more clinical attacks; subject 004 only had a single attack (clinically isolated syndrome) but was judged to have relapsing–remitting multiple sclerosis (RRMS) on the basis of serial magnetic resonance imaging (MRI) manifestations strictly meeting the McDonald criteria for dissemination in time and space. All clinical and MRI findings were typical of multiple sclerosis in all subjects.
Time in years from a) first probable relevant historical symptom and b) explicit diagnosis of RRMS by a neurologist after full workup.
Cerebrospinal fluid (CSF) results were either positive (CSF-specific oligoclonal bands and/or increased IgG index) in our laboratory (L+), were positive by remote outside historical report which could not be verified (H+), or were not tested (NT).
Brain MRI performed with 3-T GE scanner and with double-dose gadolinium contrast (0.2 mmol/kg body weight).
TSO production and testing
TSO was produced at GMP quality control standards, which assured purity and sterility at pharmaceutical grade, and purchased from Ovamed, Hamburg, Germany. The 2500 microscopic ova are visible only as a slight haze when suspended in 15 ml of sterile of phosphate buffered saline (PBS), pH3.0; subjects reported that the solution had a salty, not unpleasant taste, similar to a mildly acidic sports drink. After TSO was received from Ovamed, additional testing for microbial contamination was conducted by the Waisman Clinical Biomanufacturing Facility (WCBF) at the University of Wisconsin; assays performed included those for endotoxin by kinetic turbidimetric assay (US Pharmacopeia standard <USP 85>), bacterial microbial limits (USP <61>), hepatitis E by PCR, and porcine adventitious agents by cytopathology, immunofluorescence, and hemadsorption determinations (per Code of Federal Regulations, 9 CRF 113 standard). After the conclusion of the clinical trial, assay of a vial from the same lot of TSO demonstrated 2505 ± 84 total ova, with an egg embryonation rate of 86.4 ± 4.7% and a larval viability of 85.7% (assays kindly performed by Dr David Elliott, University of Iowa, Iowa City, IA, USA).
Clinical and safety assessments
The clinical status of each subject was determined monthly by masked neurological and general medical examinations, the Multiple Sclerosis Functional Composite (MSFC),25 and the Expanded Disability Status Scale (EDSS).26 Adverse effects were assessed, as recommended by the FDA, by the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers.27 Routine safety laboratory determinations, including complete blood count; serum creatinine, liver function, and pregnancy tests, as well as stool ova and parasites, were performed monthly by the clinical laboratory service at the University of Wisconsin Hospitals and Clinics.
MRI assessments
Brain MRIs were performed with and without intravenous contrast (double-dose gadolinium, 0.2mmol/kg body weight) on a 3-Tesla scanner (GE Healthcare, Waukesha, WI. USA). New gadolinium-enhancing lesions (n-Gd+) were scored by the attending neuroradiologist (ASF), who was masked to subject clinical and treatment status.
Immunological assessments
Eosinophil counts and high-sensitivity C-reactive protein (hs-CRP) were performed monthly by the clinical laboratory service at the University of Wisconsin Hospitals and Clinics.
Antibodies specific for T. suis excretory secretory products (ESP) (a generous gift from Drs. Joseph Urban and Dolores Hill USDA, Bethesda MD, USA) were determined by ELISA as previously described,28 with the assay modified by substitution of secondary antibodies for anti-human IgG1 or IgA (BD Biosciences, San Jose, CA, USA) or anti-human IgE, (Mabtech, Mariemont, OH, USA). Serum cytokine levels were determined by an array-based multiplex sandwich ELISA (Quantibody Human Th1/Th2 array, RayBiotech, Norcross, GA, USA) following the instructions of the manufacturer. In order to enumerate cells with phenotypes corresponding to alternatively activated macrophages (AAM) or T regulatory (Treg) cells, peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by Ficoll density gradient centrifugation; subsequently cells were stained and flow cytometry performed as described previously.29 In the case of AAM, cells were stained with CD14-FITC (monocyte-macrophage, eBioscience, San Diego, CA, USA), CD23-APC (low-affinity FcεRII, eBioscience, San Diego, CA, USA), and CD124-PE (IL-4R, BD Biosciences, San Jose, CA, USA). Staining was performed at 4°C for 30 min, followed by washes with 0.1% BSA in PBS. For Treg cell enumeration, cells were stained with anti-human CD4-FITC and anti-human CD25-APC (BD Biosciences, San Jose, CA, USA), fixed and permeabilized, and subsequently stained with anti-human Foxp3-PE (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. The stained cells were acquired on a FACS-Calibur cytometer (BD Biosciences, San Jose, CA, USA) and analyzed by using FlowJo analysis software (Tree Star, Ashland, OR, USA).
