Abstract
We analyzed the outcomes of 248 (61% male) adult recipients of HLA-matched unrelated and HLA-mismatched related donor hematopoietic cell transplantation (HCT) for non-Hodgkin lymphoma (NHL) after reduced or lower intensity conditioning (RIC), reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1997 to 2004. Median age was 52 (range, 18–72 yrs); 31% had a Karnofsky performance score <90. Follicular NHL (43%) was the major histology. Incidence of grades II–IV acute graft-versus-host disease (GVHD) was 43% at 100 days; and chronic GVHD was 44% at three years. Treatment-related mortality (TRM) at 100 days was 24%. Three-year overall survival (OS) and progression-free survival (PFS) were 41% and 32%, respectively. In multivariate analysis, use of anti-thymocyte globulin (ATG) and HLA mismatch were associated with increased TRM. High-grade histology, ATG use and chemotherapy resistance were associated with lower progression-free survival (PFS). Older age, shorter interval from diagnosis to HCT, non-TBI conditioning regimens, ex vivo T-cell depletion and HLA-mismatched unrelated donors were associated with mortality. GVHD did not influence relapse or PFS. Older age, aggressive histology and chemotherapy resistance correlated with poorer survival. For selected patients with NHL, lack of an available sibling donor should not be a barrier to allogeneic HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) can be curative for those with high-risk or recurrent hematologic cancers including non-Hodgkin lymphoma (NHL). (1, 2) For patients lacking an HLA-matched related donor, alternative hematopoietic cell sources include HLA-matched unrelated donors and HLA-mismatched related donors. (3, 4) Over the past decade, traditional myeloablative conditioning has been increased replaced by lower intensity conditioning in an effort to reduce treatment-related mortality (TRM). The possibility of lower regimen-related toxicity makes these regimens particularly attractive for older persons and those with co-morbidities. Lower intensity conditioning regimens have been extended to older patients, employing alternative donors and all hematopoietic cell sources including cord blood cells. Most published experience with these regimens in NHL patients, particularly their outcomes with respect to disease recurrence and TRM, is limited to single institution studies with few patients. With this in mind, we performed a non-comparative, retrospective study to evaluate the outcomes of adult recipients of alternate donor HCT for NHL following a variety of lower intensity conditioning regimens commonly referred to as reduced intensity conditioning (RIC).
METHODS
Data collection
Data used in this study were obtained from the Statistical Center of the CIBMTR. CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all consecutive transplants; compliance is monitored by on-site audits. Subjects are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Study population
This study was restricted to adult subjects (≥18 yrs) with NHL undergoing a first allogeneic HCT with an RIC regimen from 1997 to 2004. Subjects receiving allogeneic HCT after relapse from prior autologous HCT and cord blood graft recipients were excluded. The classification of degree of HLA match was based on the previously validated model for grouping the degree of HLA match proposed by Weisdorf et al (5). In this schema “partially matched,” cases were missing either high-resolution or HLA-C data or had a defined single-locus mismatch. Mismatched unrelated cases had ≥2 allele or antigen mismatches. The study population included 248 subjects with NHL, with the following characteristics: 26 (10%) received HLA-mismatched related grafts, 151 (61%) matched unrelated grafts, 47 (19%) partially-matched unrelated grafts, and 24 (9%) received mismatched unrelated donor grafts according to criteria proposed by Weisdorf et al (6). Definitions and categorization of conditioning regimens were assigned according to consensus criteria (7, 8). Regimens which did not involve full myeloablative chemo/radiation therapy were included in the schema of RIC for this analysis. All subjects received calcineurin-based GVHD prophylaxis with or without methotrexate. The follow-up completeness index for this study cohort was 90%. Patient-, disease-, and transplant- related characteristics are listed in Table 1.
Table 1.
