Abstract
Tissue damage during the neonatal period evokes long-lasting changes in nociceptive processing within the adult spinal cord which contribute to persistent alterations in pain sensitivity. However, it remains unclear if the observed modifications in neuronal activity within the mature superficial dorsal horn (SDH) following early injury reflect shifts in the intrinsic membrane properties of these cells. Therefore, the present study was undertaken to identify the effects of neonatal surgical injury on the intrinsic excitability of both GABAergic and presumed glutamatergic neurons within lamina II of the adult SDH using in vitro patch clamp recordings from spinal cord slices prepared from Gad-GFP mice. The results demonstrate that hindpaw surgical incision at postnatal day (P) 3 altered the passive membrane properties of both Gad-GFP and adjacent, non-GFP neurons in the mature SDH, as evidenced by decreased membrane resistance and more negative resting potentials in comparison to naïve littermate controls. This was accompanied by a reduction in the prevalence of spontaneous activity within the GABAergic population. Both Gad-GFP and non-GFP neurons displayed a significant elevation in rheobase and decreased instantaneous firing frequency after incision, suggesting that early tissue damage lowers the intrinsic membrane excitability of adult SDH neurons. Isolation of inward-rectifying K+ (Kir) currents revealed that neonatal incision significantly increased Kir conductance near physiological membrane potentials in GABAergic, but not glutamatergic, lamina II neurons. Overall, these findings suggest that neonatal tissue injury causes a long-term dampening of intrinsic firing across the general population of lamina II interneurons, but the underlying ionic mechanisms may be cell-type specific.
The processing of noxious stimuli within the CNS begins in the superficial dorsal horn (SDH) of the spinal cord, where a complex network of excitatory and inhibitory interneurons integrates sensory inputs and strongly regulates the output of the spinal pain circuit by modulating the excitability of a small population of neurons which send ascending projections to the brain (Todd, 2010). Mounting evidence suggests that the level of activity within mature dorsal horn neurons is significantly influenced by sensory experience during the early postnatal period. For example, in vivo electrophysiological studies using extracellular recordings have demonstrated that skin wounding in the newborn rat leads to enlarged receptive fields in dorsal horn neurons at 6 weeks post-injury (Torsney and Fitzgerald, 2003). Elevated rates of spontaneous activity and exaggerated firing in response to mechanical stimulation have also been reported in the adult dorsal horn in vivo after peripheral inflammation during the neonatal period (Peng et al., 2003). This documented hyperexcitability following early tissue damage could be explained by long-term alterations in the balance of synaptic excitation vs. inhibition onto adult SDH neurons and/or modifications in their intrinsic membrane properties which in turn modulate their excitability in a cell-autonomous manner. While in vivo extracellular recordings are invaluable in measuring the responses of dorsal horn cells to natural sensory stimuli, this technique cannot distinguish between these potential underlying mechanisms.
Recent studies have focused on identifying changes in synaptic connectivity occurring within the mature SDH network following transient injuries sustained during the neonatal period. Deficits in both phasic and tonic glycinergic transmission have been observed in the adult SDH following neonatal surgical injury (Li et al., 2013a), while stronger descending inhibition to the mature dorsal horn has been reported after peripheral inflammation during early life (Zhang et al., 2010), which may be mediated by a potentiation in opioidergic tone in the CNS (Laprairie and Murphy, 2009). However, it remains unclear whether neonatal tissue damage evokes persistent alterations in the intrinsic firing properties of developing SDH neurons. It is known that the intrinsic membrane properties of SDH neurons are developmentally regulated in a cell type-specific manner (Walsh et al., 2009; Li and Baccei, 2011; Li and Baccei, 2012), and significant changes in the transcription of genes encoding voltage-dependent and voltage-independent ion channels occur during the first postnatal weeks (Blankenship et al., 2013). Given the clear importance of neuronal activity in the modulation of gene expression (Lyons and West, 2011), perturbations in sensory input resulting from injuries during this sensitive developmental period may have long-term consequences for the electrophysiological phenotype of mature SDH neurons.
Therefore, the present study was undertaken to elucidate the persistent effects of neonatal surgical injury on the intrinsic membrane excitability of both inhibitory and presumed excitatory interneurons within lamina II of the adult mouse spinal cord.
Experimental Procedures
Ethical Approval
All experiments adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee which approved this study.
