Abstract
Patient: Female, 32
Final Diagnosis: Gastrintestinal Autonomic Nerve Tumor (GANT)
Symptoms: anemia • anorexia • fatigue • fever • hearburn • nausea • weight loss
Medication: —
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Gastrointestinal autonomic nerve tumors (GANT) are extremely rare tumors that are related to gastrointestinal autonomic nervous plexuses. They are distinguished from stromal tumors by their unique ultrastructural features. Hence, their diagnosis is usually made on electron microscopy and immunohistochemical analyses. Although they are apparently slow-growing tumors, they run an aggressive clinical course and often associated with poor prognosis which eventually leads to death.
Case Report:
We report on a case of gastric GANT in a young female who was treated surgically by total gastrectomy. The disease, however ran an aggressive course with the development of distant (nodal, liver, lung, adrenal and musculo-skeletal) metastases two months after the radical resection.
Conclusions:
We believe this could be the first reported case of adrenal and musculo-skeletal metastases from gastric GANT soon after the radical gastric resection.
Keywords: gastrointestinal autonomic nerve tumors, gastrectomy, metastases, prognosis
Background
Gastrointestinal autonomic nerve tumors (GANT) are extremely rare. They are histogenetically related to the gastrointestinal autonomic nervous plexuses [1] and sometimes considered as a rare subtype of the gastrointestinal stromal tumors (GIST) [1]. However, they can be distinguished on the basis of their unique ultrastructural features. The diagnosis of GANT is usually made on electron microscopy, and immunhistochemical analyses [2], which are not readily available in every hospital. Although GANTs are apparently slow-growing tumors, they often have poor prognosis and run an aggressive clinical course that eventually leads to death [3].
We report here one new case of gastric GANT in a young female who was treated surgically by total gastrectomy. However, the disease ran an aggressive course and she developed distant nodal, liver, adrenal and musculo-skeletal metastases two months after the radical resection. We believe this case may well be the first report in the literature of adrenal and musculo-skeletal metastases after radical resection of gastric GANT.
Case Report
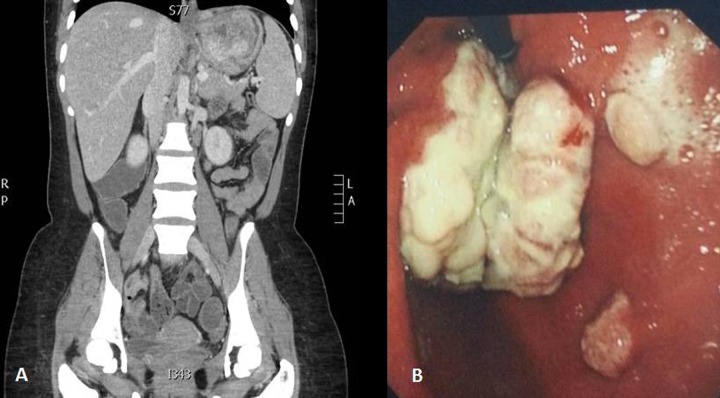
A 32-year-old female presented to the local hospital with history of anorexia, nausea, heartburn, fatigue, fever and weight loss (15 kg over 5 months). She denied any history of vomiting or change in bowel habits. Her investigations revealed anaemia (haemoglobin 7.7 g%). An upper GI endoscopy revealed chronic active gastritis with positive helicobacter pylori. Computerized tomography (CT) scan of the abdomen showed a mass in the stomach (Figure 1A) and hence was referred for further management. On examination she had a fever of 38°C and sinus tachycardia (120 beats/min). She also looked pale and emaciated, but there was no jaundice or lymphadenopathy. Abdominal examination revealed fullness in the left upper quadrant with tenderness on deep palpation.
Figure 1.
Abdominal CT scan of case 1 showing the gastric mass (A), and retroverted endoscopic view showing the tumor at the gastric fundus with adjacent two satellite lesions (B).