Characteristics of adult subjects receiving allogeneic HCT from unrelated or HLA-mismatched family member donors with a reduced-intensity or non-myeloablative conditioning regimen for NHL reported to the CIBMTR from 1997 to 2004.
| Variables | N (%) |
|---|---|
| Patient related | |
| Age, median (range), years | 52 (18 – 72) |
| Male sex | 152 (61) |
| Karnofsky score pre-transplant | |
| <90 | 78 (31) |
| Disease related | |
| Disease stage at diagnosis | |
| I | 21 (8) |
| II | 37 (15) |
| III | 45 (18) |
| IV | 133 (54) |
| Missing | 12 (5) |
| Time from diagnosis to transplant, median (range), months | 29 (4 – 196) |
| <12 months | 25 (10) |
| 12 – 24 months | 74 (30) |
| ≥24 months | 149 (60) |
| Histology | |
| Follicular | 107 (43) |
| DLCL/Immunoblastic | 58 (24) |
| Lymphoblastic/Burkitts/Burkitt-like | 55 (22) |
| Mantle Cell | 12 (5) |
| PTCL | 16 (6) |
| Transplant related | |
| Disease stage at transplant | |
| I | 122 (49) |
| II | 54 (22) |
| CR | 72 (29) |
| Disease status at transplant | |
| CR2+ | 43 (17) |
| PIF Sensitive | 48 (19) |
| PIF Resistant | 26 (10) |
| REL Sensitive | 58 (23) |
| REL Resistant | 52 (21) |
| REL untreated/unknown | 21 (9) |
| Chemo sensitivity at transplant | |
| Sensitive | 127 (51) |
| Resistant | 95 (38) |
| Not Evaluable | 26 (10) |
| Prior radiation before transplant | 69 (28) |
| TBI-based conditioning regimen | 49 (21) |
| Marrow grafts | 106 (43) |
| Conditioning regimen | |
| FludMEL±ATG | 49 (20) |
| FludBu±ATG | 31 (12) |
| FludBu±TLI | 10 (4) |
| Flud+Cy±Rituxan | 61 (25) |
| Flud+TBI=200cGY | 27 (11) |
| BuCy (reduced) | 5 (2) |
| BEAM/similar | 14 (6) |
| TBI only | 18 (7) |
| VP16+Cy | 1 (<1) |
| CBV | 19 (8) |
| Other | 13 (5) |
| Number of lines of therapy, median (range), months | 4 (1 – 6) |
| Donor type (HLA match) | |
| Unrelated well matched | 151 (61) |
| Unrelated partially matched | 47 (19) |
| Mismatched family member donors | 26 (10) |
| Unrelated mismatched | 24 (9) |
| Donor-recipient gender match | |
| Male-male | 108 (44) |
| Male-female | 58 (23) |
| Female-male | 44 (18) |
| Female-female | 38 (15) |
| Donor-recipient CMV status | |
| +/+ | 48 (19) |
| +/− | 14 (6) |
| −/+ | 75 (30) |
| −/− | 87 (35) |
| Year of transplant | |
| 1997–1998 | 7 (3) |
| 1999–2000 | 40 (16) |
| 2001–2002 | 80 (32) |
| 2003–2004 | 121 (49) |
| ATG in conditioning | 74 (30) |
| GVHD prophylaxis | |
| T-cell depletion ± other (ex vivo) | 11 (4) |
| FK506 +MTX± Other | 98 (40) |
| FK506 ± Other | 44 (18) |
| CsA+ MTX ± other | 32 (13) |
| CsA ± other | 61 (24) |
| Other/unknown | 2 (1) |
Abbreviations: DLCL = diffuse large cell lymphoma; PIF = primary induction failure; CR = complete remission; REL = relapse; TBI = total body radiation; LPAM = melphalan; Flud = fludarabine; Cy = Cyclophosphamide; CBV=cyclosphamide+BCNU+VP16 = etoposide; BM = bone marrow; PBSC = peripheral blood stem cell; GVHD = graft vs. host disease; CsA = cyclosporine; FK506 = tacrolimus; MTX = methotrexate.
Endpoints and Definitions
The primary objective was to describe the outcomes after allogeneic HCT for NHL using RIC regimens and alternate donor grafts. We analyzed time to engraftment, incidence of acute and chronic GVHD, relapse, TRM, progression-free survival (PFS) and overall survival (OS). Neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count of ≥ 0.5 × 109/L; platelet engraftment was defined as platelet count ≥ 20 × 109/L for seven consecutive days without transfusion support. TRM was defined as death from any cause in the first 28 days or death without evidence of lymphoma progression/relapse. Progression was defined as an increase of ≥ 25% in the sites of lymphoma or development of new sites of lymphoma. Relapse was defined as recurrence of lymphoma after a complete response (CR). For calculating PFS, patients were considered treatment failures at relapse or progression, or death.. Patients alive without evidence of disease relapse or progression were censored at last follow up, and PFS was summarized by a survival curve. The OS interval variable was defined as the time from date of transplant to date of death or last contact, and summarized by a survival curve. Other outcomes analyzed included acute and chronic graft-versus-host disease and cause of death. Acute GVHD was defined and graded based on the pattern and severity of organ involvement using established criteria (9). Chronic GVHD was defined as the development of any chronic GVHD based on clinical criteria (10). Both these events were summarized by the corresponding cumulative incidence estimate, with death without development of GVHD as the competing risk.
