Abstract
Imatinib and other BCR-ABL1 inhibitors are effective therapies for chronic myeloid leukemia (CML), but these inhibitors target additional kinases including KIT, raising the question of whether off-target effects contribute to clinical efficacy. Based on its involvement in CML pathogenesis, we hypothesized that KIT may govern responses of CML cells to imatinib. To test this, we assessed the growth of primary CML progenitor cells under conditions of sole BCR-ABL1, sole KIT and dual BCR-ABL1/KIT inhibition. Sole BCR-ABL1 inhibition suppressed mature CML progenitor cells, but these effects were largely abolished by stem cell factor (SCF) and maximal suppression required dual BCR-ABL1/KIT inhibition. In contrast, KIT inhibition did not add to the effects of BCR-ABL1 inhibition in primitive progenitors, represented by CD34+38− cells. Long term culture-initiating cell (LTC-IC) assays on murine stroma revealed profound depletion of primitive CML cells by sole BCR-ABL1 inhibition despite the presence of SCF, suggesting primitive CML cells are unable to use SCF as a survival factor upon BCR-ABL1 inhibition. In CD34+38+ cells, SCF strongly induced pAKTS473 in a phosphatidylinositol 3′ kinase (PI3K)-dependent manner, which was further enhanced by inhibition of BCR-ABL1 and associated with increased colony survival. In contrast, pAKTS473 levels remained low in CD34+38− cells cultured under the same conditions. Consistent with reduced response to SCF, KIT surface expression was significantly lower on CD34+38− compared to CD34+38+ CML cells, suggesting a possible mechanism for the differential effects of SCF on mature and primitive CML progenitor cells.
Introduction
The BCR-ABL1 tyrosine kinase inhibitor (TKI), imatinib, induces profound responses in most patients with newly diagnosed chronic phase chronic myeloid leukemia (CML-CP) (1). Imatinib inhibition of BCR-ABL1 correlates with response, and reactivation of BCR-ABL1 signaling by kinase point mutations with relapse (2). In addition to BCR-ABL1, imatinib targets the tyrosine kinases ABL1, KIT, ARG (ABL2), DDR1/2, PDGFR, CSF-1R, and LCK (2–4). In contrast to BCR-ABL1, we detected no mutations in KIT or PDGFR in patients with imatinib resistance (5).
Imatinib’s capacity to inhibit non-BCR-ABL1 targets has expanded its utility to malignancies driven by mutations of KIT or PDGFR (6, 7), but inhibition of physiological kinase signaling within normal cells may be the cause of side effects such as anemia (8), myelosuppression (9) and fluid retention (10). It is largely unknown whether co-inhibition of non-BCR-ABL1 targets within CML cells has therapeutic benefits.
KIT has been implicated in CML pathogenesis. BCR-ABL1 expressing progenitors were shown to be hypersensitive to stem cell factor (SCF) due to BCR-ABL1-induced upregulation of its receptor, KIT, (11, 12) (11, 12) (11, 12) (11, 12) (11, 12) (11, 12) (11, 12) and SCF was reported to support growth of cytokine-dependent CML but not normal progenitors (13). Furthermore, culture of CML stem and progenitor cells on SCF-deficient stroma favors normal progenitors, suggesting CML progenitors may be more SCF responsive than their normal counterparts (14). Accordingly, KIT-expressing BCR-ABL1-transduced murine myeloid cells were less sensitive to sole inhibition of either BCR-ABL1 or KIT compared to simultaneous inhibition of both kinases (15). In primary CML CD34+ cells, SCF reduced apoptosis in response to nilotinib (16), but it is unknown which specific pathways are activated by SCF to confer relative TKI resistance, and whether the requirement for KIT inhibition extends to more primitive CML cells. We sought to determine the contribution of KIT inhibition to the effects of TKIs on CML cells at various differentiation stages. We find that dual inhibition of BCR-ABL1 and KIT is required for suppression of mature but not primitive CML progenitors. This differential effect is due to the inability of primitive CML cells to activate AKT in response to SCF upon inhibition of BCR-ABL1.
Materials And Methods
Patient samples
Bone marrow or leukapheresis was obtained from newly diagnosed CML-CP patients. All patients provided informed consent to research protocols approved by the Institutional Review Boards of the participating institutions. Normal bone marrow mononuclear cells (MNC) were from All Cells (Emeryville, CA). Cell selection was as described (17) (details in Supplementary Methods).
Inhibition of BCR-ABL1, KIT, mitogen-activated ERK kinase (MEK) and phosphatidyl inositol 3′ kinase (PI3K)
Sole BCR-ABL1 inhibition was achieved with PPY-A (a gift of ARIAD Pharmaceuticals, Boston, MA) (18). Sole KIT inhibition was achieved by three methods: (a) use of a SCF-blocking antibody K44.2 (SCF-block, Sigma Aldrich, St. Louis, MO), a human-specific antibody that binds extracellularly to KIT and prevents SCF-induced dimerization; (b) BAW667, a small molecule that targets KIT but not BCR-ABL1, the chemical structure of which is still proprietary. The activity profile of BAW667 was determined as previously described (19, 20) and is provided in Supplementary Table 1. Requests to obtain BAW667 should be directed to Paul Manley, Novartis; (c) downregulation of KIT using a lentivirally delivered shRNA construct. Dual BCR-ABL1/KIT inhibition was achieved with imatinib or a combination of PPY-A+SCF-block, BAW667 or KIT shRNA. MEK inhibition was achieved with PD98059 (Cell Signaling Technology, Beverly, MA) and PI3K inhibition with LY294002 (Cell Signaling Technology). For details on vector construction, see Supplementary Methods.
Immunoblot analysis of cell lines and patient samples
Analysis of primary cells and cell lines was as previously published (17, 21). For details and a list of antibodies see Supplementary Methods.
Colony assays
Hematopoietic colony forming assays were performed as described (17). For details see Supplementary Methods.
Long-term culture-initiating cell (LTC-IC) assays
Murine M210B4 stromal cells plated at 5 × 104 cells/well in a 24-well format were irradiated with 40 Gy. 104 Lin-depleted CML cells were cultured in triplicate wells in LTC-IC media (IMDM, horse serum, FBS, hydrocortisone) in the following conditions: untreated, 2 μM imatinib, 1 μM PPY-A, 200 ng/mL SCF-block, 1 μM PPY-A+200 ng/mL SCF-block. The medium was supplemented with 5 ng/mL human SCF. Half medium changes were performed weekly and included inhibitors at the relevant concentrations. At intervals of one, three and six weeks, cells were detached with trypsin, plated in cytokine-supplemented methylcellulose (Stem Cell Technologies) and colony-forming cells (CFC) were analyzed at 2 weeks. CFC frequencies for all patients are reported relative to starting cell number. Individual colonies (10 per condition or as many as available) were genotyped by FISH using a dual color/dual fusion BCR-ABL1 LSI probe (Abbott Molecular) (17). Ph+ CFC relative to untreated are reported.
Cell proliferation assays
Viable cells were quantified using MTS as described (21). For details see Supplementary Methods.
Flow cytometric analysis of KIT expression
CD34+ cells were labeled with CD117/KIT-PerCP-Cy5.5, Lin-FITC, CD34-APC, CD38-PE antibodies (BD Biosciences, San Jose, CA) and mean fluorescence intensity of CD117 in Lin−CD34+38+ and Lin−CD34+38− cells were measured.