Blood tests revealed iron deficiency anaemia (haemoglobin 7 g%) and hypoalbuminaemia (17 g/L; normal 34–50). Repeated upper GI endoscopy showed a mass occupying the proximal part of the stomach with 2 satellite lesions (Figure 1B). Biopsy of the lesion confirmed a low grade spindle/epitheloid cell tumor, probably of neural origin; gastric autonomic nerve tumor (GANT). Endoscopic ultrasound (EUS) revealed a gastric mass arising from the mucosa and submucosa with perigastric, and splenic lymph nodes. Biopsy from the lymph node was taken, but was inconclusive.
As part of preoperative preparation, total parenteral nutrition was started and two units of blood were transfused. She underwent exploratory laparotomy which showed a large fundal tumor infiltrating the hilum of the spleen and tail of pancreas with multiple perigastric lymph nodes. However, there were no free peritoneal fluid, peritoneal nodules or visible distant visceral metastasis. An en-block total gastrectomy, splenectomy and distal pancreatectomy was performed (Figure 2A, 2B) with Roux-en-Y oesophago-jejunal anastmosis. On postoperative day (POD) 6, the contrast swallow showed no leak, the patient was fed and discharged home on POD 7.
Figure 2.
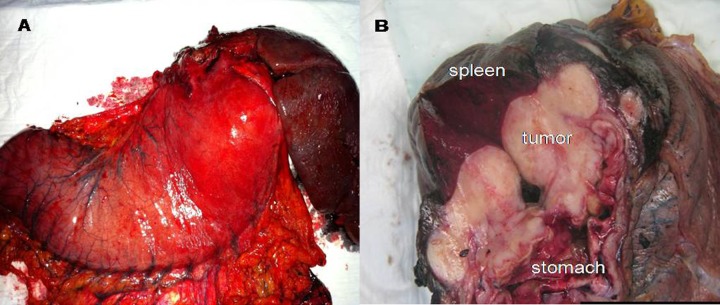
The operative specimen showing the en-block resection of stomach, spleen and tail of pancreas (A). The cut surface of the specimen eliciting the large nodular whitish tumour (B).
Histopathology
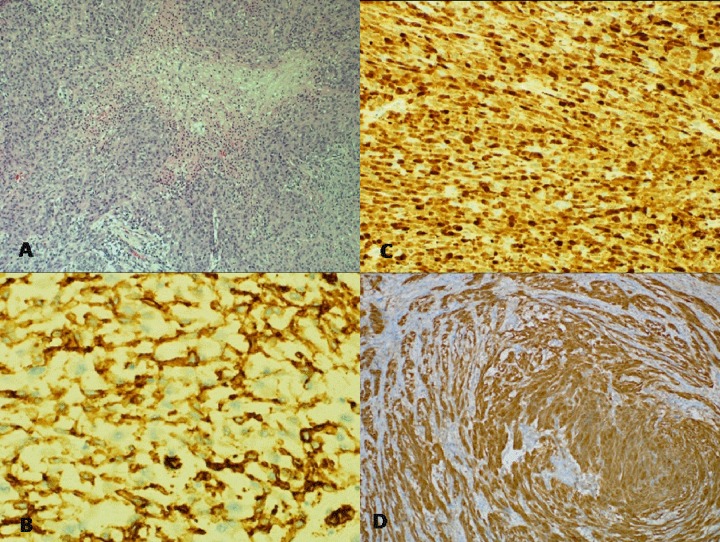
Histology of the specimen showed a heterogeneous multifocal malignant spindle cell/epithelioid cell tumor exhibiting variable patterns ranging from low grade sclerostic spindle cell areas to high grade epithelioid cell areas/nodules with high cellularity, geographical/palisading necrosis (Figure 3A) and frequent mitoses (Mitotic rate is 40/10 HPF). The tumor cells show rhabdoid morphology and in areas it shows fascicular arrangement with neural like areas. The tumor arises from the gastric fundus and invades throughout the gastric mucosa, submucosa, and muscle layer involving the subserosal fat and surrounding large serosal blood vessels and abutting the splenic capsule. All the harvested perigastric lymph nodes were reactive and negative for metastatic disease (0/6). All surgical resection margins were free of tumor which tethered the splenic capsule, without splenic or pancreatic parenchyma invasion. The two satellite mucosal nodules show similar appearances to the main tumor. The background gastric tissue is unremarkable and negative for dysplasia. Immunohistochemical staining revealed focal positive staining with for Vimentin, CD34 (Figure 3B), and S100 (Figure 3C), but were negative for c-kit (CD117), DOG1, SMA, Desmin, HMB45, ALK1, EMA, Pancytokeratin and NSE. Ki 67 was variable ranging from 10% to 70%. The histological immunohistologic findings (Figure 3C, 3D) are consistent with a gastrointestinal autonomic nerve tumor which represents a phenotypic variant of gastrointestinal stromal tumor.