Statistical analysis
Patient-, disease-, and transplant- related variables (Table 1) were described with median and range for continuous variables, and percent of total for categorical variables. Occurrence of acute and chronic GVHD, TRM, and disease recurrence/progression were calculated using cumulative incidence estimates, taking into account the competing risk (11). Probabilities of PFS and OS were estimated from the time of transplantation using the Kaplan-Meier estimator (12).
Associations between patient-, disease-, and transplant-related factors and outcomes of interest were assessed using multivariate Cox proportional hazards regression. A stepwise forward selection multivariate model was built to identify covariates that influenced outcomes. Covariates with a p-value <0.05 were considered significant. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome, and then interactions between the type of donor and the other covariates were checked. Covariates that violated the proportional hazard assumption were adjusted by stratification. Results were expressed as relative risk (RR) or the relative rate of occurrence of the event. Stepwise forward-backward selection was used to build the models from the prognostic factors under consideration. All p-values were two-sided. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC). The multivariate analyses are summarized in Table 2.
Table 2.
Variables considered in multivariate analysis
| Patient related: |
| Age at transplant: ≤60 years* vs. >60 years |
| Karnofsky performance at transplant: <90%* vs. ≥90% vs. missing |
| Gender: male* vs female |
| Disease related: |
| Histological type of NHL |
| Immunophenotype: B-cell* vs. T cell vs. missing |
| Time from diagnosis to transplant: <12 months* vs. ≥ 24 months vs. 12–24 months |
| Number of lines of therapy: ≤2* vs. 3–4 vs. ≥5 |
| Disease status at transplant: CR2* vs. PIF sens vs. PIF res vs. Rel sens vs. Rel res vs. |
| Rel untreated/unknown/missing |
| Chemosensitive disease at transplant: Sensitive* vs. resistant vs. not evaluable/untreated/missing |
| Marrow involvement at diagnosis: yes* vs no |
| Duration of CR1: continuous |
| Transplant related: |
| Conditioning regimen: TBI* vs. non-TBI |
| Conditioning regimen: ATG given vs no ATG given* |
| Donor type/HLA match: Unrelated well matched* vs. Unrelated partially matched vs. Unrelated mismatched vs mismatched family member donor |
| Source of stem cells: Bone marrow* vs. peripheral blood |
| GVHD prophylaxis: FK506+MTX±Others* vs. MTX+CsA+others vs. CsA+others vs. T cell depletion+others vs. FK506 ± Other |
| Donor-recipient CMV status: +/+* vs. +/− vs. −/+ vs. −/− |
| Donor-recipient gender match: M-M* vs. M-F vs. F-M vs. F-F |
| Year of transplant: 1997–2000* vs. 2001–2002 vs. 2003–2004 |
Abbreviations: DLCL = diffuse large cell lymphoma; PTCL = peripheral T cell lymphoma; PIF = primary induction failure; CR = complete remission; REL = relapse; res = resistant; sens = sensitive; TBI = total body radiation; GVHD = graft vs. host disease; CsA = cyclosporine; FK506 = tacrolimus; MTX = methotrexate; CMV = cytomegalovirus; M= male; F = female.
RESULTS
Subjects, disease, transplant characteristics
Median age was 52 years (range 18–72 years); 31% had a Karnofsky performance score <90. Median follow up was 44 months (range 1–123 months).
Engraftment
Incidence of neutrophil engraftment at day 28 and day 100 after HCT was 92% (95% confidence interval [CI]; 88–95%) and 94% (95% CI; 90–96%), respectively. 50% of patients received myeloid growth factors after HCT. The incidences of platelet engraftment at day 28 and day 100 after HCT were 67% (95% CI; 61–73%) and 79% (95% CI; 73–83%), respectively. 15 subjects had primary graft failure and 33 had secondary graft failure.
