Abstract
Background
Challenges in accurate, in vivo quantification of multi-planar knee kinematics and relevant timing sequence during high-risk injurious tasks pose challenges in understanding the relative contributions of joint loads in non-contact injury mechanisms. Biomechanical testing on human cadaveric tissue, if properly designed, offers a practical means to evaluate joint biomechanics and injury mechanisms. This study seeks to investigate detailed interactions between tibiofemoral joint multi-planar kinematics and anterior cruciate ligament strain in a cadaveric model of landing using a validated physiologic drop-stand apparatus.
Methods
Sixteen instrumented cadaveric legs, 45(SD 7) years (8 female and 8 male) were tested. Event timing sequence, change in tibiofemoral kinematics (position, angular velocity and linear acceleration) and change in anterior cruciate ligament strain were quantified.
Findings
The proposed cadaveric model demonstrated similar tibiofemoral kinematics/kinetics as reported measurements obtained from in vivo studies. While knee flexion, anterior tibial translation, knee abduction and increased anterior cruciate ligament strain initiated and reached maximum values almost simultaneously, internal tibial rotation initiated and peaked (p<0.015 for all comparisons) significantly later. Further, internal tibial rotation reached 1.8(SD 2.5)°, almost 63% of its maximum value, at the time that peak anterior cruciate ligament strain occurred, while both anterior tibial translation and knee abduction had already reached their peaks.
Interpretation
Together, these findings indicate that although internal tibial rotation contributes to increased anterior cruciate ligament strain, it is secondary to knee abduction and anterior tibial translation in its effect on anterior cruciate ligament strain and potential risk of injury.
Keywords: Anterior cruciate ligament, Biomechanics, Cadaveric experiments, Landing, Injury
Introduction
Over 125,000 anterior cruciate ligament (ACL) injuries occur annually in the United States (Kim et al., 2011), mainly affecting the young athletic population. Non-contact injuries are reported to be the predominant mechanism of ACL injury (>70% of ACL injuries)(Griffin et al., 2000, Henrichs, 2004). These injuries often occur during landing with high ground reaction forces, muscle forces and segmental inertia (Olsen et al., 2004, Boden et al., 2000). Injury prevention strategies are an appealing option to avoid long-term joint instability, pain, and early development of osteoarthritis associated with ACL injury(Agel et al., 2005, Arendt and Dick, 1995, Hewett et al., 1999, Malone et al., 1993), as well as potential loss of sports participation(Maquirriain and Megey, 2006, van Lent et al., 1994) and high costs associated with surgical reconstruction(de Loes et al., 2000).
Noncontact ACL injury mechanisms are multi-planar in nature, involving tibiofemoral joint articulation in all three anatomical planes (Koga et al., 2010, Quatman et al., 2010, Kiapour, 2013). Despite considerable efforts to characterize ACL injury mechanisms (Ford et al., 2003, Griffin et al., 2000, Krosshaug et al., 2007, Hewett et al., 2005, Agel et al., 2005, Arendt and Dick, 1995, Boden et al., 2000, Chappell et al., 2002, Decker et al., 2003, Hewett et al., 1999, Joseph et al., 2011, Koga et al., 2010, Malone et al., 1993, Moran and Marshall, 2006, Olsen et al., 2004, Kiapour et al., 2013a, Kiapour et al., 2013b), the relative contribution of each loading axis in the multi-axial (multi-planar) injury mechanism during landing is unclear. Due to the high-rate dynamic environment of injurious events, precise in vivo measurements of tibiofemoral joint six-degrees of freedom kinematics, its interaction with ACL tension and the associated timing sequence remain a challenge.
While clinical studies ultimately represent the gold standard for the evaluation of ACL injuries, studies of cadaveric biomechanics (ex vivo) under controlled laboratory conditions complement and often precede such work. Biomechanical testing of human cadaveric tissue offers a practical means for investigation of various disorders, and can evaluate associated conservative and non-conservative treatments. Ex vivo techniques serve to enhance our knowledge of joint biomechanics and ligament functions, and generate direct measurements of mechanical parameters (i.e. force and strain) that are challenging, if not impossible to obtain in vivo. Further, these techniques provide a standard framework in which to conduct robust parametric studies.
Over the past three decades, extensive efforts have been undertaken to study ACL biomechanics utilizing ex vivo approaches (Bach and Hull, 1998, Berns et al., 1992, Butler et al., 1980, DeMorat et al., 2004, Durselen et al., 1995, Gabriel et al., 2004, Hashemi et al., 2010, Markolf et al., 2004, Wu, 2010, Csintalan et al., 2006, Draganich and Vahey, 1990, Fukubayashi et al., 1982, Mazzocca et al., 2003, Meyer and Haut, 2008, Oh et al., 2012, Renstrom et al., 1986, Romero et al., 2002, Wall et al., 2012, Yeow et al., 2009, Zantop et al., 2007, Kiapour et al., 2012a). The majority of these studies simulate low-rate, sub-injurious tasks through the application of static and/or quasi-static loading conditions(Bach and Hull, 1998, Berns et al., 1992, Butler et al., 1980, Csintalan et al., 2006, Draganich and Vahey, 1990, Durselen et al., 1995, Fukubayashi et al., 1982, Gabriel et al., 2004, Markolf et al., 2004, Mazzocca et al., 2003, Renstrom et al., 1986, Romero et al., 2002, Wu, 2010, Zantop et al., 2007, Kiapour et al., 2012a). Reported findings from such studies help to understand ACL biomechanics and overall joint function. However, they are not strong representations of high-rate (dynamic) injurious conditions that occur during high-risk activities (i.e. landing and cutting maneuvers).
Experimental strategies have been developed to replicate high-risk, potentially injurious conditions and reproduce ACL injury (Wall et al., 2012, DeMorat et al., 2004, Hashemi et al., 2010, Meyer and Haut, 2008, Oh et al., 2012, Withrow et al., 2006, Yeow et al., 2009). Such experiments have focused on a variety of causative factors including muscle loading (DeMorat et al., 2004, Hashemi et al., 2010, Wall et al., 2012, Withrow et al., 2008), axial compression (Yeow et al., 2009, Wall et al., 2012, Meyer and Haut, 2008), and off-axis external loads (Withrow et al., 2006, Oh et al., 2012, Meyer and Haut, 2008) to simulate landing. Yet, such models are primarily limited by non-physiologic simulation of dynamic loading conditions (i.e. sharp impact peaks generated by a small mass, lack of muscle forces and insufficient magnitudes of off-axis external loads), unlike those experienced during actual ACL injuries.