Immunofluorescence
FACS-sorted CD34+ or CD34+38+ and CD34+38− cells were incubated with or without PPY-A (1 μM) for 2 hours, followed by SCF (25 ng/mL) for 30 minutes. Cells were cytospun onto glass slides, fixed with paraformaldehyde (4% in PBS), permeabilized with methanol, and blocked in 2% BSA at 4°C for 18 hours. Slides were incubated with pAKTS473 antibody (Cell Signaling) or pERK1/2Y202/204 antibody (Cell Signaling) overnight, washed twice for 5 minutes with PBS, stained with an AlexaFluor-488 antibody (Invitrogen) for 2 hours, washed 3X with PBS, stained with Hoechst stain, covered with ProLong Gold anti-fade reagent (Invitrogen) and coverslip, and allowed to cure overnight. Fluorescent images were then taken with a Nikon Eclipse E600 microscope using a CRI NUANCE multispectral imaging system with identical exposure time for each slide. Images were compiled using IP Lab and Adobe Photoshop software.
Quantitative RT-PCR for SCF
Details are described in Supplementary Methods.
Results
Imatinib inhibits both BCR-ABL1 and KIT, PPY-A inhibits BCR-ABL1 but not KIT, and BAW667 inhibits KIT but not BCR-ABL1
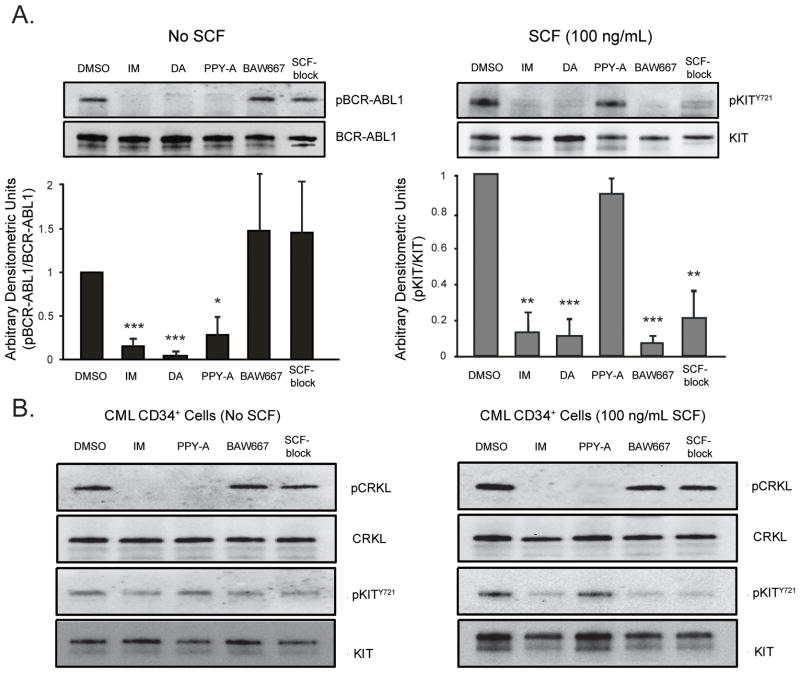
We initially determined the specificity of imatinib, dasatinib, PPY-A (22), BAW667 (a compound with activity against KIT, but not BCR-ABL1) and a SCF-blocking antibody (SCF-block). SCF-stimulated Mo7e or Mo7ep210BCR-ABL1 cells were treated with inhibitors at concentrations reported to effectively inhibit BCR-ABL1 (22, 23) (if applicable) and cell lysates were immunoblotted for pKITY721 or pBCR-ABL1. SCF-block was titrated against KIT to determine an appropriate working concentration (not shown). Imatinib (2 μM) and dasatinib (50 nM) inhibited both BCR-ABL1 and KIT, while PPY-A (1 μM) only inhibited pBCR-ABL1 and BAW667 (1 μM) only inhibited pKITY721 (Fig. 1A). No KIT inhibition was seen with 10 μM PPY-A, while 10 μM BAW667 slightly reduced pBCR-ABL1 (Supplementary Fig. 1). We concluded that PPY-A and BAW667 at 1 μM selectively inhibit BCR-ABL1 or KIT, respectively. SCF-block at 200ng/mL suppressed KIT phosphorylation without affecting BCR-ABL1 activity. Similar results were obtained in CD34+ CML cells, using CRKL as a marker for BCR-ABL1 activity (Fig. 1B). KIT was phosphorylated in CML CD34+ cells in the absence of SCF and this phosphorylation was reduced by imatinib or BAW667, but not PPY-A, suggesting that some KIT activation occurs without SCF, independent of BCR-ABL1 kinase activity. The band corresponding to pKITY721 was not completely suppressed in CML CD34+ cells under any conditions, including imatinib and BAW667 treatment, suggesting that a kinase other than BCR-ABL1 or KIT may maintain a low level of KIT phosphorylation in primary CML cells.
Figure 1. BCR-ABL1 and KIT inhibitor profile.
(A) Mo7ep210BCR-ABL1 or Mo7e cells stimulated with SCF were treated overnight with 2 μM imatinib (IM), 50 nM dasatinib (das), 1 μM BAW667, 1 μM PPY-A or 200 ng/mL SCF blocking antibody (SCF-block). Lysates were immunoblotted for phosphotyrosine or pKITY721 respectively. Total BCR-ABL1 and total KIT are shown as loading controls. Mean relative signal intensity in the presence of inhibiting agents, determined by densitometric quantitation of band intensity, is shown for n=3 replicates. Error bars represent SEM; *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). (B) CD34+ cells from newly diagnosed chronic phase CML patients (n=3) were treated for 4 hours with 2 μM IM, 1 μM BAW667, 1 μM PPY-A, or 200 ng/mL SCF-block ± SCF stimulation as indicated. Lysates were immunoblotted for pCRKL or pKITY721. Total CRKL and total KIT are shown as loading controls. One representative experiment is shown. Dasatinib was not tested due to cell number limitations.
Dual inhibition of BCR-ABL1 and KIT is required for maximal suppression of CFU-GM colony formation by CML CD34+ cells
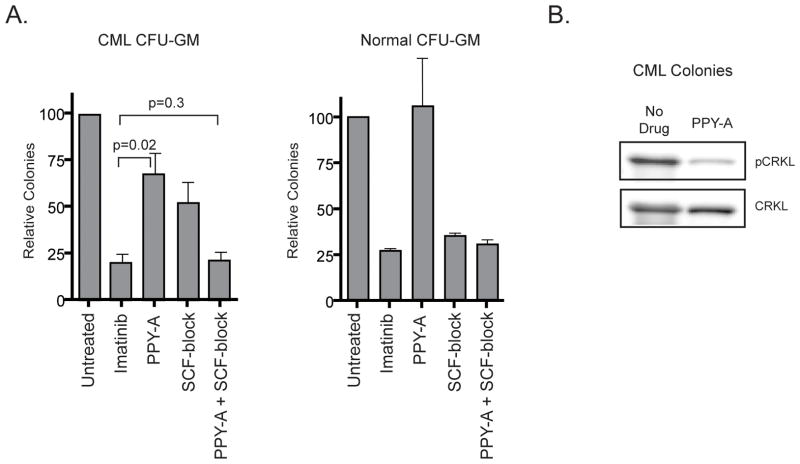
We initially compared CFU-GM colony formation upon sole BCR-ABL1 inhibition (PPY-A), sole KIT inhibition (SCF-block) or dual BCR-ABL1/KIT inhibition (imatinib or PPY-A+SCF-block). Cells were plated in IL-3, GM-CSF and SCF. Unlike imatinib, which reduced colonies by ~80%, PPY-A suppressed CFU-GM colony formation by only ~30% and SCF-block by ~50% (Fig. 2A). Dual BCR-ABL1 and KIT inhibition by PPY-A and SCF-block, however, reduced colony numbers by ~80%, suggesting that both BCR-ABL1 and SCF/KIT contribute independently to colony growth. Normal CFU-GM colony formation was unaffected by sole BCR-ABL1 inhibition (PPY-A), but suppressed by imatinib or SCF-block, consistent with dependence on KIT signaling. The lack of efficacy of PPY-A was not due to drug instability, since pCRKL was inhibited in PPY-A-treated colonies harvested following the culture period (Fig. 2B). We also tested inhibitor effects on BFU-E colony formation. PPY-A had no effect, while sole SCF-block reduced BFU-E colony numbers to those observed with imatinib (Supplementary Fig. 2A). Thus, CML erythroid colony growth is independent of BCR-ABL1 and its suppression by imatinib is due entirely to KIT inhibition. Responses of normal BFU-E were identical, confirming that growth inhibition was cell-type rather than CML-specific. The insensitivity of CML BFU-E to PPY-A is not due to autocrine SCF production, since SCF is not expressed by CML CD34+ cells and not induced by PPY-A (Supplementary Fig. 2B).