Graft-versus-host disease
The incidence of grade II–IV acute GVHD was 43% (95% CI; 37–49%). The incidence of chronic GVHD was 36% (95% CI; 30–42%) at one year and 44% (95% CI; 37–50%) at three years. Recipients of HLA-mismatched unrelated donor grafts had a greater risk of acute GVHD (HR= 2.70; 95% CI; 1.50–4.84; p<0.001) in multivariate analysis. The use of TBI increased the risk of chronic GVHD (HR= 1.62; 95% CI; 1.05–2.49; p=0.03). GVHD was the primary cause of death in 23 subjects (15%).
Relapse
Cumulative incidences of lymphoma progression or relapse at 1, 3 and 5 years post-HCT were 26% (95% CI; 20–31%), 30% (95% CI; 24–36%), and 31% (95% CI; 26–38%), respectively. Older age (> 60 years) (RR= 1.93; 95% CI; 1.07–3.48; p=0.028), diffuse large cell histology (RR=3.46; 95% CI; 1.80 – 6.34) and resistant relapsed disease status at HCT (RR= 5.05; 95% CI; 2.13–11.99) were associated with a higher risk of progression/relapse (Table 3). Patients transplanted in the most recent years (2003–2004) had a higher risk of progression/relapse (HR= 2.87; 95% CI; 1.26–6.58; p=0.013). GVHD did not correlate with disease progression or relapse. Median interval from HCT to relapse was 6 months.
Table 3.
Multivariate analysis
| Variables | Relative Risk (95% CI) | P-value |
|---|---|---|
| TRM | ||
| ATG: Yes vs. No | 2.13 (1.40 – 3.25) | <0.001 |
| Donor type: Unrelated mismatched vs well matched | 2.07 (1.17 – 3.84) | 0.02 |
| Relapse | ||
| Age: >60 vs. ≤60 | 1.93 (1.07 – 3.48) | 0.028 |
| Histology: Diffuse Large B Cell vs. Follicular | 3.46 (1.80 – 6.34) | <0.001 |
| Status: REL Resistant vs. CR2+ | 5.05 (2.13 – 11.99) | <0.001 |
| Year of transplant: 2003–4 vs. 1997 -00 | 2.87 (1.25 – 6.58) | 0.013 |
| Risk of Treatment Failure | ||
| Histology: Lymphoblastic/Burkitts/Burkitt-like vs. Follicular | 2.11 (1.40 – 3.18) | <0.001 |
| Status: REL Resistant vs. CR2+ | 2.54 (1.50 – 4.31) | 0.001 |
| ATG: Yes vs. No | 1.50 (1.07 – 2.10) | 0.020 |
| Time from diagnosis to transplant: 12 – 24 months vs. ≥ 24 | 1.58 (1.09 – 2.31) | 0.017 |
| Risk of Mortality | ||
| Age: >60 vs. ≤ 60 | 1.77 (1.16 – 2.70) | 0.009 |
| Time from diagnosis to transplant: 12 – 24 months vs. ≥ 24 | 2.26 (1.56 – 3.27) | <0.001 |
| TBI: No vs. Yes | 2.17 (1.36 – 3.48) | 0.001 |
| Donor type: Unrelated mismatched vs. well matched | 2.20 (1.24 – 3.90) | 0.007 |
| GVHD prophylaxis: ex vivo T-cell depletion vs. FK506/MTX | 6.0 (2.68 – 13.45) | <0.001 |
Abbreviations: ATG = anti-thymocyte globulin; rel = relapse; CR = complete remission; GVHD = graft vs. host disease; FK506 = tacrolimus; MTX = methotrexate.
Treatment-related mortality
TRM at 28 and 100 days post-HCT was 11% (95% CI; 7–15%) and 24% (95% CI= 19–30%), respectively. TRM gradually increased from 31% (95% CI; 25–37%) at one year to 39% (95% CI; 32–45%) at three years, to 43% (95% CI; 36–50%) at five years post-HCT. Use of ATG (RR= 2.13; 95% CI; 1.40–3.25; p<0.001), HLA-mismatched unrelated grafts (RR= 2.07; 95% CI; 1.17–3.84; p=0.02), and HLA-partially matched unrelated grafts (RR= 1.85; 95% CI; 1.13–3.01; p = 0.014) were associated with greater risk of TRM in multivariate analysis (Table 3).