Due to the complex, multi-factorial dynamic nature of knee injuries, validated experimental models that simulate realistic inciting events leading to consistent physiologic injuries are essential. Such models can be utilized to study the overall interaction between knee joint kinematics/kinetics with ACL tension and further investigate the relative contribution of each loading axis in overall risk of ACL injury. Hence, this study aims to develop a novel, physiologic cadaveric model of landing (as a well-established high-risk task in non-contact ACL injury (Olsen et al., 2004, Boden et al., 2000)) in order to investigate detailed interactions between tibiofemoral joint multi-planar kinematics and ACL strain. We hypothesized that there are significant differences in temporal knee joint kinematics in different planes such that the peak knee sagittal and frontal planes motion coincides with peak ACL strain, while knee axial rotation peaks significantly later. Detailed understanding of knee joint dynamic motion during high-risk activities can lead to improved knowledge of ACL injury mechanisms and associated risk factors. This may in turn help clinicians to optimize current prevention and rehabilitation strategies in an effort to minimize the high incidence of ACL injury and early-onset post-traumatic osteoarthritis.
Methods
Specimen Preparation
Sixteen unembalmed fresh frozen cadaveric lower limbs, 45(SD 7) years (8 female and 8 male), were acquired. Each specimen was inspected visually, and by computed tomography (CT) and magnetic resonance imaging (MRI) for signs of soft or hard tissue pathology including indications of prior surgery, mal-alignment deformities and ACL disruption. Specimens were stored at −20°C. Specimens were slowly thawed to room temperature 24 hours prior to testing. Thawed specimens were sectioned at the mid-femoral shaft (30 cm above the joint line) and all soft tissues up to 15 cm proximal to the joint line were dissected. Subsequently, the remaining segment of the proximal femur of each specimen was potted in a 3.8 cm (1.5 inch) diameter polyvinyl chloride (PVC) tube with polyester resin for rigid attachment to the testing frame.
The quadriceps (rectus femoris) and hamstrings (semitendinosus, biceps femoris and semimembranosus) tendons were then isolated, and clamped inside metal tendon grips to allow for the application of simulated muscle loads. The remaining musculature along with the skin were maintained intact. The foot and ankle were also maintained intact to provide a realistic load transfer interface. Exposed tissue about the knee joint was kept moist with 0.9% buffered saline solution at all times.
Testing Apparatus
A novel testing apparatus was designed to maintain specimens in an orientation that simulates lower extremity posture during ground strike while landing from a jump (Figure 1) (Levine et al., 2013, Kiapour et al., 2013c). The unconstrained nature of this experimental setup allows for a broad range of loading conditions (i.e. anterior shear force, knee abduction and tibial axial rotation) to be applied during simulated landing (Levine et al., 2013, Quatman et al., 2013). Each specimen was rigidly fixed at the proximal femur to a fixture with an embedded custom-designed six-axis load cell (B9401, Denton, Rochester Hills, MI, USA). Specimens were positioned inverted with the tibia orientated vertically and the foot positioned above the tibia. The knee was positioned at 25° of flexion to simulate the orientation of this joint during injurious events, as reported by video analyses of ACL injuries (Koga et al., 2010). The femoral fixture was able to rotate and translate about five axes (no translation in Z-direction) in order to orient the tibia in line with the axis of the impactor, while maintaining 25° of knee flexion.
Figure 1.
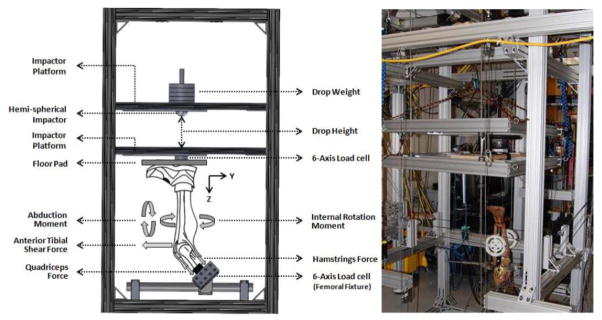
Custom designed drop-stand testing apparatus.
As shown in Figure 1, the drop stand is comprised of two independent platforms (floor and impactor). The lower platform (floor platform) acts to simulate floor contact, while the upper platform (impactor platform) imparts a simulated ground reaction force (GRF) during landing. Six vertically aligned linear bearings (three on each platform) were used to maintain platform alignment and guide the motion of each platform during the simulated landing. A second six-axis load cell (2586, Denton, Rochester Hills, MI, USA) incorporated into the floor platform captured all forces and moments applied to the specimen during simulated landing representing the GRF.
Muscle forces were simulated by multiple cable-pulley systems along with static weights that served to apply constant forces to the quadriceps and hamstrings tendons. Adjustable pulley systems were used to maintain the physiologic line of action of each muscle group (Figure 2). In order to simulate different postures during landing, an external fixation frame with an integrated pulley system was rigidly attached to the tibia. Additional cable-pulley systems along with static weights were designed to produce forces to generate anterior tibial shear, and force couples to generate pure abduction/adduction and internal/external rotation moments about the knee without a fixed center of rotation (Figure 2) (Levine et al., 2013, Quatman et al., 2013). To allow for the unconstrained application of external loads, the distal extremity (lower leg and foot) was free to rotate and translate during loading. Following the application of the muscle and other external loads, the specimen was repositioned through translation/rotation of the femoral fixture in order to vertically align the tibia. An athletic shoe was placed on the foot to provide a more realistic load transfer interface during initial contact. Subsequently, the floor platform was set upon the shoe to simulate a foot-planted position.
Figure 2.
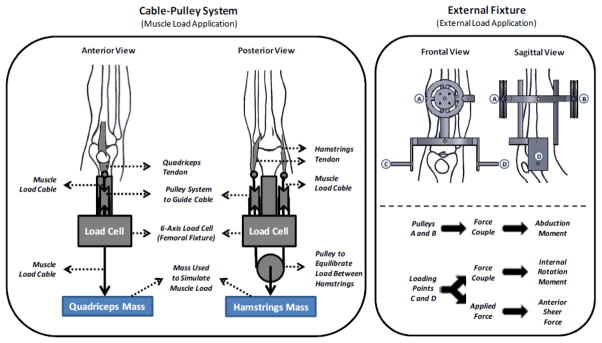
Cable-pulley system used for application of the simulated muscle loads (left). External fixation frame with the embedded cable-pulley system used for application of external loads.