Hindpaw surgical incision
At postnatal day (P)3, female Gad-GFP mice (FVB-Tg(GadGFP)4570Swn; Jackson Labs; Bar Harbor, ME), which express enhanced GFP under the control of the GAD67 promoter (Oliva, Jr. et al., 2000), were anesthetized with isoflurane (2–3%) and a small incision made through the skin and underlying muscle of the plantar hindpaw as described previously (Brennan et al., 1996). The skin was immediately closed with 7-0 suture (Ethicon; Cornelia, GA) and the wound fully healed in ≤ 2 weeks. Females were chosen based on previous work demonstrating that the long-term effects of neonatal injury on pain sensitivity are more pronounced in females (Laprairie and Murphy, 2007).
Preparation of spinal cord slices
At P49–63, Gad-GFP mice were deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused with ice-cold dissection solution consisting of (in mM): 250 sucrose, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, and 25 glucose continuously bubbled with 95% O2 / 5% CO2. The lumbar spinal cord was isolated and immersed in low-melting-point agarose (3% in above solution; Life Technologies, Carlsbad, CA) and parasagittal slices (350–400 µm) were cut from the ipsilateral side using a vibrating microtome (7000smz-2; Campden Instruments, Lafayette, IN). The slices were placed in a chamber filled with oxygenated dissection solution for 30 min then allowed to recover in an oxygenated artificial CSF (aCSF) solution containing the following (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose for ≥ 1 hour at room temperature.
Patch clamp recordings
After recovery, slices were transferred to a submersion-type recording chamber (~0.5 mL volume; RC-22; Warner Instruments, Hamden, CT) and mounted on the stage of an upright microscope (BX51WI, Olympus, Center Valley, PA) which was equipped with fluorescence to allow for the identification of GFP-expressing neurons. Slices were then perfused at room temperature with oxygenated aCSF at a rate of 3–6 ml/min.
Patch electrodes were constructed from thin-walled single-filamented borosilicate glass (1.5 mm outer diameter; World Precision Instruments, Sarasota, FL) using a microelectrode puller (P-97; Sutter Instruments, Novato, CA). Pipette resistances ranged from 4 to 6 MΩ and seal resistances were >1 GΩ. Patch electrodes were filled with a solution containing the following (in mM): 130 K-gluconate, 10 KCl, 10 HEPES, 10 Na-phosphocreatine, 4 MgATP, and 0.3 Na2-GTP, pH 7.2 (305 mOsm).
Dorsal horn neurons were visualized with infrared-differential interference contrast and patch clamp recordings were obtained from the L4/L5 dorsal horn using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Approximately 1 min after establishment of the whole-cell configuration, the spontaneous firing patterns of dorsal horn neurons were classified at the resting membrane potential (Vrest). Membrane capacitance was calculated using the built-in pClamp membrane test, while membrane resistance was measured using the hyperpolarization produced by a −20 pA current injection from Vrest. To characterize the properties of evoked action potential (AP) discharge, intracellular current injections (from −20 to +150 pA in 10 pA increments; 800 ms duration) were applied from Vrest. Instantaneous firing frequency (IF) was calculated as 1 / interspike interval (ISI), while rheobase was defined as the minimum current step (delivered in 2.5 pA increments at 50 ms duration) which evoked AP discharge.
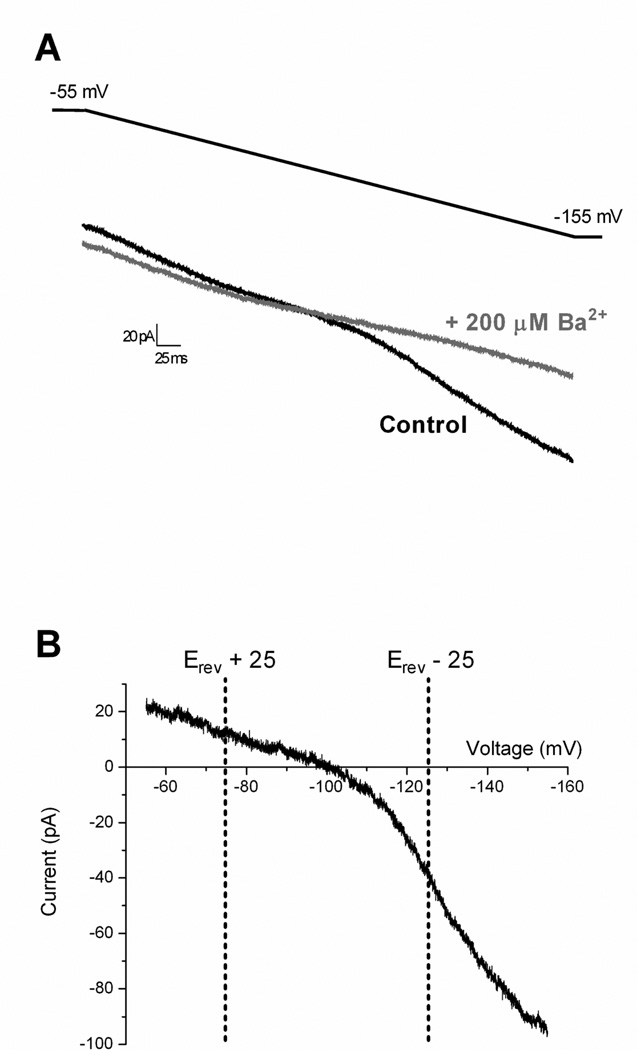