Figure 2. Suppression of CML and normal CFU-GM colony formation by PPY-A, imatinib, SCF-block or PPY-A+SCF-block.
(A) CFU-GM were assessed in samples from newly diagnosed chronic phase CML patient cells (left) or normal mononuclear cells (right) cultured for 14 days in semisolid medium containing IL-3, GM-CSF and SCF. Imatinib, PPY-A, SCF-block or PPY-A+SCF-block were added as indicated. Mean colony number of triplicate plates is shown normalized relative to untreated for n=4 samples. Error bars represent SEM. (B) CFU-GM colonies from untreated and PPY-A-treated plates were pooled and lysates were immunoblotted for pCRKL to assess BCR-ABL1 activity (n=3). Total CRKL is shown as a loading control. A representative immunoblot is shown.
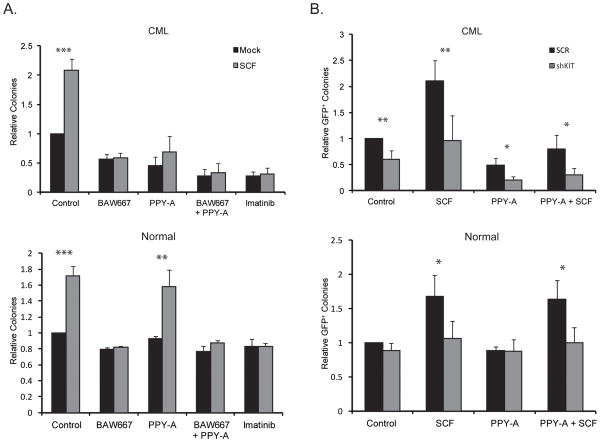
In a second independent series of experiments we included BAW667 and shKIT as alternative means of suppressing KIT activity. CD34+ cells from CML patients or normal controls were plated with or without SCF and BAW667, PPY-A, BAW667+PPY-A, or imatinib were added (Fig. 3A). SCF increased CML and normal CFU-GM colonies by ~2.1-fold and ~1.7-fold, respectively. BAW667 abrogated the colony increase imparted by SCF in CML and normal cells. Additionally, BAW667 reduced CML colony formation in the absence of SCF by ~50%, while effects on normal colonies were minimal, suggesting that KIT is constitutively active in CML but not normal progenitor cells and contributes to their growth. PPY-A inhibited colony formation by CML progenitor cells, and this was partially rescued by SCF, but had no effect on normal progenitor cells. Combination of PPY-A and BAW667 had effects similar to imatinib. To specifically inhibit KIT without concerns about possible off-target effects of biochemical inhibitors, we used a lentiviral vector for simultaneous expression of shKIT and GFP in human cells (Supplementary Fig. 3). Without SCF, shKIT had little effect on normal cells, but reduced colony formation of CML CD34+ cells by ~45% (Fig. 3B), similar to BAW667 alone (Fig. 3A). shKIT also abrogated the increase in colony formation caused by SCF in both normal and CML CD34+ cells. Lastly, combining shKIT and PPY-A had similar effects as PPY-A+BAW667 or imatinib. Altogether these data demonstrate that CML CD34+ progenitor cells are slightly more responsive to SCF than normal CD34+ cells and that KIT is intrinsically active in CML but not normal cells. As a result, KIT inhibition differentiates between normal and CML CD34+ progenitor cells in the presence and absence of SCF, and this differential is further increased by inhibition of BCR-ABL1.
Figure 3. Effects of genetic or biochemical KIT inhibition on PPY-A sensitivity in normal and CML CD34+ cells.
(A) CD34+ cells from newly diagnosed CML-CP patients (top, n=4) or healthy controls (bottom, n=4) were cultured for 14 days in semisolid medium containing IL-3 and GM-CSF ± SCF. BAW667, PPY-A, BAW667+PPY-A or imatinib were added as indicated. CFU-GM colonies were scored on day 14. Untreated controls (without SCF) were set to 1. Error bars represent SEM; *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). (B) CD34+ cells from the same patients (top) and controls (bottom) as in (A) were infected with lentivirus for simultaneous expression of GFP and either shKIT or shSCR. Cells were plated in semisolid medium containing IL-3 and GM-CSF. PPY-A, SCF or both were added as indicated. GFP-positive colonies were scored after 14 days. shSCR controls cultured with IL-3 and GM-SCF only were set to 1. Error bars represent SEM; *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test).
For additional validation, we analyzed CML CFU-GM colony growth following removal of the individual cytokines SCF, GM-CSF or IL-3. We found that removal of SCF had the most pronounced effect; combining SCF removal with PPY-A had effects comparable to imatinib, suggesting that the differential sensitivity of CML CFU-GM to imatinib and PPY-A is due exclusively to their differential effects on KIT (Supplementary Fig. 4A,B). It should be noted that some colony growth is due to IL-3 or GM-CSF, evidenced by an ~15% reduction of colony growth upon removal of IL-3 or GM-CSF in the presence of PPY-A (Supplementary Fig. 4A,B). Removal of individual cytokines did not enhance PPY-A effects against BFU-E colony formation (Supplementary Fig. 4C), unsurprising given our finding that these cells are dependent on KIT but not BCR-ABL1.