Figure 3.
Histological photograph of the tumour. (A) Gastric tumor showing necrosis (Haematoxylin and Eosin stain 10×), (B) CD34 immunostain is positive (20×), (C) S100 immunostain is positive (10×), (D) Immunohistochemistry picture showing positivity of the tumor cells to S100 indicating its neural origin.
She presented 2 months later, with mild dysphagia. Gastroscopy was advised to exclude an anastomotic stricture but she refused. Therefore, a CT scan with oral contrast was performed and showed a newly developed hypo-attenuating lesion at segment IVb (1.7×1.4 cm) consistent with hepatic metastasis. There was also a left chest solitary metastasis (Figure 4A) and an enlarged necrotic lymph node in the hepato-gastric ligament. A PET/CT 18F-FDP whole body scan showed FDG-avid pulmonary, upper abdominal, hepatic, adrenal, bony and muscular metastases (Figure 4B, 4C). She presented again 3-month later with a pathological fracture of the left humerus. Resection biopsy of the bone lesion revealed metastatic GANT invading bone and skeletal muscle tissue.
Figure 4.
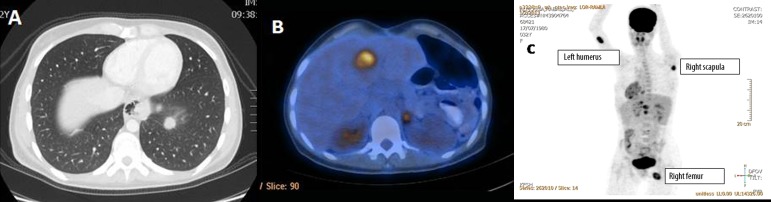
CT scan showing the lung metastatic lesion (A), and PET/CT showing the liver and adrenal metastases appearing 2 months after operative resection of the primary lesion (B). (C) Showing PET body scan in which the bony metastases in the left humorous, right scapula and right femur are clearly demonstrated.
Her clinical condition deteriorated 3 months later and was admitted for palliative care management. She was later lost to follow-up and presumed dead.
Discussion
GANT was first described by Herrera et al. in 1984 [4]. They are extremely rare accounting for 1% of all malignant gastrointestinal tumors and occurring at any age, but most commonly in younger patients; around 20 years of age [1]. Several cases were also reported in the paediatric age group [5,6], and some were described in association with neurofibromatosis, Carney’s syndrome and in patients with adrenal ganglioneuromas [7]. Histologically, they are very similar to and may overlap with other gastrointestinal stromal tumors (GIST), with spindle and large epithelioid cells with clear cytoplasm which are arranged in sheets, fascicles, or nests [3]. The cells show intense staining for Vimentin and focal staining for neuron-specific enolase, chromogranin, synaptophysin, gastrin, P substance and S-100 protein. Hence, they are considered by some researchers as a variant of GISTs [1]. The diagnosis is usually made on electron microscopy, and immunohistochemical analyses [2]. However, the real distinction between the two entities (GIST vs. GANT) is made on the basis of the unique ultrastructural features of GANTs [1]. The ultrastructural diagnostic characteristics of other gastrointestinal tumors, such as those originating from smooth muscle, Schwann cell, or endocrine cell type are absent and immunocytochemically the tumor is diffusely positive for vimentin and neuron-specific enolase and focally positive for neurofilament triplet protein (NFTP) 160 [1,3]. Immunohistochemical studies usually display evidence of neural differentiation and negativity for smooth muscle antigens, but the key for identifying a tumor as GANT is the presence of dendritic processes with dense neuroendocrine granules on ultrastructural examination [7]. Furthermore, in contrast to GIST, GANT is rather more common in the small intestine than stomach [8], but has been reported form the other sites such as oesophagus [9], colon [10], retroperitoneum, omentum, mesentery and peritoneum [3,11].