Due to the small number of subjects, the brief period of treatment, and the exploratory nature of the first-use safety study, formal statistical analyses were not considered appropriate, and data are presented for each subject.
Results
Clinical and safety assessments
All testing at Ovamed and WCBF showed no microbiological contamination of TSO. No subject had a relapse or significant change in baseline versus treatment neurological functioning as judged by the EDSS or MSFC performed by masked examiners (data not shown). Three subjects experienced the onset of mild gastrointestinal symptoms (FDA scale27 grade 1, no interference with activities of daily living such as school or work, 2–3 loose stools per day) at about 30 days after the first dose of TSO (study day 1). Spontaneous resolution of these minor symptoms occurred after 6, 1, and 4 days for subjects 002, 003, and 004, respectively, similar to the transient, low-level gastrointestinal symptoms noted in a prior clinical trial of TSO in allergic rhinitis.15 We suspect that the transition from minor symptoms to asymptomatic status in these subjects corresponded to the onset of strong adaptive immune responses to T. suis. No abnormality was detected in standard laboratory tests of hematological, renal, and liver function.
No subject became pregnant. All ova and parasite stool determinations were negative, except for a single sample in subject 003, which showed scant ova at study day 151. On follow-up at study day 169, this subject’s stool ova and parasite sample was negative. It should be noted that even in the rare event of egg passage by a subject, ova are not infectious for contacts30 since ova are likely dead pass-through eggs; even in the event that new, viable eggs were to be passed in stool, prolonged incubation after excretion is required for ova to become embryonated (i.e. infectious), and modern sanitation blocks this step in the whipworm life cycle.31 All data were reviewed by the independent DSMB, and no safety concerns were identified, although more intensive analysis of stool samples in future studies was recommended.
MRI assessments
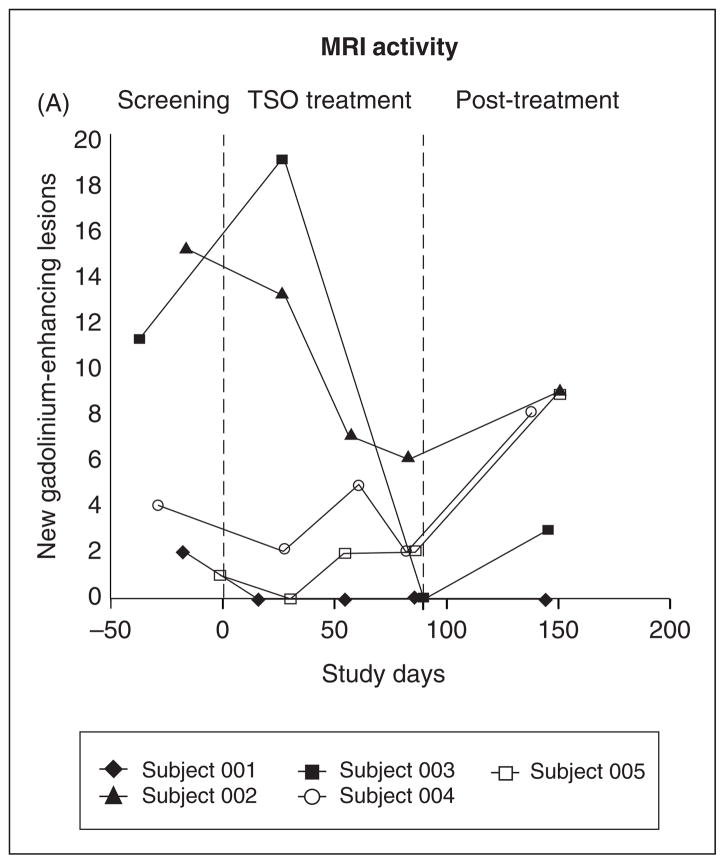
The mean number of new (unique to each MRI) gadolinium- enhancing lesions on masked reading of double-dose gadolinium studies was 6.6 at baseline screening, 2.0 at the end of 3 months of TSO treatment, and 5.8 at 2 months after treatment was stopped (Figure 1). No subject had a change in neurological symptoms during the fluctuations of MRI activity shown in Figure 1.
Figure 1.
MRI Results. Masked reading of the number of new (unique to each scan) enhancing lesions after double-dose gadolinium (0.2 mmol/kg body weight) administration on a 3-Tesla MRI brain scan by study day, with study day 1 being the first day of Trichuris suis ova (TSO) administration. Shown in the figure are the screening period (1 month), TSO treatment period (3 months), and the post-treatment observation period (2 months). Subjects are designated by the symbols shown in the insert box.