A hemi-spherical impactor combined with the weight stack was designed to drop each specified weight from each specified height onto the floor pad (embedded within the floor platform) using varying weight and drop-height magnitudes to achieve different levels of impact severity (Levine et al., 2013, Quatman et al., 2013). The drop weight exerted an impulsive axial compressive force that simulated GRF during landing from a jump. In this study, neutral bi-pedal landing was simulated by releasing half body weight (350 N) from a height of 30 cm in the presence of the pre-tensioned quadriceps (1200 N) and hamstrings (800 N; 400 to each medial and lateral group). It is important to note that the pre-landing knee extension due to the quadriceps to hamstrings force imbalance was resisted by preventing anterior translation of the foot using a high stiffness cable connecting the foot to the back of the test frame. This fixation only constrained the anterior translation of the foot while preserving the other five-degrees of freedom (2 translations and 3 rotations) motion across the ankle joint.
Instrumentation
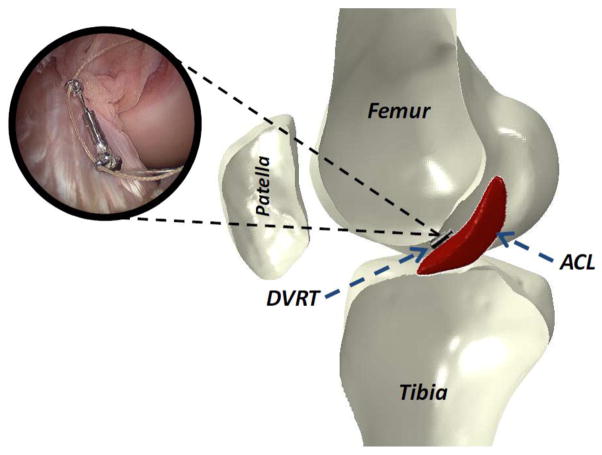
ACL strain was calculated based on the measurements of a differential variable reluctance transducer (DVRT) (MicroStrain Inc., Williston, VT, USA) that was arthroscopically placed on the distal third of the anterior medial (AM) bundle through two para-patellar incisions (Figure 3). This system allows for quantification of displacement with an accuracy of 0.1% and the repeatability of 1 μm. In order to calculate absolute strain values, ACL reference length was calculated based on established methods (Howe et al., 1990, Fleming et al., 1994) as the distinct inflection point in the force versus DVRT displacement curve. These data were collected by placing each specimen through four cycles of anterior-posterior (A-P) shear prior to the testing. The selected inflection point was chosen as the proper reference between ligament taut and slack conditions. Therefore reference length is not dependent on the initial gauge length of the DVRT at the time of insertion. It was assumed that the average strain across the ACL AM-bundle is equal to the change in length of the measured segment divided by the reference length obtained from DVRT measurements using the following equation:
Figure 3.
DVRT insertion on the AM-bundle of the ACL.
Where L is the instantaneous length measured across the DVRT, and L0 is the length measured across the DVRT at the reference length of the ligament.
Three-dimensional (3D) rigid body motion of the femur and tibia were tracked using arrays of three infrared-LED markers rigidly attached to each bone, and an Optotrak 3020 3D motion capture system (Northern Digital, Waterloo, Ontario, Canada). This system allows for the tracking of rigid body motion with a resolution of 0.01 mm and an accuracy of 0.1 mm. Subsequent to testing, specimens were inspected arthroscopically to document any tissue damage or failure of knee joint structures.
Data Acquisition and Processing
Data collection from all data acquisition units were synchronized utilizing a simultaneous trigger. Analog data (load cells and the DVRT) were collected at 4 kHz, while motion data were collected at 400 Hz. A custom macro was developed in Matlab 7.1 (The MathWorks Inc., Natick, MA, USA) to process the data. Six-degree of freedom tibiofemoral joint kinematics were calculated from marker position data. Kinematics were then low-pass filtered using a 4 pole Butterworth filter with a cut-off frequency of 50 Hz(Woltring et al., 1985). Segmental angular velocity and linear acceleration were calculated from rotation and displacement data using a central difference method.
Statistical Analysis
A paired sample t-test was used to analyze relative changes in tibiofemoral kinematics and ACL strain due to simulated landing under axial impact. Analysis of variance (ANOVA) with a post-hoc Bonferroni Correction for multiple comparisons was used to compare the initiation time from initial contact, time to peak from initial contact, and time to peak from peak axial impact between all measured kinematics and kinetics components. Differences were considered to be statistically significant for p<0.05.
Results
Peak axial impact force, ACL strain and tibiofemoral joint kinematic measures are presented in Table 1. Prior to impact, force imbalance between the quadriceps and hamstrings muscle groups produced an average anterior tibial translation of 3.8(SD 3.1) mm and mean ACL strain of 2.1(SD 2.1)%. Simulated pre-impact quadriceps and hamstrings force ratio did not change the initial frontal and axial plane tibiofemoral alignment, and the average initial knee flexion angle was maintained at 25.0(SD 0.2)°.
Table 1.