The diagnostic differentiation between GANTs and Schwannomas is difficult by light microscopic and immunohistochemical examination, as many of their immunohistolochemical features are similar; all are vimentin positive, most show positive reaction to NSE, and a number are also synaptophysin, S-100, and PGP 9.5 positive [1,3,4,7,12]. Nevertheless, positive S-100 and Leu-7 antigen indicate Schwannoma [13]. It has also been suggested the presence of both Antoni A/Verocay bodies (cells forming an organised palisade pattern) and Antoni B (small lacunar foci with loss of palisade architecture) areas to be very specific for schwannoma [14]. The differentiation is, however easy on electron microscopy [15], which can discriminate safely between GANTs and Schwannomas by the presence of dense core granules, cell processes, neurotubules, dystrophic axons, synapse-like structures and skeinoid fibres in the former [16]. Moreover, Schwannoma lacks features, such as Luse bodies and basal lamina and has less irregular cell processes [16].
Clinical symptoms at presentation are usually non-specific. The most common presentation is abdominal pain, fullness and fever, as well as gastrointestinal bleeding, chronic iron deficiency anaemia with pallor, weight loss, fatigue and malaise [3,8,17]. In this case, high persistent (non-swinging) fever, tachycardia, iron-deficiency anaemia and weight loss were the dominant presenting features. Interestingly, the fever settled immediately after the surgical resection raising suspicion that GANT is acting as a functioning tumor that may secrete pyretic agents, but this warrants substantiation by future research.
Endoscopic ultrasound is considered reliable in predicting malignancy and the predictive features being irregular margins, deep penetration, and malignant-looking lymph nodes [18]. However, it does not differentiate GANT from the other stromal tumours [11]. Endoscopic ultrasound-guided fine needle aspiration with immunohistochemical analysis may be useful in the preoperative diagnosis of GIST [19]. This was carried out in this case, but the diagnostic yield was inconclusive.
GANT – though considered benign – are apparently slow-growing tumors with an aggressive clinical course and often poor prognosis that eventually leads to death [3]. This aggressive behavior correlates very well with tumor size (>10 cm), proliferative activity, mitotic count (at least five per 10 HPF), degree of necrosis, surgical respectability and DNA ploidy [12,16]. This aggressive behavior is translated by frequent tumor recurrence and/or metastasis to the liver that occurs in more than 55% and tumor-related death in more than 33% of patients [1]. This poor prognosis was also evident in a series of 20 patients followed for at least 5 months with 70% having either local recurrence or hepatic metastasis [17]. In a smaller series of 9 patients, two patients died due to tumor recurrence, two died of unknown causes and five were alive, 2–44 months after presentation [3]. Four of the five survivors have recurrent/ residual intra-abdominal tumour [3]. Kodet et al. reported a metastatic gastric GANT to the liver in an adolescent girl who was treated by subtotal gastrectomy with chemotherapy but the liver metastases did not respond significantly. However, she lived with unresectable liver metastases 10 months after the gastrectomy [6]. Similar to this case, Drews et al. reported a case of a metastatic gastric GANT in a 40-year-old woman. En-bloc resection of the retroperitoneal mass, spleen, pancreatic tail, and a part of the posterior gastric wall adhering to the mass were performed [20]. Liver metastases were detectable on CT scan 14 months after surgery. In spite of radical excision more than 72% of patients died few months after surgery with local recurrences and/or metastases in regional lymph nodes and in the liver [20]. The disease is therefore considered fatal and the response to chemotherapy or radiotherapy was poor. Lauwers et al. collected 12 GANTs; 3 cases (40%) were gastric. Tumor recurred or metastasized to the liver in seven patients (58%) and four patients (33%) died with tumor [16]. This again confirmed that GANT is aggressive and fatal [12]. Therefore radical surgical resection is the cornerstone of treatment and offers the only chance of cure as the response to chemotherapy is unpredictable.