Immunological assessments
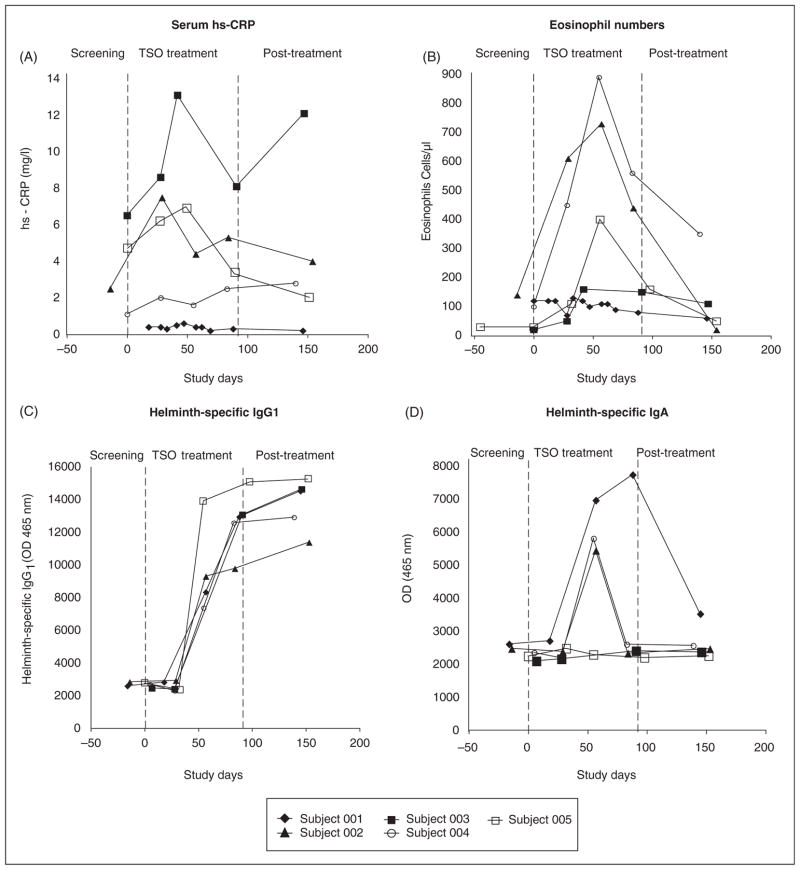
Serum hs-CRP and blood eosinophils initially rose during the first two months of TSO administration and fell thereafter in most subjects during and after the last month of ova exposure (Figure 2A and 2B), as might be expected for an acute-phase reactant and an indicator of Th2 responses, respectively. It is possible that these trends reflect an early inflammatory response during the initiation of gastrointestinal infection, followed by a later, anti-inflammatory response induced by persistent, low-level T. suis colonization and repeated oral dosing of TSO.
Figure 2.
Systemic acute-phase reactants. (A) Serum high-sensitivity C-reactive protein (hs-CRP) levels in mg/l. (B) Blood eosinophil levels in cells/μl. (C) Specific IgG1 for Trichuris suis in optical density units (OD 465nm). (D) Specific IgA for T. suis in optical density units (OD 465nm).
Increasing levels of IgG1 antibodies specific for T. suis ESP were detected in all subjects during HINT therapy (Figure 2C); the IgG1 antibodies peaked at the end of TSO administration and remained elevated thereafter. IgA antibodies specific for T. suis ESP peaked at day 60 in subjects 002 and 004 and at day 100 in subject 001. In contrast to IgG1, IgA dropped to baseline values after TSO treatment was terminated (Figure 2D). These antibody responses to ESP demonstrate the presence of the larval form (not merely ova) of T. suis in our subjects and show the ability of this organism to elicit strong antibody responses in a non-natural host. T. suis-specific IgE was not detected in any subject (data not shown), possibly due to the short period of TSO administration or weak IgE-inducing ability of the helminth.