Dual inhibition of BCR-ABL1 and KIT is required to suppress CML progenitor cell growth, while sole BCR-ABL1 inhibition is sufficient to suppress CML stem cell growth
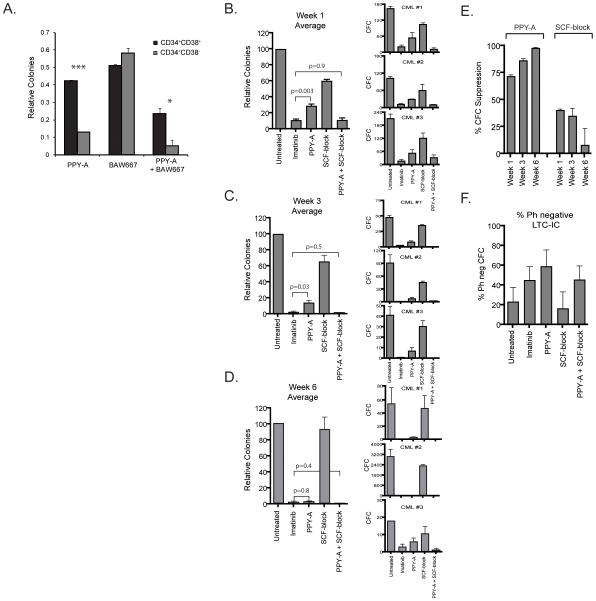
Since BCR-ABL1 and KIT signaling both contribute to survival of CML progenitor cells, we determined whether this was also the case for more primitive CML cells, using immunophenotype or functional capacity to distinguish between mature and primitive CML progenitor cells. Lin−CD34+38+ (representing relatively mature progenitor cells) and Lin−CD34+38− cells (representing a primitive population, including stem cells) (17) from newly diagnosed CML-CP patients were plated in semisolid medium supplemented with 1 μM PPY-A, 1 μM BAW667 or a combination thereof (Fig. 4A). KIT inhibition with BAW667 reduced colony formation by ~49% in progenitor cells and ~42% in primitive cells, respectively. In contrast, isolated BCR-ABL1 inhibition (PPY-A) had a more significant effect on primitive cells (~87% reduction) than on mature progenitor cells (~58% reduction). Combining KIT and BCR-ABL1 inhibition increased inhibition of progenitor cells by 27% to ~76%, while inhibition of primitive cells was only mildly increased by about 8% to ~95%.
Figure 4. Suppression of mature vs. primitive CML progenitors and stem cells by sole inhibition of BCR-ABL1, sole inhibition of KIT or combined inhibition of KIT and BCR-ABL1.
(A) Lin−CD34+38+ and Lin−CD34+38− cells were isolated from viably frozen MNC from CML-CP patients (n=3) and plated in semisolid media containing IL-3 and GM-CSF with or without 1 μM PPY-A, 1 μM BAW667 or their combination. CFU-GM colonies were counted after 14 days. Untreated controls were set to 1. Error bars represent SEM. *p<0.05; ***p<0.001 (Student’s t-test). (B–E) A separate series of experiments was performed on CD34+ cells from newly diagnosed CML patients (n=3). Cells were cultured in LTC-IC assays on murine stromal cells (M2-10B4) in the presence of 2 μM imatinib, 1 μM PPY-A, 200 ng/mL SCF-block or PPY-A+SCF-block for a total duration of six weeks. At (B) 1 week, (C) 3 weeks and (D) 6 weeks, triplicate cultures were assessed for CFC growth. Total CFC derived from 10,000 input cells are shown for each sample as well as normalized mean values for each time point. Error bars represent standard error of the mean (SEM). Differences in LTC-IC numbers were evaluated by Student’s t-test. The frequency of Ph+ colonies was determined by FISH analysis of individual colonies from all treatment conditions. Data presented in parts B–C include only Ph+ colonies. (E) Suppression of mature and primitive CML cell outgrowth in the presence of sole BCR-ABL1 or sole KIT inhibition was compared for the three LTC-IC assays combined. (F) Frequency of normal (Ph−) colonies in 6-week CML LTC-IC. Mean values for the three samples are shown. Error bars represent SEM.
We also plated CML progenitors on murine stroma for 1, 3 or 6 weeks in the presence of PPY-A, SCF-block or both. Although precise assignment of these populations to a specific differentiation stage is difficult, the progressively longer duration of culture permits expansion and maturation of increasingly primitive cells that are quantified by CFC assays as a composite readout for survival, expansion and maturation (24). Resulting colonies were genotyped for BCR-ABL1 by FISH. Neither sole BCR-ABL1 (PPY-A) nor sole inhibition of KIT (SCF-block) achieved the CFC suppression seen with dual inhibition by imatinib or PPY-A+SCF-block in week 1 and week 3 colonies (Fig. 4B,C). In 6-week LTC-IC, representative of primitive CML progenitors and stem cells, sole BCR-ABL1 inhibition achieved >95% suppression of Ph+ CFC (Fig. 4D) and was comparable to imatinib (p=0.8), while minimal growth suppression of 6-week LTC-IC was observed with SCF-block (Fig. 4E). Dependence on BCR-ABL1 and KIT progressively increased or decreased, respectively, with duration of growth on murine stroma (Fig. 4E). FISH revealed a mix of CML and LTC-IC, typical of early CML-CP (25). Although growth suppression was variable between patients and in some instances the absolute number of surviving colonies was small, Ph+ colonies were observed in all treatment conditions. Either imatinib or PPY-A, but not SCF-block increased the proportion of Ph− 6-week LTC-IC relative to untreated (Fig. 4F). Altogether these data suggest that primitive cells are less dependent on KIT and more dependent on BCR-ABL1 and that the capacity of KIT signaling to rescue CML cells in the presence of sole BCR-ABL1 inhibition is largely restricted to mature CML progenitors.
Differences in sensitivity to KIT inhibition may depend on KIT expression
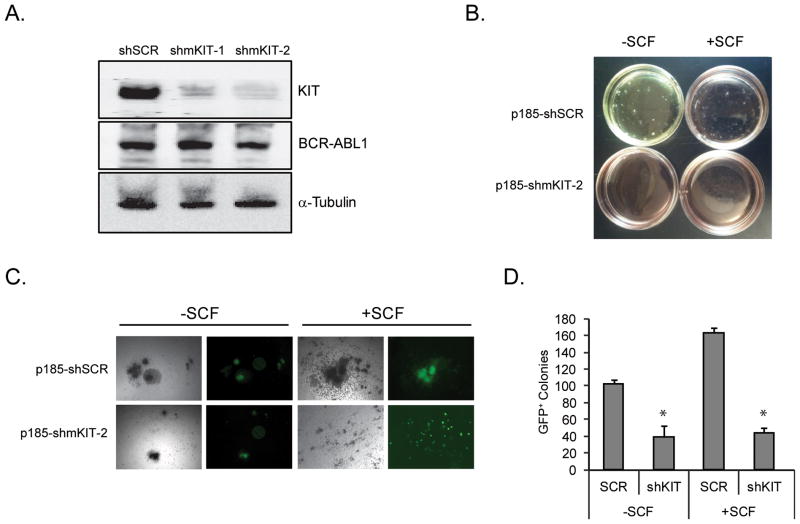
KIT has been implicated in CML pathogenesis, but it is not precisely known how KIT and BCR-ABL1 signaling interact and whether any such interaction may depend on the differentiation stage of the cells (26). Figs. 1B and 3A,B show that KIT is constitutively active in CML CD34+ cells in the absence of SCF, and that inhibition of KIT activity alone reduces colony growth, demonstrating that KIT contributes to CML progenitor cell growth independently of SCF. To confirm this, we infected bone marrow from 5-FU treated mice with retrovirus for simultaneous expression of BCR-ABL1, GFP and shKIT or scrambled shRNA. Equal numbers of GFP+ cells were plated in colony assays with or without SCF. KIT shRNA significantly reduced colony formation in the absence and presence of SCF, confirming that KIT is involved in BCR-ABL1 transformation irrespective of receptor engagement by ligand (Fig. 5A–D).
Figure 5. Effects of KIT knockdown on colony formation by murine bone marrow cells in the presence and absence of SCF.