Summary of the peak axial impact load, change in tibiofemoral kinematics and ACL strain.
| Specimens
|
Peak Impact | Knee Flexion
|
ATT*
|
Peak Abduction | Peak Int. Rotation | ACL Strain
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age | Side | Impact induced | Peak | Impact induced | Peak | Impact Induced | Peak | |||
| 1-C080044 | F | 52 | L | 3468 N | 1.1° | 25.8° | 5.8 mm | 10.9 mm | −0.9° | 1.6° | 1.4 % | 3.4 % |
| 2-C090105 | M | 38 | L | 3188 N | 0.1° | 24.8° | 3.1 mm | 8.0 mm | 0.6° | 0.8° | 1.0 % | 6.9 % |
| 3-C080033 | M | 51 | L | 3561 N | −0.1° | 24.8° | 8.6 mm | 14.6 mm | 1.3° | 2.5° | 5.6 % | 8.5 % |
| 4-S090574 | F | 49 | R | 4050 N | 0.8° | 26.0° | 12.0 mm | 16.5 mm | 1.7° | 4.4° | 7.6 % | 9.4 % |
| 5-C090155 | M | 46 | L | 4400 N | 0.4 | 25.4° | 6.6 mm | 5.2 mm | 0.9° | 1.0° | 5.7 % | 4.4 % |
| 6-C090105 | M | 38 | R | 4155 N | 1.0° | 25.9° | 4.3 mm | 5.3 mm | 0.9° | 1.9° | 9.1 % | 13.3 % |
| 7-C090155 | M | 46 | R | 3394 N | 0.7° | 25.5° | 5.4 mm | 10.8 mm | 0.9° | 2.9° | 1.7 % | 6.2 % |
| 8-C090361 | M | 34 | R | 2869 N | 1.6° | 26.5° | 6.6 mm | 7.6 mm | 3.8° | 5.1° | 4.7 % | 5.1 % |
| 9-C090508 | F | 45 | L | 5076 N | −0.2° | 25.1° | 4.9 mm | 15.2 mm | 2.8° | 1.3° | 5.7 % | 9.3 % |
| 10-C090508 | F | 45 | R | 5036 N | 0.7° | 25.8° | 8.4 mm | 8.8 mm | 3.6° | 8.4° | 8.9 % | 8.5 % |
| 11-C080044 | F | 52 | R | 3616 N | 0.1° | 25.1° | 6.9 mm | 8.5 mm | 2.4° | 2.3° | 2.2 % | 6.0 % |
| 12-C090552 | M | 45 | R | 4751 N | 0.6° | 25.3° | 8.9 mm | 13.8 mm | 3.6° | 4.4° | 6.4 % | 7.4 % |
| 13-1007889 | F | 29 | R | 4483 N | 1.3° | 26.6° | 9.1 mm | 12.1 mm | 3.9° | 6.8° | 2.9 % | 6.4 % |
| 14-1008352 | F | 54 | R | 4274 N | 2.3° | 27.3° | 9.8 mm | 17.6 mm | 4.0° | 4.7° | 2.6 % | 3.9 % |
| 15-C090552 | M | 45 | L | 4453 N | 1.6° | 26.6° | 6.4 mm | 11.2 mm | 2.0° | −0.7° | 4.4 % | 5.7 % |
| 16-S090706 | F | 50 | L | 4962 N | 3.1° | 28.2° | 9.4 mm | 9.9 mm | 1.3° | −1.8° | 3.7 % | 2.2 % |
ATT: Anterior tibial translation
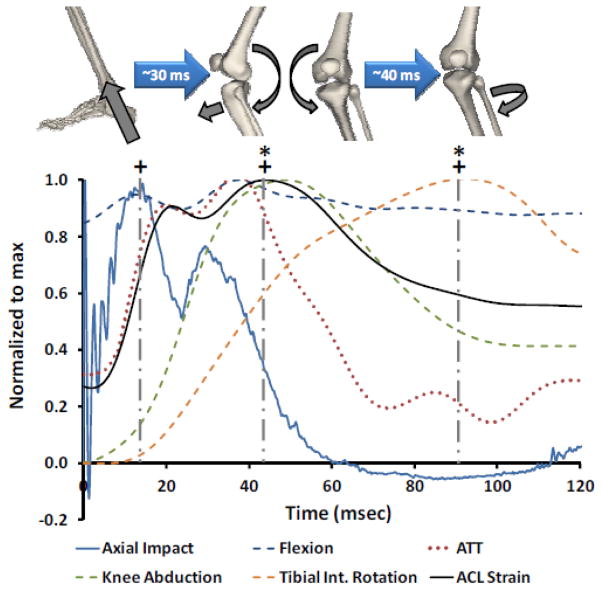
Simulated bi-pedal landing resulted in an average peak axial impact load of 4109(SD 691) N over a mean period of 72(SD 12) msec. A summary of average timing sequences for axial impact load, knee flexion, anterior tibial translation, knee abduction, internal tibial rotation and ACL strain are presented in Table 2. Simulated landings initiated knee flexion, anterior tibial translation, knee abduction, increased ACL strain and internal tibial rotation, sequentially. Internal tibial rotation was initiated significantly later than all other quantified parameters (p≤0.01 for all comparisons). No significant differences were observed between initiation time of knee flexion, anterior tibial translation, knee abduction and increased ACL strain following initial contact (p>0.35 for all comparisons).
Table 2.
Summary of the average (SD) timing sequences.
| Parameter | Initiation Time from IC* | Time to Peak
|
|
|---|---|---|---|
| From IC | From Peak Axial Impact | ||
| Axial Impact Load | --- | 13.7 (2.4) msec | --- |
| Knee Flexion | 7.1 (3.5) msec | 36.6 (7.8) msec | 22.8 (8.9) msec |
| Anterior Tibial Translation | 7.7 (1.9) msec | 37.5 (7.0) msec | 23.5 (8.1) msec |
| Knee Abduction | 10.8 (6.2) msec | 51.5 (22.6) msec | 37.6 (22.1) msec |
| Internal Tibial Rotation | 21.0 (11.7) msec | 86.5 (25.1) msec | 72.5 (25.6) msec |
| ACL Strain | 12.4 (6.3) msec | 54.2 (27.0) msec | 40.3 (28.1) msec |
IC: Initial contact
Load generated by axial impact significantly increased: knee flexion angle by 0.9(SD 0.8)° (p<0.0005; 22.8(SD 8.9) msec after peak impact), anterior tibial translation by 7.3(SD 2.3) mm (p=0.001; 23.5(SD 8.1) msec after peak impact), knee abduction by 2.0(SD 1.4)° (p<0.0005; 37.6(SD 22.1) msec after peak impact) and internal tibial rotation by 2.8(SD 2.6)° (p=0.001; 72.5(SD 25.6) msec after peak impact) compared to the pre-landing condition. Resultant change in tibiofemoral kinematics along with axial impact load increased ACL strain by 4.6(SD 2.6)% (p<0.0005; 40.3(SD 28.1) msec following peak impact) compared to the pre-landing condition. Simulated landings resulted in a peak angular velocity of 68.3(SD 32.0) deg/s (knee abduction) and 70.5(SD 32.3) deg/s (internal tibial rotation), and peak anterior tibial acceleration of 154.7(SD 179.1) m/s2. No significant difference was observed between peak abduction angular velocity and peak internal rotation angular velocity (p=0.08).