These results indicate that although whipworm colonization is limited to the gastrointestinal lumen and superficial mucosa,19 a vigorous systemic immune response was nevertheless elicited in all HINT 1 subjects. Similar results were found in a recent clinical trial of TSO treatment of allergic rhinitis.15
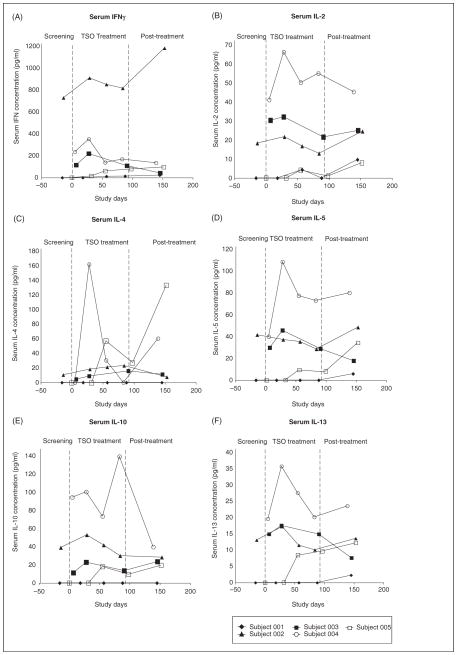
Serum cytokine levels for each subject are shown in Figure 3. Although there was individual variation in cytokine responses, in general TSO was associated with a relative increase of serum IL-4 (Figure 3C; subjects 002, 003, 004, 005), IL-5 (Figure 3D; subjects 004, 005), Il-10 (Figure 3E; subjects 002, 003, 004, 005), and IL-13 (Figure 3F; subjects 004, 005). No significant decrease in INFγ or IL-2 was found (Figure 3A and 3B). Subject 001, the only male in the study and the patient with the longest history of relevant symptoms, in general had less evidence of immunological or MRI activity than did the other subjects. These exploratory, serum-based findings are limited by the small number of subjects and because cellular responses to specific antigens, such as those of myelin, were not measured. Nevertheless, taken together, they suggest a shift to an anti-inflammatory environment and indicate the need for further investigations to test this hypothesis.
Figure 3.
Serum cytokine determinations. The cytokines shown were measured by ELISA and reported in pg/ml.
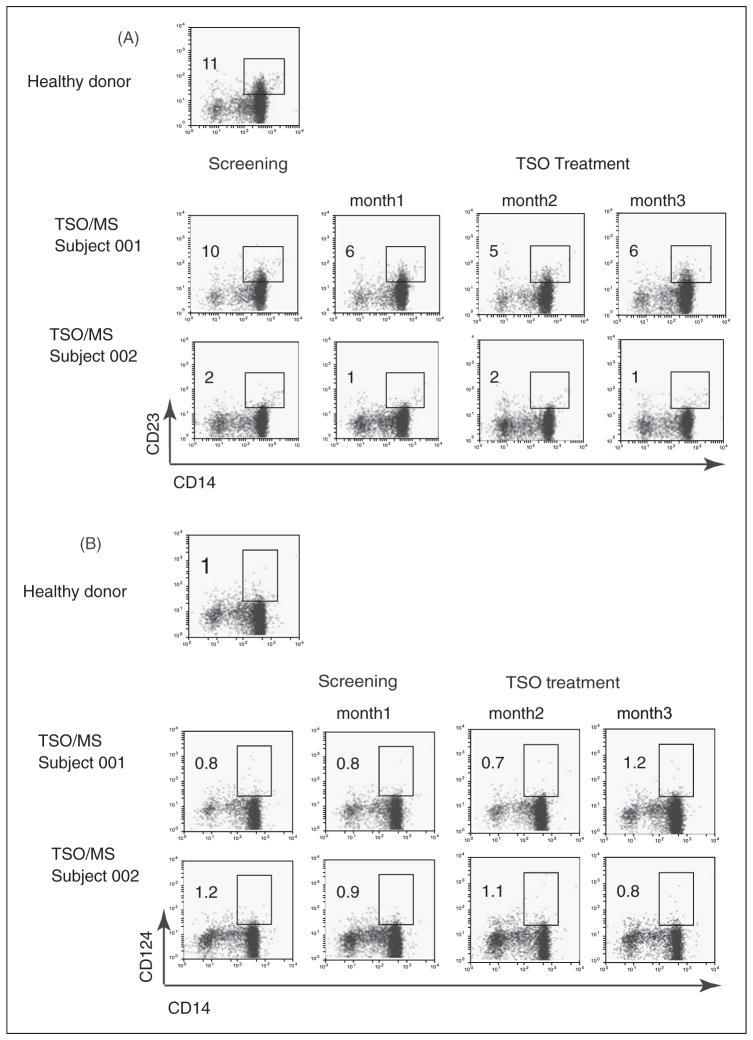
Since helminth infections or Th2-type polarized immune responses often generate high numbers of AAM, we next tested the frequency of circulating monocytes expressing typical cell surface AAM markers in PBMC of our subjects.32 We measured the relative number of CD23+ (low-affinity FcεRII) and CD124+ (IL-4R) in the CD14+ monocyte population by flow cytometry. Two representative cytofluorimetry dot plot panels (subjects 001, 002) are shown in Figure 4. Subject 001 showed an equivalent percentage of CD14+CD23+ monocytes when compared with a healthy normal donor subject (10% and 11%, respectively), and subject 002 showed lower frequency (2%). With regard to CD124 expression, only 1% of CD14+ cells were positive in all individuals, and no changes could be detected during TSO treatment. These results suggest that any AAM-inducing soluble factors at the site of T. suis infection, if present, had no effect on the phenotype of circulating monocytes.
Figure 4.