(A) Murine bone marrow MNCs were infected with MIG-p185-SCR, or MIG-p185-shmKIT-2 lentivirus. GFP+ cells were sorted and p185-BCR-ABL1 and KIT expression were detected by immunoblot. (B–D) BM from 5-FU treated mice was infected with MIG-p185-SCR or MIG-p185-shmKIT-2 lentivirus and colony assays were set up in the presence or absence of 50 ng/ml rmSCF without any other cytokines. After 15 days plates were (B) photographed, (C) GFP-positive colonies were identified and counted under a fluorescence microscope and (D) colony numbers were compared between conditions. The experiment was performed twice, with similar results. Error bars represent SEM. *p<0.05 (Student’s t-test).
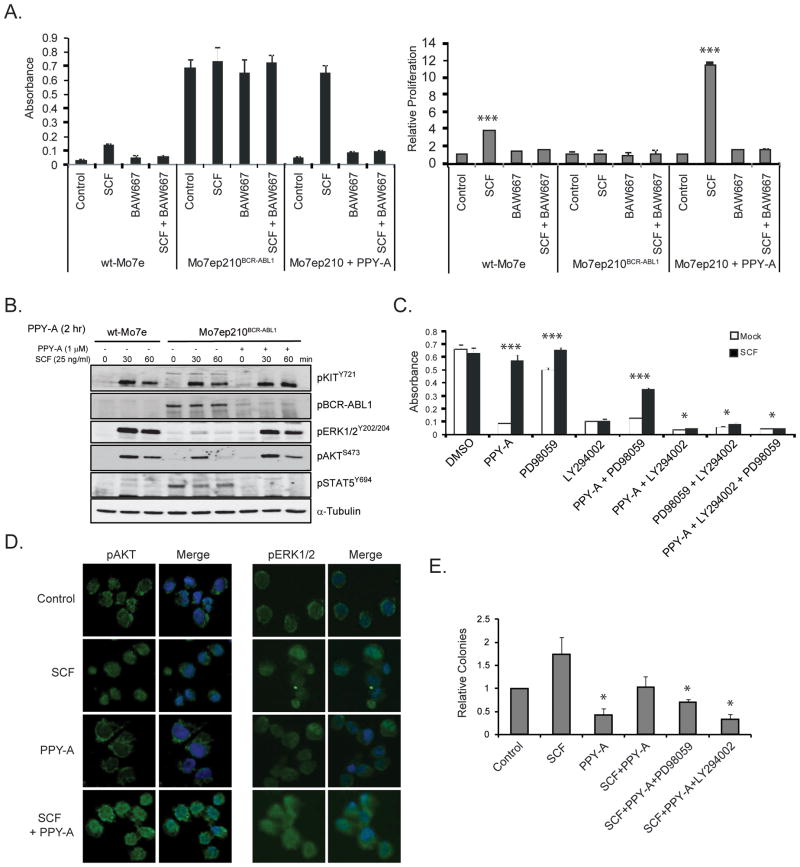
To further characterize interactions of KIT and BCR-ABL1 signaling we used Mo7ep210BCR-ABL1 cells. SCF rescued Mo7ep210BCR-ABL1 cells treated with PPY-A (Fig. 6A), similar to colony assays of CML CD34+ cells (Fig. 3A). Since KIT signals through several canonical pathways including PI3K and MEK (reviewed in (27)), we tested how BCR-ABL1 and KIT signaling influence activation of these pathways. Mo7ep210BCR-ABL1 cells were treated with SCF ± prior PPY-A inhibition of BCR-ABL1 signaling. In the presence of active BCR-ABL1, KIT activation of AKT (downstream of PI3K) and ERK1/2 (downstream of MEK) was weak and short-lived. In contrast, strong and sustained activation of AKT and ERK1/2 occurred when BCR-ABL1 was inhibited (Fig. 6B). Activation of AKT was associated with a reduction of Foxo3A (Supplementary Fig. 5). Co-treatment with a PI3K inhibitor (LY294002) completely and co-treatment with a MEK inhibitor (PD98059) partially inhibited SCF-mediated proliferation, suggesting the SCF signal is transmitted mainly via PI3K/AKT and enhanced by BCR-ABL1 inhibition (Fig. 6C). To validate this finding in primary CML cells, we performed immunofluorescence for pAKTS473 and pERK1/2 Y202/204 on CD34+ cells treated with SCF ± prior PPY-A treatment. PPY-A alone or SCF alone had moderate impact on pAKTS473 and pERK1/2Y202/204 (Fig. 6D). However, both were strongly induced by simultaneous treatment with PPY-A and SCF, confirming the results in Mo7ep210BCR-ABL1 cells (Fig. 6B). Inhibition of PI3K (50 μM LY294002) completely and inhibition of MEK (20 μM PD98059) partially rescued the SCF effect (Fig. 6E), validating the data on Mo7ep210BCR-ABL1 cells (Fig. 6C). Altogether these results show that SCF rescues CML CD34+ progenitor cells upon inhibition of BCR-ABL1, mainly via PI3K/AKT, which explains why dual inhibition of KIT and BCR-ABL1 is required to effectively target these cells.
Figure 6. Effects of SCF in combination with inhibition of BCR-ABL1, KIT, PI3K and/or MEK on Mo7ep210BCR-ABL1, Mo7e cells and primary CD34+ CML-CP cells.
(A) Mo7ep210BCR-ABL1 and Mo7e cells were treated with 25 ng/ml SCF, 1 μM PPY-A, 1 μM BAW667 or their combination(s) as indicated. Viable cell numbers were measured after 72 hours. Results at 24 hours and 48 hours were comparable (not shown). Error bars represent SEM. ***p<0.001 (Student’s t-test). (B) Total cellular lysates were harvested after 30 and 60 minutes and subjected to immunoblot analysis for pKITY721, pABLY402, pERK1/2Y202/204, pAKTS473, pSTAT5Y694 and α-Tubulin (loading control). (C) Mo7ep210BCR-ABL1 cells were treated with PPY-A in combination with 20 μM PD98059 (MEK inhibitor) or 20 μM LY294002 (PI3K inhibitor), in the presence or absence of 25 ng/mL SCF. Viable cells were measured by MTS assay at 72 hours. Error bars represent SEM. *p<0.05; ***p<0.001 (Student’s t-test). (D, E) CD34+ cells from newly diagnosed CML-CP patients (n=3) were incubated with or without PPY-A (1 3M) for 2 hours, followed by SCF (25 ng/mL) for 30 minutes. Aliquots of cells were analyzed for pAKTS473 and pERK1/2Y202/204 by immunofluorescence (D) or cultured in semisolid medium, using identical conditions, with CFU-GM colonies assessed after 15 days (E). Error bars represent SEM.*p<0.05 (Student’s t-test).