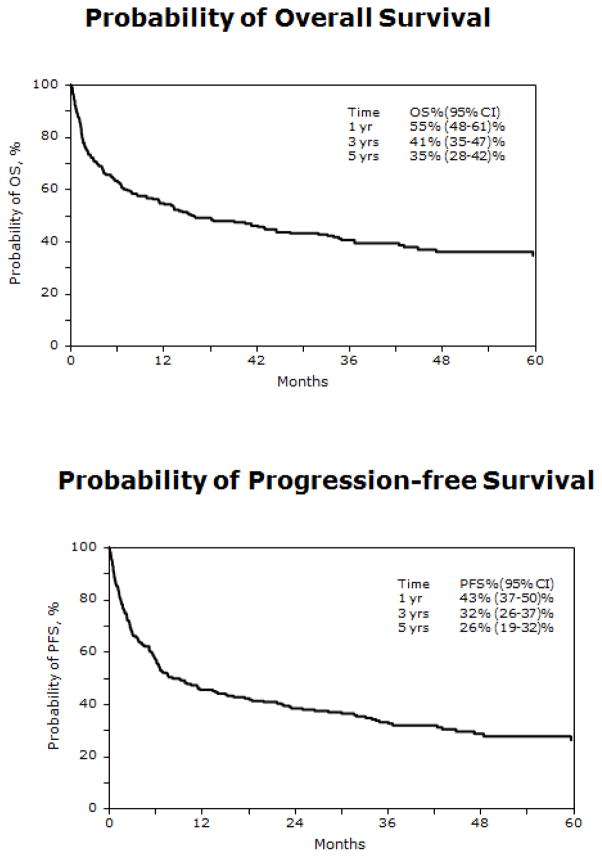
Progression-free survival
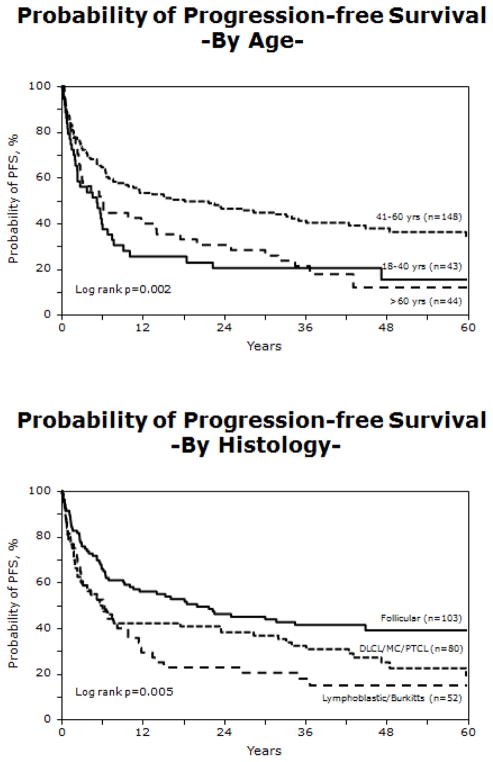
PFS was 43% (95% CI= 37–50%) at one year post HCT, 32% (95% CI; 26–37%) at three years post HCT, and 26% (95% CI; 19–32%) at five years post-HCT (Figure 1). High-grade histology, use of ATG (RR= 1.50; 95% CI; 1.07–2.10; p=0.020), and chemotherapy-resistant disease at HCT (RR = 2.54; 95% CI; 1.50–4.31; p =0.001) were associated with higher risk of treatment failure or lower PFS (Table 3). Subjects with Burkitt, Burkitt-like, lymphoblastic (RR= 2.11; 95% CI; 1.40–3.18; p<0.001) or mantle cell (RR= 2.34; 95% CI; 1.15–4.76; p=0.019) histologies were at increased risk of treatment-failure and lower PFS (Figure 2). Neither acute nor chronic GVHD correlated with PFS. PFS was significantly higher for subjects transplanted between the ages of 41 years and 60 years, when compared to the older and younger age groups (Figure 2).
Figure 1.
Overall survival and progression-free survival for all subjects
Figure 2.