Frequency of alternatively activated monocytes (AAM). After isolation of peripheral blood mononuclear cells by Ficoll density gradient and staining for CD14+ (monocytes), cells were stained with markers characteristic of AAM (Panel (A) CD23+; Panel (B) CD 124+). Representative results are shown for a control healthy donor subject and two of the study subjects, 001 and 002. Data are shown for timepoints at screening and after 1, 2, or 3 months of TSO treatment, and the frequency of doubly stained, AAM phenotypic cells is indicated by the insert box and the percent enumerations shown. No significant increases in AAM frequency were noted during TSO treatment.
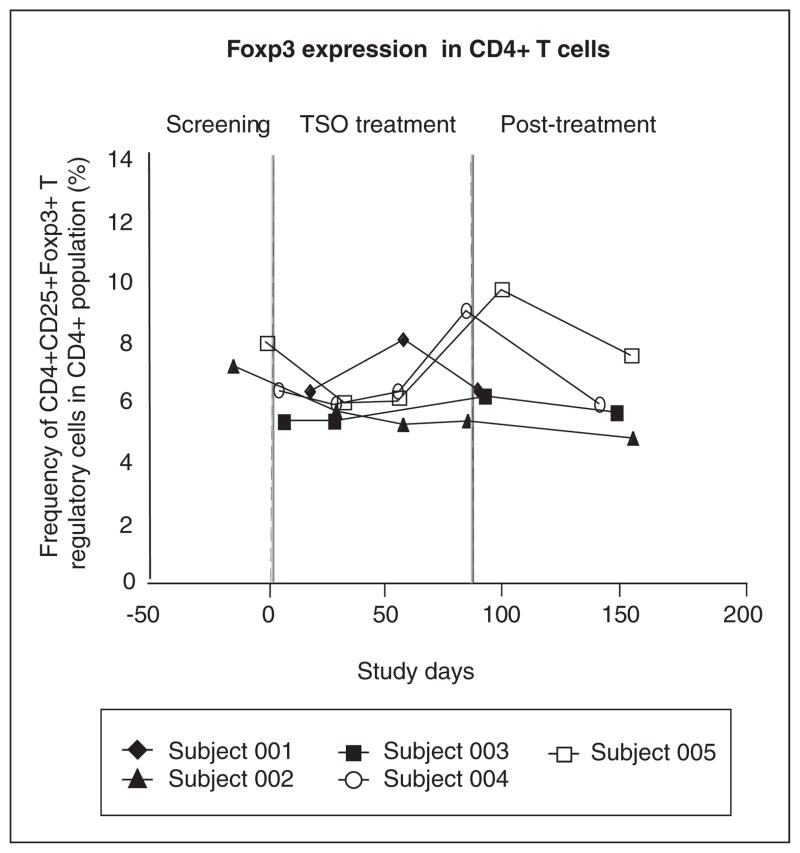
We next studied the frequency of Treg cells (CD4+CD25+Foxp3+), which have been observed following helminth infections and may suppress inflammatory responses.33 As shown in Figure 5, there was a modest increase in CD4+CD25+Foxp3+ cells in subjects 004 and 005, but no change in the other subjects. These findings indicate that if CD4+CD25+Foxp3+ cells are generated in the gut34 during T. suis infection, they do not enter the systemic circulation in significant numbers. Further studies will be required to determine if the suppressive function35,36 of circulating CD4+CD25+Foxp3+ cells is altered after TSO administration in MS subjects.
Figure 5.
Frequency of CD4+CD25+FoxP3+T regulatory cells. After isolation of peripheral blood mononuclear cells by Ficoll density gradient, cells were stained for CD4 and CD25, fixed and permeabilized, and then stained for Foxp3 expression. The frequency of FoxP3+ cells as a percentage of total CD4+ cells for all subjects during the study is shown.
Discussion
HINT 1 is the first prospective clinical trial designed to assess the effects of a hygiene hypothesis-based GMP probiotic treatment for MS. Although safety and efficacy of TSO had been demonstrated in clinical trials of IBD,13,14,19,37 an exploratory study of TSO was deemed necessary in MS because of the possibility of adverse outcomes such as gastrointestinal complications 38,39 or a paradoxical or anti-therapeutic effect on MS activity.
HINT 1 demonstrated that TSO was well tolerated by patients with RRMS. The minor gastrointestinal symptoms (FDA grade 1) observed in three of the five subjects at TSO initiation were transient and did not interfere with activities of daily living, such as work or school; these findings were similar to low-level symptoms noted in a previous TSO trial in allergic rhinitis.15 Results of MRI activity, as judged by the change in number of n-Gd+ on double-dose gadolinium scans during TSO exposure, were favorable (Figure 1), although longer studies with larger numbers of subjects will be required in order to determine whether these findings represent an early therapeutic response, regression to the mean, or coincidence. Notably, however, the increase or apparent rebound in activity in four of five subjects 2 months after TSO was discontinued suggests that helminth exposure did influence MRI measures.