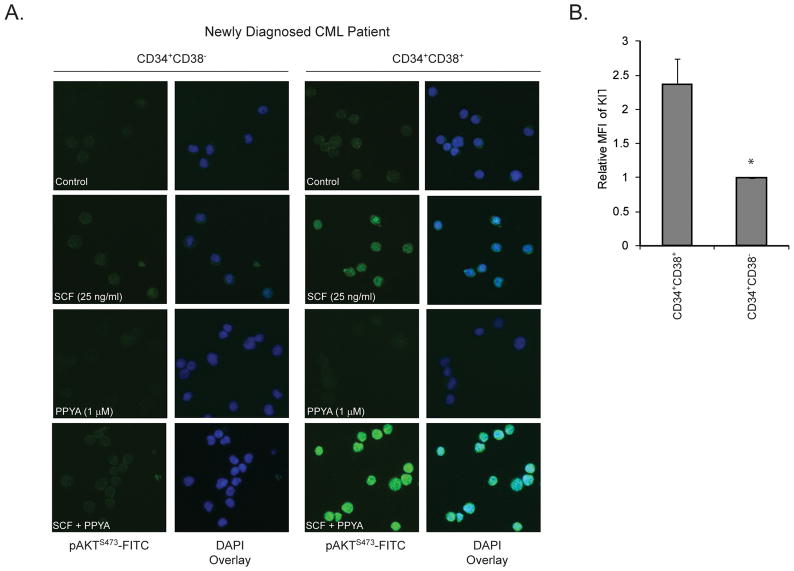
We next examined possible differences in signaling response to SCF between mature (CD34+38+) and primitive (CD34+38−) progenitor cells. CD34+ cells were cultured overnight in BIT medium without cytokines and sorted, treated with SCF, PPY-A or both, and then analyzed by immunofluorescence for pAKTS473 (Fig. 7A). pAKTS473 was lower in CD34+38− cells than CD34+38+ cells, as previously reported(17). PPY-A alone minimally reduced pAKTS473 in CD34+38+ cells. Strikingly, SCF strongly induced pAKTS473 in PPY-A-treated CD34+38+ cells, analogous to Mo7ep210BCR-ABL1 cells (Fig. 6B), but had little effect in CD34+38− cells, suggesting that upon BCR-ABL1 inhibition CD34+38− CML cells fail to launch a robust pAKTS473 response to SCF stimulation. To identify the underlying mechanism we measured CD117 (KIT) expression on Lin−CD34+38+ vs. Lin−CD34+38− cells and found significantly lower expression in the primitive Lin−CD34+38− cells, which may explain their decreased response to SCF and greater vulnerability to sole BCR-ABL1 inhibition (Fig. 7B).
Figure 7. Assessment of SCF-induced pAKTS473 and CD117 expression in mature and primitive CML progenitor cells.
(A) Lin−CD34+38+ and Lin−CD34+38− cells from newly diagnosed CML-CP patients (n=3) were simulated with 25 ng/mL SCF with or without prior treatment with 1 μM PPY-A, followed by immunofluorescence to detect pAKTS473. (B) Cryopreserved column-selected CD34+ cells from newly diagnosed CML-CP patients (n=4) were labeled with CD117-PerCP-Cy5.5, Lin-FITC, CD34-APC and CD38-PE antibodies and mean fluorescence intensity of CD117 measured. Error bars represent SEM. *p<0.05 (Student’s t-test).
Discussion
Previous studies have implicated KIT in CML pathogenesis, suggesting the efficacy of TKIs such as imatinib may be due to their combined activity against BCR-ABL1 and KIT (13, 15, 28). For example, KIT+ BCR-ABL1-transduced murine progenitor cells are more sensitive to dual inhibition of KIT and BCR-ABL1 than to inhibition of either kinase individually (15). In another study SCF rescued CML CD34+ cells from nilotinib but not imatinib effects and ascribed the difference to nilotinib’s relatively weaker anti-KIT activity (16). However, it remained unknown which specific pathways are activated by SCF to confer relative TKI resistance and whether the requirement for KIT inhibition depends on the stage of differentiation. Here we use TKIs, shRNA and blocking antibodies to examine whether the complimentary activity of imatinib against both BCR-ABL1 and KIT contributes to its efficacy in mature and primitive primary CML cells.
Sole BCR-ABL1 inhibition with PPY-A modestly suppressed CML CFU-GM colony formation in the presence of cytokines, while SCF-block caused a slightly more pronounced reduction (Fig. 2A). This reduction was not exclusively dependent on active SCF signaling, as SCF-block (Fig. 2A) or removal of SCF (Supplementary Fig. 4A) had quantitatively similar effects to BAW667 (Fig. 3A) or shKIT knockdown in SCF-free cultures (Fig. 3B). These data show that KIT contributes to the proliferation of mature CML progenitors in the absence of ligand, in accord with previous reports in BCR-ABL1 expressing cell lines (26). Consistent with this, we detected low levels of pKITY721 in serum-starved CML CD34+ cells that was reduced by imatinib and BAW667 (Fig. 1B). The effects of BAW667 inhibition of KIT were maximal in cultures of CML CD34+ cells that were supplemented with SCF (Fig. 3A, left panel: compare dark bars in control vs. BAW667). Expression of BCR-ABL1 with or without simultaneous KIT knockdown in murine bone marrow (Fig. 5) reproduced the data on primary human CML cells, indicating that both SCF-induced and SCF-independent KIT activation contribute to CML progenitor cell growth. Surprisingly, shKIT significantly enhanced the effects of PPY-A on CML CD34+ colony formation in the absence of SCF (Fig 3B, upper panel). This could reflect persistence of a low level of BCR-ABL1 kinase activity not detected by pCRKL immunoblots (29) or constitutive KIT activation that is independent of BCR-ABL1 kinase activity. Additionally, the band corresponding to pKITY721 was not completely abolished by BAW667 or imatinib (Fig. 1B), suggesting that a kinase other than BCR-ABL1 or KIT may phosphorylate KIT on tyrosine 721, although this residue is generally regarded as an autophosphorylation site. For example, SRC family kinases have been shown to phosphorylate tyrosine 900 of KIT (30). Additional studies will be required to distinguish between these two possibilities.
In contrast to the limited effects of targeting either BCR-ABL1 or KIT in isolation, simultaneous inhibition of both kinases dramatically reduced CML CFU-GM growth. Disruption of KIT signaling was achieved with four independent approaches (BAW667; SCF-block; SCF removal; KIT knockdown), all of which produced similar effects when combined with the BCR-ABL1 inhibitor PPY-A. Dual dependence of primary CML progenitor cells on BCR-ABL1 and KIT signaling is restricted to granulocyte precursors. Although erythroid progenitors express both BCR-ABL1 and KIT (31), erythroid colony formation was maximally suppressed by inhibition of KIT alone and independent of BCR-ABL1 activity, identical to normal BFU-E (Supplementary Fig. 2A). Thus, imatinib suppression of leukemic BFU-E is due entirely to KIT inhibition and BCR-ABL1 expression in erythroid lineage cells is not synonymous with dependence on BCR-ABL1 (32). Accordingly, erythrocytosis is not a feature of CML.