Conventional chemotherapy and radiotherapy is ineffective in the treatment of GANT. The pattern of tumor aggression and recurrence after radical surgical resection calls for the urgent need to develop new adjuvant non-surgical therapies. However, for metastatic CD117-positive tumors, tyrosine kinase inhibitors might be an appropriate palliative treatment [21,22]. Imatinib mesilate, or Glivec (a tyrosine kinase inhibitor), was confirmed to be effective against metastatic or unresectable CD117-positive GANT. Imatinib – though expensive – was given to this case despite the negativity of tumor CD117. Hence, it was not surprising to see poor clinical response to this treatment.
The liver is the most common reported site for GANT metastases. Wide spread metastases including the adrenal gland, muscles and bone are however rare; none of the previously reported cases identified the musculo-skeletal system or adrenal glands as sites of metastases after radical gastric resection of GANT. To the best of our knowledge, this is the first case of gastric GANT metastasizing to the adrenal gland and musculo-skeletal system, two months after radical resection.
Conclusions
GANTs are extremely rare tumors that arise from the autonomic nerve plexuses. The diagnosis is often made by electron microscopy and immunohistochemical analysis. They may run an aggressive course with poor prognosis even after radical surgical resection. This aggressive behavior correlates with the tumor size, presence of high mitotic figures, degree of necrosis and DNA ploidy. Metastases are common to the liver, but may – based on this case – also affect lungs, adrenals, and even bone and muscles.
Footnotes
Conflict of interest
Nothing to declare.
References:
- 1.MacLeod CB, Tsokos M. Gastrointestinal autonomic nerve tumor. Ultrastruct Pathol. 1991;15(1):49–55. doi: 10.3109/01913129109021303. [DOI] [PubMed] [Google Scholar]
- 2.Pinedo Moraleda F, Martínez González MA, Ballestín Carcavilla C, Vargas Castrillón J. Gastrointestinal autonomic nerve tumours: a case report with ultrastructural and immunohistochemical studies. Histopathology. 1992;20(4):323–29. doi: 10.1111/j.1365-2559.1992.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 3.Shanks JH, Harris M, Banerhee SS, Eyden BP. Gastrointestinal autonomic nerve tumours: a report of nine cases. Histopathology. 1996;29:111–21. doi: 10.1046/j.1365-2559.1996.d01-502.x. [DOI] [PubMed] [Google Scholar]
- 4.Herrera GA, Pinto De Moraes H, Grizzle WE, Han SG. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma). Light and electron microscopic study confirming the origin of the neoplasm. Dig Dis Sci. 1984;29:275–84. doi: 10.1007/BF01296263. [DOI] [PubMed] [Google Scholar]
- 5.Kerr JZ, Hicks MJ, Nuchtern JG, et al. Gastrointestinal autonomic nerve tumors in the pediatric population: a report of four cases and a review of the literature. Cancer. 1999;85(1):220–30. doi: 10.1002/(sici)1097-0142(19990101)85:1<220::aid-cncr30>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Kodet R, Snajdauf J, Smelhaus V. Gastrointestinal autonomic nerve tumor: a case report with electron microscopic and immunohistochemical analysis and review of the literature. Pediatr Pathol. 1994;14(6):1005–16. doi: 10.3109/15513819409037697. [DOI] [PubMed] [Google Scholar]