The changes in acute-phase reactants and antibodies shown in Figure 2 indicate a vigorous systemic response to the transient colonization of the superficial intestinal mucosa by T. suis. Although our investigations showed an increase in serum IL-4 and IL-10 levels in most subjects during HINT 1, the mechanism of putative immunomodulation by T. suis is unknown and will require further investigation.
The MRI and immunological changes after helminth exposure in the HINT 1 trial were less dramatic than those noted in the previous double-cohort prospective observational study of Correale and Farez,11 although there were significant methodological differences between the two studies. In the observational study, serial MRI studies were performed every 6 months for an average follow-up of 4.5 years; during this period of observation, only four n-Gd+ MRI lesions were found in 12 MS patients with asymptomatic gastrointestinal helminth infections, in comparison with 78 n-Gd+ MRI lesions which were seen in 12 uninfected, demographically matched MS control subjects, a 95% relative reduction in MRI activity.11 It should be noted, however, that the first time-point in this investigation was 6 months after the onset of eosinophilia, whereas the HINT 1 safety study examined the earliest phases of helminth exposure, i.e. 3 months after first TSO administration and just 2 months after eosinophilia appeared. Since the magnitude of MRI effects by agents such as copolymer may take 3–6 months before becoming fully apparent,40,41 it is possible that the observations in the pilot HINT 1 study were of insufficient duration to adequately test the eventual favorable or unfavorable effects of TSO on MRI measures.
In experimental and clinical autoimmune conditions, helminths have been shown to modulate dysregulated and pathogenic immune responses through multiple mechanisms.11,16,33,42–47 Helminth infection also may be associated with laboratory evidence of decreased immune activity48 and attenuated responses to vaccination. 49 However, clinical immunosuppression sufficient to result in morbidity and mortality of the host would not be expected during prolonged co-evolution with a parasite.46,50 In fact, overt immunosuppression by helminths rarely occurs unless one or both of the following are present: (1) concomitant immunosuppressive therapy, such as that employed for transplantation or rheumatological disorders;51,52 and (2) an agent, such as Stronglyloides stercoralis, which is able to complete a full lifecycle within the host and therefore has the potential for autoinfection and hyperinfection.53 Empirically, in an observational study of RRMS patients with asymptomatic, natural gastrointestinal parasitism, immunosuppression was not evident during a mean follow-up of 4.5 years.11 With specific regard to whipworms, it has been found that these organisms generally have low pathogenicity in adult humans,31 and clinical trials with the porcine whipworm (TSO) have not demonstrated clinical immunosuppression. 13–15,18 During the HINT 1 study there was no increase in the expected number or severity of infections, and the principal arms of the immune system appeared to be intact, as judged by total leukocyte counts, total CD4+ lymphocyte numbers (data not shown), humoral responses (Figure 2), cytokine production (Figure 3), and macrophage phenotype (Figure 4).
Maizels et al. have suggested that the long co-evolution of helminths with humans has made these organisms master regulators of host immune responses.54 At present, the most practical means of exploring the potential of helminth-induced immunomodulation has been observational and clinical trials with live organisms;11,13–15,55 however, if these investigations indicate therapeutic promise and reveal mechanisms of action, it is realistic to hope that eventually specific helminth-associated immunomodulatory molecules may be identified, purified, and studied as potential pharmaceuticals.
The principal finding of this pilot study was that during 3 months of probiotic TSO administration, RRMS subjects did not experience any early, major toxicity or paradoxical increase in clinical or MRI measures of MS activity. Favorable trends in MRI results and some immunological determinations were noted in this first-use exploratory trial, and indicate the potential promise of the novel GMP immunomodulatory agent. However, only follow-up studies of longer duration and with larger numbers of subjects will adequately determine safety and efficacy of TSO and similar biologics in RRMS. We strongly discourage administration of this or other helminth preparations outside of controlled clinical trials which have appropriate regulatory oversight and continuous monitoring for possible adverse effects. In addition to the possible application of this new class of immunomodulatory agents, as well as molecules derived from them,44 to the treatment of IBD, MS, and other disorders, research in this field may address the fundamental immunological issue of the relevance of the hygiene hypothesis to the etiopathogenesis of allergic and autoimmune conditions in general.