Unlike the balanced contribution of BCR-ABL1 and KIT inhibition to suppression of CFU-GM colonies, effects on primitive CML cells, defined either by a CD34+38− phenotype (Fig. 4A) or LTC-IC functionality (Fig. 4B) were mostly due to BCR-ABL1 inhibition. In particular, in 6-week LTC-IC assays, which select primitive CML progenitor cells (24), both imatinib and PPY-A reduced Ph+ LTC-IC colonies by >95%, consistent with an effect that requires inhibition of BCR-ABL1, but not KIT. On the surface, the capacity of sole BCR-ABL1 inhibition to suppress primitive CML cells seems to contradict reports by us and others that that CML stem cells are insensitive to BCR-ABL1 inhibitors (33, 34). Furthermore, previous studies reported only modest imatinib effects on CML LTC-IC (35, 36). The differences are readily explained by the fact that prior studies evaluated the effects of short-term (72–96 hours) drug treatment of CML progenitors followed by 6-week culture on stroma without TKIs. These assays demonstrate the inability of TKIs to effectively induce apoptosis in primitive cells, but do not reflect conditions of long-term imatinib treatment. In contrast, we examined how continuous suppression of BCR-ABL1, KIT or their combination throughout the 6-week culture period would affect LTC-IC outgrowth. Importantly, to generate an environment devoid of human cytokines, we performed the LTC-IC assays using unmanipulated murine (M210B4) stromal cells (i.e. not engineered to express human cytokines). Since most cytokines and chemokines are not cross-reactive between species (37), these conditions minimize extrinsic factors that might support CML stem cells despite BCR-ABL1 inhibition. In these conditions, imatinib and PPY-A resulted in profound suppression of the most primitive cells. Notably, the differential effects of sole BCR-ABL1 vs. sole KIT inhibition on mature vs. primitive CML progenitor cells were consistent irrespective of whether the cell populations were defined by immunophenotype (Fig. 4A) or functionality (Fig. 4B-F). Given the overall profound effect of sole BCR-ABL1 inhibition on primitive CML progenitor cells, it is impossible to exclude a small contribution of KIT inhibition to the suppression of this population. Despite small numbers of colonies, in all samples Ph+ LTC-IC survived in the presence of BCR-ABL1 inhibitors, consistent with reports of residual BCR-ABL1+ LTC-IC and CD34+38− cells in patients with sustained molecular response to imatinib (38, 39).
The differential sensitivity of mature and primitive CML progenitors to sole BCR-ABL1 vs. combined BCR-ABL1/KIT inhibition suggested cell type specific differences in the response to SCF. We initially studied Mo7ep210BCR-ABL1 cells and found that SCF rescued these cells from the effects of PPY-A inhibition of BCR-ABL1 (Fig. 6A). While active BCR-ABL1 blunted SCF activation of AKT and MEK, key pathways downstream of KIT (27), inhibition of BCR-ABL1 sensitized cells to SCF. SCF rescue was completely blocked by PI3K inhibition, but only partially by MEK inhibition, implicating PI3K/AKT as the critical pathway downstream of KIT (Fig. 6C). Similar results were obtained in primary CML CD34+ cells: while PPY-A or SCF alone had little effect on pAKTS473, their combination greatly increased pAKTS473 (Fig. 6D). LY294002 abrogated the SCF-induced increase in colony formation (Fig. 6E). In contrast, basal pAKTS473 in CD34+38− cells was low, as previously reported in BCR-ABL1 expressing murine stem cells and CD34+38− CML cells (17, 40), unchanged upon PPY-A treatment and minimally increased by SCF alone or in combination with PPY-A (Fig. 7A). This is in stark contrast to CD34+38+ CML cells (Fig. 6D) and suggests that the PPY-A sensitivity of CD34+38− CML cells is due to their inability to strongly activate SCF signaling upon BCR-ABL1 inhibition, possibly reflecting the lower CD117 expression on CD34+38− compared to CD34+38+ CML cells (Fig. 7B). As the most primitive CML are not strongly KIT dependent, this may explain why no KIT mutations have been observed in patients with imatinib resistance (5). Additional mechanisms may be involved in blunting the SCF response of primitive CML progenitor cells in vivo. For example, Naka et al. reported that transforming growth factor β (TGFβ) blocks BCR-ABL1-induced AKT activation in BCR-ABL1 transduced murine stem cells (40). Since TGFβ is reported to prevent SCF rescue of mast cells after IL-3 withdrawal (41), it is possible that TGFβ in the microenvironment may further reduce SCF-induced rescue of CD34+38− CML cells.
From the drug development perspective, it is important to consider whether there is benefit to inhibiting non-oncogenic targets, or whether ‘surgical’ inhibitors with the narrowest possible target spectrum are preferred. Amongst the second and third generation BCR-ABL1 inhibitors, dasatinib, nilotinib and ponatinib directly inhibit KIT, while bosutinib has no activity against KIT (42). The clinical activity of bosutinib (43, 44) can be explained by its inhibitory activity toward SRC kinases, which play a critical role in KIT signaling (45, 46). Altogether our data suggest that, in vivo, CML stem cells may survive BCR-ABL1 inhibition through a pathway other than SCF/KIT that is not activated by M210B4 stromal cells. Therefore, the TKI resistance of primitive CML cells may simply reflect the fact that these inhibitors fail to target a second critical pathway, in contrast to the fortuitous situation in mature progenitor cells. Identifying pathways that support CML stem cells in the presence of BCR-ABL1 TKIs should open new therapeutic options.
Supplementary Material
Acknowledgments
We thank Kimberly Reynolds for help with cell processing and Sarah Bowden and Kimberly Snow for clerical assistance. We also thank Dr. Lars Rönnstrand, Lund University, Sweden, for helpful discussion.
Grant Support
This work was supported by HL082978-01 (M.W.D.) and CA04963920A2 (M.W.D.), Leukemia and Lymphoma Society grant 7036-01 (M.W.D.), P01 CA049639 (J.E.C., M.W.D.) and Howard Hughes Medical Institute. We acknowledge support of funds in conjunction with grant P30 CA042014 awarded to Huntsman Cancer Institute. A.M.E. is a Fellow and M.W.D. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Footnotes
Authors’ Contributions
A.S.C. designed experiments, performed research, analyzed data and wrote the paper, T.O. designed experiments and wrote the paper, Z.G. performed research and analyzed data, and I.L.K., A.D.P. and T.Y.Z. performed research. A.M.E. and C.E. analyzed data and wrote the paper, J.S.K., P.W.M. and B.J.D. designed experiments. J.E.C. designed experiments and provided vital reagents. M.W.D. designed experiments, analyzed data and wrote the paper.
Disclosure of Potential Conflicts of Interest:
M.W.D. is a consultant for BMS, Novartis, ARIAD and Incyte and his laboratory receives research funding from BMS and Novartis. B.J.D. is principal investigator or co-investigator on Novartis, Bristol-Myers Squibb, and ARIAD clinical trials. His institution has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead. He does not derive salary, nor does his lab receive funds from these contracts. OHSU and B.J.D. have a financial interest in MolecularMD. OHSU has licensed technology used in some of these clinical trials to MolecularMD. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. P.W.M. is an employee of Novartis Pharma, Basel, Switzerland. J.E.C. receives research funding from ARIAD, Novartis, BMS, Pfizer, Chemgenex, Deciphera, and Incyte.