Progression free survival by age group and histology
Survival
The 100-day mortality rate was 30% (95% CI; 24–35%). Survival at one year post HCT was 55% (95% CI; 48–61%) and 41% (95% CI; 35–47%) at three years post HCT (Figure 1). On multivariate analysis, older age (>60 years) (RR= 1.77; 95% CI; 1.16–2.70; p=0.009), use of a non-TBI conditioning regimen (RR= 2.17; 95% CI; 1.36–3.48; p=0.001), grafts from unrelated mismatched donors (RR = 2.20; 95% CI; 1.24–3.90; p=0.007), and female recipients (RR= 1.47; 95% CI; 1.03–2.10; p=0.035) were associated with decreased OS (Table 3). Survival was superior for those receiving tacrolimus/methotrexate GVHD prophylaxis compared to those receiving other GVHD prophylaxis regimens. Use of T-cell depletion was associated with higher risk of death (RR= 6.0; 95% CI; 2.68 – 13.45; p<0.001). Similar to PFS, subjects between the ages of 41 and 60 had superior survival compared to other age groups, and subjects with indolent histologies had improved survival when compared to those with high or intermediate grade histology NHL (Figure 3).
Figure 3.
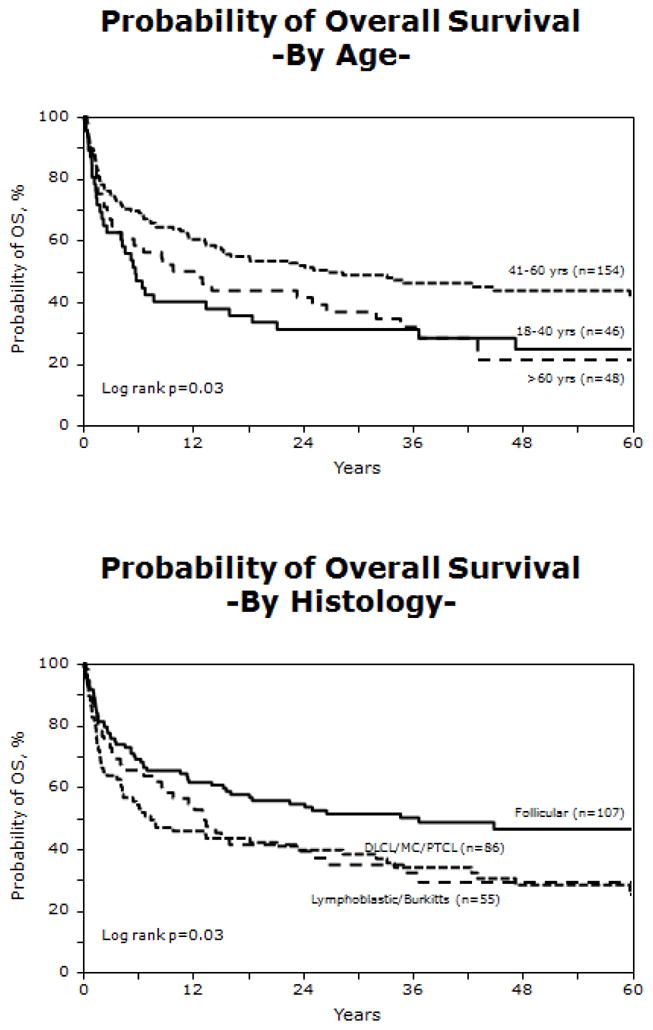
Overall survival by age group and histology
Cause of death
153 transplant recipients died from disease recurrence (n=40, 26%), organ failure (n=29, 19%), GVHD (n=23, 15%), infection (n=22, 14%), other causes (n=21, 14%), pulmonary syndrome (n=12, 8%), hemorrhage (n=5, 3%), and new malignancy (n=1, <1%).
DISCUSSION
In this cohort of patients with high risk NHL, 26% (95% CI; 19–32%) of subjects were alive without disease progression at five years after HCT. However, graft failure, TRM and disease recurrence were common obstacles to transplant success. It is important to recognize that this study population included only first allogeneic transplants, and excluded those who had undergone prior autologous or allogeneic HCT. Therefore, this population is unique in that physicians proceeded to HCT with an alternative donor rather than autologous transplantation. This analysis studied a patient population at very high risk of treatment failure: most were heavily pretreated leading to resistant disease and a high risk of transplant-related mortality.