Acknowledgments
The authors gratefully acknowledge the assistance of Andrea Maser and Benjamin Savage for clinical coordination of the study, Stacey Balousek for regulatory review, Jennifer Broihahn for the illustrations, and Dr Simon Oh for neurological examinations. The investigators assume complete responsibility for the design and conduct of the trial, but thank Drs. David Elliott and Robert Summers at the University of Iowa, Dr Joel Weinstock at Tufts University, as well as Drs. Jeffrey Cohen, Gary Cutter, Fred Lublin, John Noseworthy, Hillel Panitch, and Steven Schwid of the National Multiple Sclerosis Society Committee on Clinical Trials for helpful discussions and suggestions. Drs. Joseph Urban and Dolores Hill generously provided T. suis ESP antigen for the ELISA antibody test. Dr Derek Hei, Lisa Byrne, and colleagues at WCBF provided valuable direction and assistance with post-production testing of TSO preparations to exclude microbial contamination. Dr David Elliott kindly performed assays for ova counts, egg embryonation, and larval viability at the University of Iowa. Helpful guidance was provided by Drs. Paul Rutecki (Neurology, Chair), Lindel Gentry (Neuroradiology), Michael Lucey (Gastroenterology), Daniel Muller (Immunology), Andrew Urban (Infectious Diseases), and Patricia O’Looney (National Multiple Sclerosis Society) of the DSMB.
Funding
This study was supported by National Multiple Sclerosis Society (research grant RG 3613A4/1 to JF) and NIH (grant number 1R56AI091462-01 to ZF).
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Conflict of interest statement
The authors declare that they have no conflicts of interest.
References
- 1.Bach JF. The effect of infections on susceptibility to auto-immune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard TM. Western Diseases: An Evolutionary Perspective. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 4.Leibowitz U, Antonovsky A, Medalie JM, Smith HA, Halpern L, Alter M. Epidemiological study of multiple sclerosis in Israel. II. Multiple sclerosis and level of sanitation. J Neurol Neurosurg Psychiatry. 1966;29:60–68. doi: 10.1136/jnnp.29.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
- 6.Johannsen H, Prescott SL. Practical prebiotics, probiotics and synbiotics for allergists: how useful are they? Clin Exp Allergy. 2009;39:1801–1814. doi: 10.1111/j.1365-2222.2009.03368.x. [DOI] [PubMed] [Google Scholar]
- 7.Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160:70–79. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstock J, Elliott DE. Inflammatory bowel disease and the hygiene hypothesis: an argument for the role of helminths. In: Rook GAW, editor. The Hygiene Hypothesis and Darwinian Medicine. Basel: Birkhauser; 2004. [Google Scholar]
- 9.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 11.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 12.Fleming J, Fabry Z. The hygiene hypothesis and multiple sclerosis. Ann Neurol. 2007;61:85–89. doi: 10.1002/ana.21092. [DOI] [PubMed] [Google Scholar]
- 13.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. e1–e3. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med. 2010;2:60ra88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 17.Beer RJ. Experimental infection of man with pig whipworm. Br Med J. 1971;2:44. doi: 10.1136/bmj.2.5752.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers RW, Elliott DE, Qadir K, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 19.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–464. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Miller DH, Albert PS, Barkhof F, Francis G, Frank JA, Hodgkinson S, et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann Neurol. 1996;39:6–16. doi: 10.1002/ana.410390104. [DOI] [PubMed] [Google Scholar]
- 21.Sormani MP, Miller DH, Comi G, Barkhof F, Rovaris M, Bruzzi P, et al. Clinical trials of multiple sclerosis monitored with enhanced MRI: new sample size calculations based on large data sets. J Neurol Neurosurg Psychiatry. 2001;70:494–499. doi: 10.1136/jnnp.70.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 23.Roepstorff A, Murrell KD. Transmission dynamics of helminth parasites of pigs on continuous pasture: Ascaris suum and Trichuris suis. Int J Parasitol. 1997;27:563–572. doi: 10.1016/s0020-7519(97)00022-2. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen S, Saeed I. Experimental infection of pigs with three dose levels of Trichuris suis. Parasite. 2000;7:275–281. doi: 10.1051/parasite/2000074275. [DOI] [PubMed] [Google Scholar]
- 25.Rudick RA, Cutter G, Reingold S. The Multiple Sclerosis Functional Composite: a new clinical outcome measure for multiple sclerosis trials. Mult Scler. 2002;8:359–365. doi: 10.1191/1352458502ms845oa. [DOI] [PubMed] [Google Scholar]