- 7.Dhimes P, Lopez-Carreira M, Ortega-Serrano MP, et al. Gastrointestinal autonomic nerve tumours and their separation from other gastrointestinal stromal tumours: an ultrastructural and immunohistochemical study of seven cases. Virchows Arch. 1995;426:27–35. doi: 10.1007/BF00194695. [DOI] [PubMed] [Google Scholar]
- 8.Lee JR, Joshi V, Griffin JW, Jr, et al. Gastrointestinal autonomic nerve tumor: immunohistochemical and molecular identity with gastrointestinal stromal tumor. Am J Surg Pathol. 2001;25:979–87. doi: 10.1097/00000478-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lam KY, Law SY, Chu KM, Ma LT. Gastrointestinal autonomic nerve tumor of the esophagus. A clinicopathologic, immunohistochemical, ultrastructural study of a case and review of the literature. Cancer. 1996;78(8):1651–59. [PubMed] [Google Scholar]
- 10.Veloso FT, Pereira P, Saraiva A, et al. Colonic gastrointestinal autonomic nervous tumor in a patient with Crohn’s disease. Dig Dis Sci. 2005;50:1476–80. doi: 10.1007/s10620-005-2865-5. [DOI] [PubMed] [Google Scholar]
- 11.Rueda O, Escribano J, Vicente JM, et al. Gastrointestinal autonomic nerve tumors (plexosarcomas). Is a radiological diagnosis possible? Eur Rad. 1998;8:458–60. doi: 10.1007/s003300050413. [DOI] [PubMed] [Google Scholar]
- 12.Lauwers GY, Erlandson RA, Casper ES, et al. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1993;17(9):887–97. doi: 10.1097/00000478-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sugimura H, Suzuki M, Iwase T, et al. Benign schwannoma of the esophagus: report of two cases with immunohistochemical and ultrastructural studies. Pathol Int. 1994;44:460–65. doi: 10.1111/j.1440-1827.1994.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 14.DasGupta TK, Brasfield RD, Strong EW, Hajdu SI. Benign solitary Schwannomas (neurilemomas) Cancer. 1969;24(2):355–66. doi: 10.1002/1097-0142(196908)24:2<355::aid-cncr2820240218>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Min W, Yamada N, Asano G. Gastrointestinal autonomic nerve tumors: immunohistochemical and ultrastructural studies in cases of gastrointestinal stromal tumors. Pathol Int. 1997;47:308–14. doi: 10.1111/j.1440-1827.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 16.Tornoczky T, Ka’lman E, Hegedus G, et al. High mitotic index associated with poor prognosis in gastrointestinal autonomic nerve tumour. Histopathology. 1999;35:121–28. doi: 10.1046/j.1365-2559.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer DE, Jr, Erickson RA. Plexosarcoma: endoscopic ultrasound and electron-microscopic characteristics of a stromal tumor. Am J Gastroenterol. 2001;96(3):906–9. doi: 10.1111/j.1572-0241.2001.03642.x. [DOI] [PubMed] [Google Scholar]
- 18.Palazzo L, Landi B, Cellier C, et al. Endoscopic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobuhiro A, Hidemi G, Yasumasa N, et al. The diagnosis of stromal tumours with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 20.Drews G, Kiene S, Emmrich P, Lehmann J. Malignant tumor of the autonomic nervous system of the stomach (GAN-tumor) – case report of a new entity. Zentralbl Chir. 1992;117(10):564–68. [PubMed] [Google Scholar]
- 21.Stift A, Friedl J, Gnant M, et al. Gastrointestinal autonomic nerve tumors: A surgical point of view. World J Gastroenterol. 2004;10(16):2447–51. doi: 10.3748/wjg.v10.i16.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]