RIC regimens have been proposed to produce equivalent or superior survival rates when compared to myeloablative regimens in subjects with myeloid and lymphoid cancers (13–15), yet published literature on outcomes of RIC regimens for NHL are limited to relatively small series. PFS is reported to range from 22–59% at three years after HCT, with the best outcomes observed for indolent histologies. More aggressive NHL histologies and Hodgkin lymphoma had outcomes approximating 20% (16–19) PFS. The PFS rates reported in our study compare favorably to single center studies reported in the literature, given the degree of HLA mismatch, poor performance status, and extensive prior therapy of our patient population.
These outcomes, however, are certainly less than optimal. Although 43% of subjects were alive in remission at one year after HCT, only 26% were free of disease progression at five years post HCT. Single institution studies reporting higher PFS rates generally included smaller patient numbers and subjects with closer HLA matching to donors, or included predominately low-risk NHL such as those with indolent lymphoma or in remission (15–18). The low PFS in our cohort likely results from the high-risk disease, extensive prior therapy, older median age of this patient population, and the high graft failure and TRM rates. In this study, 47% of subjects had chemotherapy-refractory disease and 31% had a KPS < 90.
It is noteworthy that even patients with chemotherapy-resistant disease are curable in some instances with RIC regimens followed by allogeneic HCT (17). Our study supports the observation that chemotherapy-resistant disease is a marker for poor outcome, but should be viewed as a prognostic factor and not an absolute contraindication to allogeneic HCT. Disease progression occurring prior to full donor chimerism may contribute to relapse in patients with more aggressive histologies allowing the malignant cells to outpace the donor immunologic response (16, 18). In addition, the use of ATG, alemtuzumab, or T-cell depleted grafts may have delayed lymphocyte recovery after HCT, potentially abrogating an immune-mediated graft-versus-lymphoma effect. Alternatively, delayed immunological recovery following HLA-disparate HCT or in the setting of GVHD may have played a role (20, 21), although our data do not support a potent graft-versus-lymphoma dependent on GVHD.
Disease progression occurred most commonly in the first year after HCT and was infrequent thereafter. Acute or chronic GVHD did not influence the risk of disease progression. This observation suggests that GVHD may not be related to a graft-versus-lymphoma effect, or that the graft-versus-lymphoma effect may be more active in certain histologies, and that this could not be observed because of the wide variety of indolent and aggressive histologies in this study (22). Alternatively, the rates of disease progression in aggressive histologies may outpace immunologic recovery after HCT so that tumor cells escape immunologic destruction. As expected, subjects with more advanced disease and more aggressive histologies had greater risks of disease recurrence. Surprisingly, those transplanted more recently had greater risk of relapse than those transplanted earlier in the study. This association of year of HCT and relapse is not readily explainable, but may reflect the fact that higher-risk subjects were being transplanted in the later years of the study as transplant physicians became more comfortable with these regimens. Alternatively, this may reflect transplant physician bias, whereby subjects who may benefit more from myeloablative regimens receive RIC regimens to decrease TRM.
TRM was 24% at 100 days after HCT and gradually rose throughout the study period, reaching 43% at five years post HCT, with no evidence of a plateau. Late TRM was most frequently due to infectious complications or chronic GVHD. The use of ATG, alemtuzumab or T-cell depleted grafts, commonly used to reduce GVHD, may have delayed immune reconstitution leading to an increased risk of infectious complications (20). In our study, infection was responsible for 22 patient deaths (14%), 8 in ATG recipients. GVHD also has been reported to be a significant problem after HCT with an RIC regimen. As expected, an HLA-mismatched unrelated donor graft source increased the risk of acute GVHD. The use of TBI increased the risk of chronic GVHD; this effect may be due to thymic damage from TBI that impairs T cell reconstitution in the thymus after HCT. (23) It is also possible that patients who received TBI-containing regimens were more likely to be mixed chimeras following HCT or that disease control after these regimens was poorer. Withdrawal of immunosuppressive therapy in the setting of early disease recurrence could lead to chronic GVHD.
Most recipients had neutrophil engraftment by day 100 after HCT. However, primary and secondary graft failures were more common than anticipated in this population undergoing first transplantation. This may be due to the use of in vivo T-cell depletion agents in the conditioning regimen, the presence of marrow disease, and/or donor-recipient HLA disparity. Alternatively, some recipients may have had marrow stromal damage, since many of them likely were unable to undergo autologous HCT, which suggests a mobilization problem in some subjects.