Peak axial impact occurred significantly earlier than peak knee flexion, anterior tibial translation, knee abduction, internal tibial rotation and ACL strain (P≤0.013 for all comparisons). While peak anterior tibial translation, knee abduction and ACL strain occurred at approximately 45 msec following initial contact, peak internal tibial rotation occurred significantly later (p<0.015 for all comparisons; 86.5(SD 25.1) msec after initial contact). The time-history graph of normalized ACL strain, tibiofemoral kinematics and generated axial impact load for one of the specimens is shown in Figure 4. No tissue failure was observed across anatomical structures of the knee following testing.
Figure 4.
Time-history graph of the normalized generated axial impact, tibiofemoral kinematics and ACL strain for a typical specimen during simulated landing. (+) Demonstrating significant delay (~30ms) in occurrence of the peak anterior tibial translation, knee abduction, ACL strain and internal tibial rotation following peak axial impact load (p<0.013). (*) Shows significant delay (~40ms) in occurrence of the peak internal tibial rotation subsequent to the concurrent peak anterior tibial translation, knee abduction and ACL strain (p<0.015).
Discussion
Challenges in accurate, in vivo quantification of multi-planar knee kinematics and timing sequence during injury hinder the understanding of the relative contributions of each loading axis to the overall injury mechanisms. Biomechanical testing of human cadaveric tissue, if properly designed, offers a practical means to evaluate joint biomechanics and injury mechanisms. The purpose of this study was to investigate the interaction between tibiofemoral joint kinematics and ACL strain in addition to their timing sequence using a novel, physiologic cadaveric model of landing.
A unique, custom-designed drop-stand with physiologically relevant drop weights and drop heights was employed. Simulated landings from a jump were conducted on sixteen instrumented cadaveric specimens. Event timing sequence, change in tibiofemoral kinematics and change in ACL strain during a simulated bi-pedal landing task were quantified. The proposed cadaveric model of landing demonstrated similar tibiofemoral kinematics and kinetics as reported by in vivo biomechanical and video analysis studies, Table 3. Comparisons (in vivo validation) were conducted on: landing duration (landing stance) (Chappell et al., 2002, Joseph et al., 2011), time to peak axial impact load (GRF) (Decker et al., 2003), peak knee abduction angular velocity (Joseph et al., 2011), time to peak knee abduction (as percent landing stance) (Joseph et al., 2011), peak anterior tibial acceleration (Moran and Marshall, 2006) and time to peak ACL strain (ACL rupture) (Koga et al., 2010, Krosshaug et al., 2007). These comparisons are compelling, especially in light of the lack of complete active neuromuscular control in cadaveric model, intra-specimen variability in joint geometry and tissue mechanical properties, and the limited sample size compared to in vivo biomechanical studies. Moreover, this cadaveric model has been reported to consistently reproduce clinically relevant injury patterns to the ACL (ACL failure in almost 90% of the specimens) and surrounding soft tissue structures (Levine et al., 2013) under injurious conditions. Finally, the resultant tibial plateau injury patterns (both articular cartilage and subchondral bone) were shown to be similar to clinically observed bone bruise patterns across the tibial plateau during actual cases of non-contact ACL injury (Levine et al., 2013, Kiapour et al., 2012c). As a result, the current cadaveric model can be considered a valid approach in simulating landing biomechanics.
Table 3.
Cadaveric model Vs. in vivo biomechanical data (in vivo validation).
| Parameter | Ex vivo | In vivo | References |
|---|---|---|---|
| Landing duration (landing stance) | 72 (11) msec | 75 ms | (Joseph et al., 2011) |
| 55 (15) msec | (Chappell et al., 2002) | ||
|
| |||
| Time to 1st and 2nd peak axial impact load (GRF) following initial contact | 13 (2) msec and 31 (6) msec | 10 (3) msec and 40 (10) msec | (Decker et al., 2003) |
|
| |||
| Peak abduction angular velocity | 68 (32) msec | 57 (20) msec | (Joseph et al., 2011) |
|
| |||
| Time to peak knee abduction (% of landing duration) | 55 (22) % | 60 (10) % | (Joseph et al., 2011) |
|
| |||
| Peak anterior tibial acceleration | 154 (179) m/sec2 | ~150 (100) m/sec2 | (Moran and Marshall, 2006) |
|
| |||
| Time to peak ACL strain (ACL rupture) following initial contact | 54 (27) msec | 39 (10) msec | (Krosshaug et al., 2007) |
| ~40 msec | (Koga et al., 2010) | ||
The results of this study demonstrate an increase in both anterior tibial translation and ACL strain due to anterior-posterior imbalance in simulated knee muscle loads prior to impact. This is in agreement with previous findings suggesting that anterior translation of the tibia with respect to the femur and increased levels of ACL strain/force or risk of ACL injury under aggressive quadriceps force (Berns et al., 1992, Beynnon et al., 1995, DeMorat et al., 2004, Draganich and Vahey, 1990, Durselen et al., 1995, Hashemi et al., 2010, Li et al., 1999, Wall et al., 2012, Quatman et al., 2012). Simulated landings in this study sequentially resulted in increased knee flexion, anterior tibial translation, knee abduction, ACL strain and internal tibial rotation. This is in agreement with our hypothesis that temporal differences exist in multi-planar knee kinematics during dynamic landing. Previous clinical, video analysis and in vivo biomechanical studies indicate that knee flexion, anterior tibial translation, knee abduction and internal rotation of the tibia are associated with landing (Ford et al., 2003, Hewett et al., 2005, Koga et al., 2010, Krosshaug et al., 2007, Moran and Marshall, 2006). Additionally, these factors have been shown to contribute to non-contact ACL injuries at shallow knee flexion angles (Levine et al., 2013, Meyer and Haut, 2008, Oh et al., 2012, Kiapour et al., 2013c).