References
- 1.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 3.Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–32. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 4.Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, et al. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18:6977–86. doi: 10.1016/j.bmc.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ali HK, Heinrich MC, Lange T, Krahl R, Mueller M, Muller C, et al. High incidence of BCR-ABL kinase domain mutations and absence of mutations of the PDGFR and KIT activation loops in CML patients with secondary resistance to imatinib. Hematol J. 2004;5:55–60. doi: 10.1038/sj.thj.6200319. [DOI] [PubMed] [Google Scholar]
- 6.Braconi C, Bracci R, Cellerino R. Molecular targets in Gastrointestinal Stromal Tumors (GIST) therapy. Curr Cancer Drug Targets. 2008;8:359–66. doi: 10.2174/156800908785133169. [DOI] [PubMed] [Google Scholar]
- 7.Cross NC, Reiter A. Fibroblast growth factor receptor and platelet-derived growth factor receptor abnormalities in eosinophilic myeloproliferative disorders. Acta Haematol. 2008;119:199–206. doi: 10.1159/000140631. [DOI] [PubMed] [Google Scholar]
- 8.Cortes J, O’Brien S, Quintas A, Giles F, Shan J, Rios MB, et al. Erythropoietin is effective in improving the anemia induced by imatinib mesylate therapy in patients with chronic myeloid leukemia in chronic phase. Cancer. 2004;100:2396–402. doi: 10.1002/cncr.20292. [DOI] [PubMed] [Google Scholar]
- 9.Sneed TB, Kantarjian HM, Talpaz M, O’Brien S, Rios MB, Bekele BN, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–21. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- 10.Deininger MW, Manley P. What do kinase inhibition profiles tell us about tyrosine kinase inhibitors used for the treatment of CML? Leuk Res. 2012;36:253–61. doi: 10.1016/j.leukres.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Pierce A, Spooncer E, Ainsworth S, Whetton AD. BCR-ABL alters the proliferation and differentiation response of multipotent hematopoietic cells to stem cell factor. Oncogene. 2002;21:3068–75. doi: 10.1038/sj.onc.1205424. [DOI] [PubMed] [Google Scholar]
- 12.Hallek M, Danhauser-Riedl S, Herbst R, Warmuth M, Winkler A, Kolb HJ, et al. Interaction of the receptor tyrosine kinase p145c-kit with the p210bcr/abl kinase in myeloid cells. Br J Haematol. 1996;94:5–16. doi: 10.1046/j.1365-2141.1996.6102053.x. [DOI] [PubMed] [Google Scholar]
- 13.Moore S, Haylock DN, Levesque JP, McDiarmid LA, Samels LM, To LB, et al. Stem cell factor as a single agent induces selective proliferation of the Philadelphia chromosome positive fraction of chronic myeloid leukemia CD34(+) cells. Blood. 1998;92:2461–70. [PubMed] [Google Scholar]
- 14.Agarwal R, Doren S, Hicks B, Dunbar CE. Long-term culture of chronic myelogenous leukemia marrow cells on stem cell factor-deficient stroma favors benign progenitors. Blood. 1995;85:1306–12. [PubMed] [Google Scholar]
- 15.Wong S, McLaughlin J, Cheng D, Zhang C, Shokat KM, Witte ON. Sole BCR-ABL inhibition is insufficient to eliminate all myeloproliferative disorder cell populations. Proc Natl Acad Sci U S A. 2004;101:17456–61. doi: 10.1073/pnas.0407061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belloc F, Airiau K, Jeanneteau M, Garcia M, Guerin E, Lippert E, et al. The stem cell factor-c-KIT pathway must be inhibited to enable apoptosis induced by BCR-ABL inhibitors in chronic myelogenous leukemia cells. Leukemia. 2009;23:679–85. doi: 10.1038/leu.2008.364. [DOI] [PubMed] [Google Scholar]
- 17.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou T, Parillon L, Li F, Wang Y, Keats J, Lamore S, et al. Crystal structure of the T315I mutant of AbI kinase. Chem Biol Drug Des. 2007;70:171–81. doi: 10.1111/j.1747-0285.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 19.Melnick JS, Janes J, Kim S, Chang JY, Sipes DG, Gunderson D, et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci U S A. 2006;103:3153–8. doi: 10.1073/pnas.0511292103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804:445–53. doi: 10.1016/j.bbapap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Snead JL, O’Hare T, Adrian LT, Eide CA, Lange T, Druker BJ, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–63. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou T, Parillon L, Li F, Wang Y, Keats J, Lamore S, et al. Crystal structure of the T315I mutant of AbI kinase. Chem Biol Drug Des. 2007;70:171–81. doi: 10.1111/j.1747-0285.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110:2242–9. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 24.Bock TA. Assay systems for hematopoietic stem and progenitor cells. Stem Cells. 1997;15 (Suppl 1):185–95. doi: 10.1002/stem.5530150824. [DOI] [PubMed] [Google Scholar]
- 25.Petzer AL, Eaves CJ, Lansdorp PM, Ponchio L, Barnett MJ, Eaves AC. Characterization of primitive subpopulation of normal and leukemic cells present in the blood of patients with newly diagnosed as well as established chronic myeloid leukemia. Blood. 1996;88:2162–71. [PubMed] [Google Scholar]
- 26.Hallek M, Danhauser Riedl S, Herbst R, Warmuth M, Winkler A, Kolb HJ, et al. Interaction of the receptor tyrosine kinase p145c-kit with the p210bcr/abl kinase in myeloid cells. Br J Haematol. 1996;94:5–16. doi: 10.1046/j.1365-2141.1996.6102053.x. [DOI] [PubMed] [Google Scholar]
- 27.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–48. doi: 10.1007/s00018-004-4189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strife A, Wisniewski D, Liu C, Lambek CL, Darzynkiewicz Z, Silver RT, et al. Direct evidence that Bcr-Abl tyrosine kinase activity disrupts normal synergistic interactions between Kit ligand and cytokines in primary primitive progenitor cells. Mol Cancer Res. 2003;1:176–85. [PubMed] [Google Scholar]
- 29.O’Hare T, Eide CA, Agarwal A, Adrian LT, Zabriskie MS, Mackenzie RJ, et al. Threshold levels of ABL tyrosine kinase inhibitors retained in chronic myeloid leukemia cells define commitment to apoptosis. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennartsson J, Wernstedt C, Engstrom U, Hellman U, Ronnstrand L. Identification of Tyr900 in the kinase domain of c-Kit as a Src-dependent phosphorylation site mediating interaction with c-Crk. Exp Cell Res. 2003;288:110–8. doi: 10.1016/s0014-4827(03)00206-4. [DOI] [PubMed] [Google Scholar]
- 31.von Lindern M, Schmidt U, Beug H. Control of erythropoiesis by erythropoietin and stem cell factor: a novel role for Bruton’s tyrosine kinase. Cell Cycle. 2004;3:876–9. [PubMed] [Google Scholar]
- 32.Deininger M, Goldman JM, Lydon NB, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL positive cells. Blood. 1997;90:3691–8. [PubMed] [Google Scholar]
- 33.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2011 doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 36.Copland M, Pellicano F, Richmond L, Allan EK, Hamilton A, Lee FY, et al. BMS-214662 potently induces apoptosis of chronic myeloid leukemia stem and progenitor cells and synergises with tyrosine kinase inhibitors. Blood. 2007 doi: 10.1182/blood-2007-09-112573. [DOI] [PubMed] [Google Scholar]
- 37.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–41. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Chomel JC, Bonnet ML, Sorel N, Bertrand A, Meunier MC, Fichelson S, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood. 2011;118:3657–60. doi: 10.1182/blood-2011-02-335497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu S, McDonald T, Lin A, Chakraborty S, Huang Q, Snyder DS, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118:5565–72. doi: 10.1182/blood-2010-12-327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 41.Mekori YA, Metcalfe DD. Transforming growth factor-beta prevents stem cell factor-mediated rescue of mast cells from apoptosis after IL-3 deprivation. J Immunol. 1994;153:2194–203. [PubMed] [Google Scholar]
- 42.Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–22. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 43.Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–12. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–5. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y, Jones N, Kluppel M, Hirashima M, Tachibana K, Cohn JB, et al. Targeted mutations of the juxtamembrane tyrosines in the Kit receptor tyrosine kinase selectively affect multiple cell lineages. Proc Natl Acad Sci U S A. 2004;101:6015–20. doi: 10.1073/pnas.0305363101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.