- 26.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 27.FDA. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. doi: 10.1016/j.vaccine.2023.07.072. http://www.fda.gov.cber/guidelines.htm. [DOI] [PubMed]
- 28.Hill DE, Romanowski RD, Urban JF., Jr A Trichuris-specific diagnostic antigen from culture fluids of Trichuris suis adult worms. Vet Parasitol. 1997;68:91–102. doi: 10.1016/s0304-4017(96)01055-2. [DOI] [PubMed] [Google Scholar]
- 29.Nikodemova M, Lee J, Fabry Z, Duncan ID. Minocycline attenuates experimental autoimmune encephalomyelitis in rats by reducing T cell infiltration into the spinal cord. J Neuroimmunol. 2010;219:33–37. doi: 10.1016/j.jneuroim.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Winsberg GR, Sonnenschein E, Dyer AR, Schnadig V, Bonilla E. Prevalence of intestinal parasites in Latino residents of Chicago. Am J Epidemiol. 1975;102:526–532. doi: 10.1093/oxfordjournals.aje.a112190. [DOI] [PubMed] [Google Scholar]
- 31.Bundy DA, Cooper ES. Trichuris and trichuriasis in humans. Adv Parasitol. 1989;28:107–173. doi: 10.1016/s0065-308x(08)60332-2. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buning J, Homann N, von Smolinski D, Borcherding F, Noack F, Stolte M, et al. Helminths as governors of inflammatory bowel disease. Gut. 2008;57:1182–1183. doi: 10.1136/gut.2008.152355. [DOI] [PubMed] [Google Scholar]
- 35.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. J Clin Immunol. 2008;28:697–706. doi: 10.1007/s10875-008-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summers RW, Elliott DE, Weinstock JV. Therapeutic colonization with Trichuris suis. Arch Pathol Lab Med. 2006;130:1753. doi: 10.5858/2006-130-1753b-IR. author reply 1753–1754. [DOI] [PubMed] [Google Scholar]
- 38.Kradin RL, Badizadegan K, Auluck P, Korzenik J, Lauwers GY. Iatrogenic Trichuris suis infection in a patient with Crohn disease. Arch Pathol Lab Med. 2006;130:718–720. doi: 10.5858/2006-130-718-ITSIIA. [DOI] [PubMed] [Google Scholar]
- 39.Van Kruiningen HJ, West AB. Iatrogenic Trichuris suis infection. Arch Pathol Lab Med. 2007;131:180. doi: 10.5858/2007-131-180-ITSI. [DOI] [PubMed] [Google Scholar]
- 40.Rovaris M, Codella M, Moiola L, Ghezzi A, Zaffaroni M, Mancardi G, et al. Effect of glatiramer acetate on MS lesions enhancing at different gadolinium doses. Neurology. 2002;59:1429–1432. doi: 10.1212/01.wnl.0000033800.93899.e1. [DOI] [PubMed] [Google Scholar]
- 41.Mancardi GL, Sardanelli F, Parodi RC, Melani E, Capello E, Inglese M, et al. Effect of copolymer-1 on serial gadolinium-enhanced MRI in relapsing remitting multiple sclerosis. Neurology. 1998;50:1127–1133. doi: 10.1212/wnl.50.4.1127. [DOI] [PubMed] [Google Scholar]
- 42.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 43.Correale J, Farez M. Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms. J Immunol. 2009;183:5999–6012. doi: 10.4049/jimmunol.0900897. [DOI] [PubMed] [Google Scholar]
- 44.Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol. 2010;10:278–284. doi: 10.1038/nri2730. [DOI] [PubMed] [Google Scholar]
- 45.Johnston MJ, MacDonald JA, McKay DM. Parasitic helminths: a pharmacopeia of anti-inflammatory molecules. Parasitology. 2009;136:125–147. doi: 10.1017/S0031182008005210. [DOI] [PubMed] [Google Scholar]
- 46.McKay DM. The therapeutic helminth? Trends Parasitol. 2009;25:109–114. doi: 10.1016/j.pt.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J Infect Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Chan JV, Rosado-Vallado M, Dumonteil E. Malaria vaccine efficacy: overcoming the helminth hurdle. Expert Rev Vaccines. 2010;9:707–711. doi: 10.1586/erv.10.63. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel RM, Hengartner H. Regulation of the immune response by antigen. Science. 2001;293:251–253. doi: 10.1126/science.1063005. [DOI] [PubMed] [Google Scholar]
- 51.Barsoum RS. Parasitic infections in transplant recipients. Nat Clin Pract Nephrol. 2006;2:490–503. doi: 10.1038/ncpneph0255. [DOI] [PubMed] [Google Scholar]
- 52.Huang NC, Fang HC, Chou KJ, Chung HM. Trichuris trichiura: an unusual cause of chronic diarrhoea in a renal transplant patient. Nephrol Dial Transplant. 2003;18:2434–2435. doi: 10.1093/ndt/gfg328. [DOI] [PubMed] [Google Scholar]
- 53.Santiago M, Leitao B. Prevention of strongyloides hyperinfection syndrome: a rheumatological point of view. Eur J Intern Med. 2009;20:744–748. doi: 10.1016/j.ejim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites – masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 55.Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]