It was further noted that while knee flexion, anterior tibial translation, knee abduction and increased ACL strain were initiated and reached their maximum values almost simultaneously, internal tibial rotation was initiated (P≤0.01 for all comparisons) and peaked (p<0.015 for all comparisons) significantly later (Figure 4). This observed timing sequence highlights the primary role of the anterior tibial translation along with knee abduction in ACL loading and potential risk of injury during landing, as suggested by others (Boden et al., 2000, Ford et al., 2003, Koga et al., 2010, Krosshaug et al., 2007, Olsen et al., 2004, Shin et al., 2009, Withrow et al., 2006, Kiapour et al., 2012d). The generated internal tibial rotation during simulated landings along with concurrent increase in both ACL strain and internal tibial rotation support internal rotation as a potential risk factor of ACL injury as previously indicated (Kiapour et al., 2012d, Meyer and Haut, 2008, Oh et al., 2012, Kiapour et al., 2012b). Further, it was demonstrated that internal tibial rotation reached an average of 1.8(SD 2.5)°, almost 63% of its maximum value, by the time that peak ACL strain occurs, while both anterior tibial translation and knee abduction have already reached their peaks supporting our hypothesis. Together, these findings imply that although internal tibial rotation contributes to increased ACL strain, it is secondary to anterior tibial translation and knee abduction in affecting ACL strain and potential risk of injury, as noted by the knee joint kinematics timing sequence. This is in agreement with previous findings reporting greater peak ACL strain and higher rates of ACL injury under anterior shear force and abduction moment compared to internal tibial rotation moment (Levine et al., 2013, Quatman et al., 2013).
This is the first study, to the authors’ knowledge, to demonstrate the detailed interaction and timing sequence between knee kinematics/kinetics and ACL strain during a simulated landing task using a validated physiologic cadaveric model. Current work builds upon previous cadaveric studies (Wall et al., 2012, DeMorat et al., 2004, Hashemi et al., 2010, Meyer and Haut, 2008, Oh et al., 2012, Yeow et al., 2009) by generating ACL loading via axial compression and muscle loading in an unconstrained manner. This setup was designed to replicate the range of loading observed in vivo. Detailed attention to real world loading/impact parameters including body mass, drop height and loading interface resulted in physiologic simulation of knee kinematics/kinetics with a timing sequence similar to in vivo data (Table 3).
This evolution in experimental design facilitates the use of a cadaveric model to independently evaluate extrinsic and intrinsic risk factors, and underlying mechanisms associated with ACL injury. Data from this study indicates that the most critical dynamic landing scenario that leads to elevated ACL strain levels and potential injury include a combination of anterior tibial translation, knee abduction and internal tibial rotation. Further, the current findings emphasize the significant role of anterior tibial translation and knee abduction as primary contributors, and internal tibial rotation as a secondary contributor to risk of ACL injury.
Study Limitations
As with any study, inherent limitations exist in the current cadaveric study. First, ACL strain was represented by local strain measurements across the AM-bundle. However, the attachment of a second DVRT to the posterolateral bundle of the ACL would have been associated with the compromise of the posterior joint capsule and potential measurement artifacts (Bach and Hull, 1998). The choice to place a single DVRT on the ACL AM-bundle was based on previous work that found AM-bundle strain to be a good representation of overall ACL strain (Markolf et al., 1990). Another limitation is the potential differences in tissue properties associated with cadaveric specimens compared with the in vivo tissue properties of young athletes, which can affect the accuracy of the absolute reported values. We have tried to minimize this artifact by testing relatively young specimens. Moreover, the effect of change in knee flexion angle was not evaluated as all the specimens were tested at 25° of knee flexion, since this flexion angle has been reported during real cases of ACL injury. Additionally, landing was simulated with the foot in a flat position with ankle joint being semi-constrained to a limited range of dorsi flexion, which does not replicate the ankle motion during landing. Finally, the primary and secondary roles of loading factors on risk of ACL injury have been identified solely based on the temporal characteristics of knee multi-planar kinematics. Despite strong agreement with previous findings, further parametric and sensitivity analyses are required to better characterize the independent role of each loading axis in ACL injury risk.
However, the current findings are least likely to be affected by this limitation as the study intended to replicate/investigate the isolated knee joint biomechanical response during the inciting event not the whole multi-joint landing phenomenon. We believe that qualitative findings and relative comparisons presented in this work minimize such artifacts. Considering the strengths and limitations of this experimental model, the authors believe that it is well suited and able to evaluate mechanisms of ACL injury.
Acknowledgments
The authors acknowledge funding support from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR049735 and R01-AR056259. The authors would also like to thank Dr. Jason Levine for his assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGEL J, ARENDT EA, BERSHADSKY B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33:524–30. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- ARENDT E, DICK R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- BACH JM, HULL ML. Strain inhomogeneity in the anterior cruciate ligament under application of external and muscular loads. J Biomech Eng. 1998;120:497–503. doi: 10.1115/1.2798020. [DOI] [PubMed] [Google Scholar]