Survival was 55% at one year and 35% at five years after HCT. The use of non-TBI conditioning regimens was associated with poorer OS. It is not readily apparent why non-TBI regimens were associated with poorer OS. TBI may have been avoided in subjects who were heavily pretreated, who had radiation therapy prior to HCT, or who had lower performance scores. TBI in RIC regimens is given to enhance immune suppression or to prevent graft failure. Both OS and PFS were counterintuitively lower in the 18 to40 year old group than in the 41 to 59 year old age group. This reduced survival may be due to the lower incidence of follicular lymphomas, which are associated with a higher survival rate, in this age group. Alternatively, many studies of adolescent and young adult (AYA) cancer patients, defined by most studies as 15 years to 39 years old, demonstrate reduced increments in survival for these patients. AYA patients have reduced compliance, late diagnosis, lower insurance rates, and may have different biologic characteristics of their cancers.
Interestingly and perhaps counter intuitively, the occurrence of acute or chronic GVHD was not significantly correlated with decreased disease recurrence after HCT. The reasons for this lack of correlation are not known. The effects of GVHD may have varied depending on the NHL histologic subtype; subjects with indolent NHL histologies have been noted in other reports to have lower disease recurrence rates after allogeneic HCT. Alternatively, the effect may have been abrogated due to the high rates of graft failure or TRM. A graft-versus-lymphoma effect has not been consistently correlated with GVHD after allogeneic HCT, suggesting that it may operate independently of GVHD (15,20).
The study is limited by its retrospective nature and the underlying reasons behind the clinical decision to proceed to allogeneic HCT as well as the rationale behind the choice of an RIC regimen are unknown to us. Many of these recipients had intermediate to advanced disease and a low performance score (Table 1). These characteristics suggest that the treating clinicians were considering an RIC regimen to reduce TRM in this high-risk group. This analysis does not attempt to compare outcomes of subjects with hematologic malignancies based on donor-recipient relationship or HLA-mismatch. Our results suggest that RIC regimens should be considered as a cautious alternative to myeloablative regimens for NHL subjects undergoing HCT from unrelated or HLA-mismatched related donors. Future trials should study the use of RIC regimens in pediatric patients and focus on interventions to further reduce TRM and disease recurrence.
Supplementary Material
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard M, Dauriac C, Drenou B, et al. Long-term follow-up of allogeneic bone marrow transplantation in patients with poor prognosis non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1999;23:329–333. doi: 10.1038/sj.bmt.1701587. [DOI] [PubMed] [Google Scholar]
- 2.Kiss TL, Panzarella T, Messner HA, et al. Busulfan and cyclophosphamide as a preparative regimen for allogeneic blood and marrow transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;31:73–78. doi: 10.1038/sj.bmt.1703790. [DOI] [PubMed] [Google Scholar]
- 3.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 5.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. New York, N.Y: Springer-Verlag; 2003. [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from in complete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 15.Patil S, Spencer A, Schwarer A, et al. Reduced-intensity conditioned allogeneic haematopoietic stem cell transplantation results in durable disease-free and overall survival in patients with poor prognosis myeloid and lymphoid malignancies: eighty-month follow-up. Bone Marrow Transplant. doi: 10.1038/bmt.2009.322. [DOI] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14:418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean RM, Fowler DH, Wilson WH, et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy-refractory non-hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:593–599. doi: 10.1016/j.bbmt.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Morris EC, Mackinnon S. Reduced intensity allogeneic stem cell transplantation for low grade non-Hodgkin’s lymphoma. Best Pract Res Clin Haematol. 2005;18:129–142. doi: 10.1016/j.beha.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 21.Glass B, Nickelsen M, Dreger P, et al. Reduced-intensity conditioning prior to allogeneic transplantation of hematopoietic stem cells: the need for T cells early after transplantation to induce a graft-versus-lymphoma effect. Bone Marrow Transplant. 2004;34:391–397. doi: 10.1038/sj.bmt.1704600. [DOI] [PubMed] [Google Scholar]
- 22.Gross TG, Hale GA, He W, et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16:223–230. doi: 10.1016/j.bbmt.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE. Single Cell Analysis of Complex Thymus Stromal Cell Populations: Rapid Thymic Epithelia Preparation Characterizes Radiation Injury. Clin Transl Sci. 2009;2:279–285. doi: 10.1111/j.1752-8062.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.