- BERNS GS, HULL ML, PATTERSON HA. Strain in the anteromedial bundle of the anterior cruciate ligament under combination loading. J Orthop Res. 1992;10:167–76. doi: 10.1002/jor.1100100203. [DOI] [PubMed] [Google Scholar]
- BEYNNON BD, FLEMING BC, JOHNSON RJ, NICHOLS CE, RENSTROM PA, POPE MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- BODEN BP, DEAN GS, FEAGIN JA, GARRETT WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- BUTLER DL, NOYES FR, GROOD ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259–70. [PubMed] [Google Scholar]
- CHAPPELL JD, YU B, KIRKENDALL DT, GARRETT WE. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am J Sports Med. 2002;30:261–7. doi: 10.1177/03635465020300021901. [DOI] [PubMed] [Google Scholar]
- CSINTALAN RP, EHSAN A, MCGARRY MH, FITHIAN DF, LEE TQ. Biomechanical and anatomical effects of an external rotational torque applied to the knee: a cadaveric study. Am J Sports Med. 2006;34:1623–9. doi: 10.1177/0363546506288013. [DOI] [PubMed] [Google Scholar]
- DE LOES M, DAHLSTEDT LJ, THOMEE R. A 7-year study on risks and costs of knee injuries in male and female youth participants in 12 sports. Scand J Med Sci Sports. 2000;10:90–7. doi: 10.1034/j.1600-0838.2000.010002090.x. [DOI] [PubMed] [Google Scholar]
- DECKER MJ, TORRY MR, WYLAND DJ, STERETT WI, RICHARD STEADMAN J. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin Biomech (Bristol, Avon) 2003;18:662–9. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- DEMORAT G, WEINHOLD P, BLACKBURN T, CHUDIK S, GARRETT W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32:477–83. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- DRAGANICH LF, VAHEY JW. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J Orthop Res. 1990;8:57–63. doi: 10.1002/jor.1100080107. [DOI] [PubMed] [Google Scholar]
- DURSELEN L, CLAES L, KIEFER H. The influence of muscle forces and external loads on cruciate ligament strain. Am J Sports Med. 1995;23:129–36. doi: 10.1177/036354659502300122. [DOI] [PubMed] [Google Scholar]
- FLEMING BC, BEYNNON BD, TOHYAMA H, JOHNSON RJ, NICHOLS CE, RENSTROM P, POPE MH. Determination of a zero strain reference for the anteromedial band of the anterior cruciate ligament. J Orthop Res. 1994;12:789–95. doi: 10.1002/jor.1100120606. [DOI] [PubMed] [Google Scholar]
- FORD KR, MYER GD, HEWETT TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35:1745–50. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- FUKUBAYASHI T, TORZILLI PA, SHERMAN MF, WARREN RF. An in vitro biomechanical evaluation of anterior-posterior motion of the knee. Tibial displacement, rotation, and torque. J Bone Joint Surg Am. 1982;64:258–64. [PubMed] [Google Scholar]
- GABRIEL MT, WONG EK, WOO SL, YAGI M, DEBSKI RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85–9. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- GRIFFIN LY, AGEL J, ALBOHM MJ, ARENDT EA, DICK RW, GARRETT WE, GARRICK JG, HEWETT TE, HUSTON L, IRELAND ML, JOHNSON RJ, KIBLER WB, LEPHART S, LEWIS JL, LINDENFELD TN, MANDELBAUM BR, MARCHAK P, TEITZ CC, WOJTYS EM. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–50. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- HASHEMI J, BREIGHNER R, JANG TH, CHANDRASHEKAR N, EKWARO-OSIRE S, SLAUTERBECK JR. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010;17:235–41. doi: 10.1016/j.knee.2009.09.010. [DOI] [PubMed] [Google Scholar]
- HENRICHS A. A review of knee dislocations. J Athl Train. 2004;39:365–9. [PMC free article] [PubMed] [Google Scholar]
- HEWETT TE, LINDENFELD TN, RICCOBENE JV, NOYES FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- HEWETT TE, MYER GD, FORD KR, HEIDT RS, JR, COLOSIMO AJ, MCLEAN SG, VAN DEN BOGERT AJ, PATERNO MV, SUCCOP P. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- HOWE JG, WERTHEIMER C, JOHNSON RJ, NICHOLS CE, POPE MH, BEYNNON B. Arthroscopic strain gauge measurement of the normal anterior cruciate ligament. Arthroscopy. 1990;6:198–204. doi: 10.1016/0749-8063(90)90075-o. [DOI] [PubMed] [Google Scholar]
- JOSEPH MF, RAHL M, SHEEHAN J, MACDOUGALL B, HORN E, DENEGAR CR, TROJIAN TH, ANDERSON JM, KRAEMER WJ. Timing of lower extremity frontal plane motion differs between female and male athletes during a landing task. Am J Sports Med. 2011;39:1517–21. doi: 10.1177/0363546510397175. [DOI] [PubMed] [Google Scholar]
- KIAPOUR A, KIAPOUR AM, KAUL V, QUATMAN CE, HEWETT TE, DEMETROPOULOS CK, GOEL VK. Finite Element Model of the Knee for Investigation of Injury Mechanisms: Development and Validation. J Biomech Eng. 2013a doi: 10.1115/1.4025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIAPOUR AM. Doctor of Philosophy Degree in Biomedical Engineering Dissertation. The University of Toledo; 2013. Non-Contact ACL Injuries during Landing: Risk Factors and Mechanisms. [Google Scholar]
- KIAPOUR AM, KAUL V, KIAPOUR A, QUATMAN CE, WORDEMAN SC, HEWETT TE, DEMETROPOULOS CK, GOEL VK. The Effect of Ligament Modeling Technique on Knee Joint Kinematics: A Finite Element Study. Applied Mathematics. 2013b;4:91–97. doi: 10.4236/am.2013.45A011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIAPOUR AM, QUATMAN CE, DITTO RC, LEVINE JW, WORDEMAN SC, HEWETT TE, GOEL VK, DEMETROPOULOS CK. Global Quasi-Static Mechanical Characterization of the Human Knee Under Single- and Multi-Axis Unconstrained Loading Conditions. Proceedings of 2012 ASME Summer Bioengineering Conference; Fajardo, Puerto Rico: American Society of Mechanical Engineers (ASME); 2012a. [Google Scholar]
- KIAPOUR AM, QUATMAN CE, DITTO RC, LEVINE JW, WORDEMAN SC, HEWETT TE, GOEL VK, DEMETROPOULOS CK. Influence of Axial Rotation Moments on ACL Strain: A Cadaveric Study of Single- and Multi-Axis Loading of the Knee. Proceedings of 37th ASB Annual Meeting; 2012b; Long Beach, CA: American Society of Biomechanics (ASB); [Google Scholar]
- KIAPOUR AM, QUATMAN CE, GOEL VK, DITTO RC, WORDEMAN SC, LEVINE JW, HEWETT TE, DEMETROPOULOS CK. Knee articular cartilage pressure distribution under single-and multi-axis loading conditions: implications for ACL injury mechanism. Proceedings of the 36th ASB Annual Meeting; Geinsville. 2012c. pp. 15–18. [Google Scholar]
- KIAPOUR AM, QUATMAN CE, LEVINE JW, WORDEMAN SC, HEWETT TE, GOEL VK, DEMETROPOULOS CK. Coupled Valgus Collapse Due to Internal Rotation: An Important Factor in the ACL injury Mechanism. Proceedings of 59th ACSM Annual Meeting; San Fransisco, CA: Lippincott Williams & Wilkins; 2012d. [Google Scholar]
- KIAPOUR AM, WORDEMAN SC, PATERNO MV, QUATMAN CE, LEVINE JW, GOEL VK, DEMETROPOULOS CK, HEWETT TE. Diagnostic Value of Knee Arthrometry in the Prediction of ACL Strain during Landing. Am J Sports Med. 2013c:42. doi: 10.1177/0363546513509961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S, BOSQUE J, MEEHAN JP, JAMALI A, MARDER R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- KOGA H, NAKAMAE A, SHIMA Y, IWASA J, MYKLEBUST G, ENGEBRETSEN L, BAHR R, KROSSHAUG T. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38:2218–25. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- KROSSHAUG T, NAKAMAE A, BODEN BP, ENGEBRETSEN L, SMITH G, SLAUTERBECK JR, HEWETT TE, BAHR R. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35:359–67. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- LEVINE JW, KIAPOUR AM, QUATMAN CE, WORDEMAN SC, GOEL VK, HEWETT TE, DEMETROPOULOS CK. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41:385–95. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G, RUDY TW, SAKANE M, KANAMORI A, MA CB, WOO SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- MALONE TR, HARDAKER WT, GARRETT WE, JAF, BASSETT FH. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J South Orthop Assoc. 1993;2:36–39. [Google Scholar]
- MAQUIRRIAIN J, MEGEY PJ. Tennis specific limitations in players with an ACL deficient knee. Br J Sports Med. 2006;40:451–3. doi: 10.1136/bjsm.2005.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOLF KL, GOREK JF, KABO JM, SHAPIRO MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72:557–67. [PubMed] [Google Scholar]
- MARKOLF KL, O’NEILL G, JACKSON SR, MCALLISTER DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32:1144–9. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- MAZZOCCA AD, NISSEN CW, GEARY M, ADAMS DJ. Valgus medial collateral ligament rupture causes concomitant loading and damage of the anterior cruciate ligament. J Knee Surg. 2003;16:148–51. [PubMed] [Google Scholar]
- MEYER EG, HAUT RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41:3377–83. doi: 10.1016/j.jbiomech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- MORAN KA, MARSHALL BM. Effect of fatigue on tibial impact accelerations and knee kinematics in drop jumps. Med Sci Sports Exerc. 2006;38:1836–42. doi: 10.1249/01.mss.0000229567.09661.20. [DOI] [PubMed] [Google Scholar]
- OH YK, LIPPS DB, ASHTON-MILLER JA, WOJTYS EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012;40:574–83. doi: 10.1177/0363546511432544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN OE, MYKLEBUST G, ENGEBRETSEN L, BAHR R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32:1002–12. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- QUATMAN CE, KIAPOUR AM, DEMETROPOULOS CK, KIAPOUR A, WORDEMAN SC, LEVINE JW, GOEL VK, HEWETT TE. Preferential Loading of the ACL Compared With the MCL During Landing: A Novel In Sim Approach Yields the Multiplanar Mechanism of Dynamic Valgus During ACL Injuries. Am J Sports Med. 2013 doi: 10.1177/0363546513506558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUATMAN CE, KIAPOUR AM, KIAPOUR A, LEVINE JW, WORDEMAN SC, DEMETROPOULOS CK, HEWETT TE, GOEL VK. Effects of Quadriceps and Hamstrings Ratio on ACL strain During Landing Activities. Proceedings of 2012 ASME Summer Bioengineering Conference; Fajardo, Puerto Rico: American Society of Mechanical Engineers (ASME); 2012. [Google Scholar]
- QUATMAN CE, QUATMAN-YATES CC, HEWETT TE. A ‘plane’ explanation of anterior cruciate ligament injury mechanisms: a systematic review. Sports Med. 2010;40:729–46. doi: 10.2165/11534950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- RENSTROM P, ARMS SW, STANWYCK TS, JOHNSON RJ, POPE MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14:83–7. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- ROMERO J, DURONIO JF, SOHRABI A, ALEXANDER N, MACWILLIAMS BA, JONES LC, HUNGERFORD DS. Varus and valgus flexion laxity of total knee alignment methods in loaded cadaveric knees. Clin Orthop Relat Res. 2002:243–53. doi: 10.1097/00003086-200201000-00029. [DOI] [PubMed] [Google Scholar]
- SHIN CS, CHAUDHARI AM, ANDRIACCHI TP. The effect of isolated valgus moments on ACL strain during single-leg landing: a simulation study. J Biomech. 2009;42:280–5. doi: 10.1016/j.jbiomech.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN LENT ME, DROST MR, WILDENBERG VDFA. EMG profiles of ACL-deficient patients during walking: the influence of mild fatigue. Int J Sports Med. 1994;15:508–14. doi: 10.1055/s-2007-1021096. [DOI] [PubMed] [Google Scholar]
- WALL SJ, ROSE DM, SUTTER EG, BELKOFF SM, BODEN BP. The role of axial compressive and quadriceps forces in noncontact anterior cruciate ligament injury: a cadaveric study. Am J Sports Med. 2012;40:568–73. doi: 10.1177/0363546511430204. [DOI] [PubMed] [Google Scholar]
- WITHROW TJ, HUSTON LJ, WOJTYS EM, ASHTON-MILLER JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21:977–83. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- WITHROW TJ, HUSTON LJ, WOJTYS EM, ASHTON-MILLER JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90:815–23. doi: 10.2106/JBJS.F.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLTRING HJ, HUISKES R, DE LANGE A, VELDPAUS FE. Finite centroid and helical axis estimation from noisy landmark measurements in the study of human joint kinematics. J Biomech. 1985;18:379–89. doi: 10.1016/0021-9290(85)90293-3. [DOI] [PubMed] [Google Scholar]
- WU JL. In-Situ Forces in the Anteromedial and Posterolateral Bundles of the Anterior Cruciate Ligament under Simulated Functional Load ing Conditions. ORS Conf. 2010;35:1995. doi: 10.1177/0363546509350110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEOW CH, NG KS, CHEONG CH, LEE PV, GOH JC. Repeated application of incremental landing impact loads to intact knee joints induces anterior cruciate ligament failure and tibiofemoral cartilage deformation and damage: A preliminary cadaveric investigation. J Biomech. 2009;42:972–81. doi: 10.1016/j.jbiomech.2009.03.026. [DOI] [PubMed] [Google Scholar]
- ZANTOP T, HERBORT M, RASCHKE MJ, FU FH, PETERSEN W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35:223–7. doi: 10.1177/0363546506294571. [DOI] [PubMed] [Google Scholar]