Inward-rectifying K+ (Kir) currents were isolated as described previously (Derjean et al., 2003; Li et al., 2013b). Briefly, neurons were voltage-clamped at −55 mV in the presence of 10 µM NBQX, 25 µM AP-5, 10 µM gabazine (GBZ) and 0.5 µM strychnine to block fast synaptic transmission in the slice. Negative voltage ramps (from −55 to −155 mV) were applied at a rate of 0.2 mV/ms. BaCl2 (200 µM) was bath-applied to block Kir (Coetzee et al., 1999) and the Ba2+-sensitive component of the current was subsequently isolated via electronic subtraction (see Fig. 4). Conductance (gBa-sensitive) was calculated as: g = I / (Vm − Erev) at two different membrane potentials that were equidistant (25 mV) from the reversal potential (Derjean et al., 2003). To estimate the degree of Kir inward rectification, a ratio of these two conductances was calculated as: g(E+25)/g(E−25).
Figure 4. Isolation of inward-rectifying K+ (Kir) currents in mature SDH neurons.
A: Representative currents recorded in a lamina II neuron during negative voltage ramps from a holding potential of −55 mV to −155 mV before (Control; black) and after (gray) the bath application of 200 µM BaCl2. B: Example of Ba2+-sensitive currents obtained by electronic subtraction (black – gray) plotted as a function of membrane voltage. Inward rectification was observed in all cases as the slope was greater at potentials negative to the observed reversal potential (Erev). The dotted vertical lines indicate representative voltages (equidistant from Erev) used to calculate the Ba2+-sensitive conductance (gBa-sensitive).
Membrane voltages were adjusted for liquid junction potentials (approximately 14 mV) calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices) unless otherwise specified. Currents were filtered at 4–6 kHz through a −3 dB, four-pole low-pass Bessel filter, digitally sampled at 20 kHz, and stored on a personal computer (ICT, Cincinnati, OH) using a commercially available data acquisition system (Digidata 1440A with pClamp 10.2 software; Molecular Devices).
Data analysis and statistics
Electrophysiological data were analyzed using Clampfit (Molecular Devices) and Origin (OriginLab Corp., Northampton, MA) software. The distribution of firing patterns was compared between the Naïve and Incision groups using the Fisher’s exact test (Prism 5.0; GraphPad Software, La Jolla, CA). Nonparametric tests were used in cases in which the distribution of data failed the D’Agostino & Pearson normality test (Prism). p < 0.05 was considered significant. n refers to the number of neurons sampled in a given group. Data are expressed as means ± SEM.
Results
Neonatal tissue injury modifies the intrinsic membrane properties of adult SDH neurons
To identify long-term changes in the intrinsic excitability of mature SDH neurons after neonatal tissue damage, unilateral hindpaw surgical incision (Brennan et al., 1996) was administered at postnatal day (P)3 in Gad-GFP mice, which selectively express enhanced GFP (eGFP) in GABAergic neurons (Oliva, Jr. et al., 2000). Naïve littermate-matched controls (handled in an identical manner including exposure to anesthesia) were used for all experiments. At P49–63, in vitro whole-cell patch clamp recordings were obtained from Gad-GFP and adjacent, non-GFP neurons within lamina II of spinal cord slices prepared from the ipsilateral side. Since eGFP labels >60% of the GABAergic neurons in the adult SDH of these mice (Dougherty et al., 2009) and GABAergic cells are thought to comprise ~30% of lamina II neurons in the rodent (Todd and Sullivan, 1990; Polgar et al., 2003), along with the observation that glycinergic neurons predominantly represent a subset of the GABAergic population (Todd and Sullivan, 1990; Huang et al., 2008), one can estimate that the vast majority (>85%) of non-GFP cells should correspond to glutamatergic neurons.
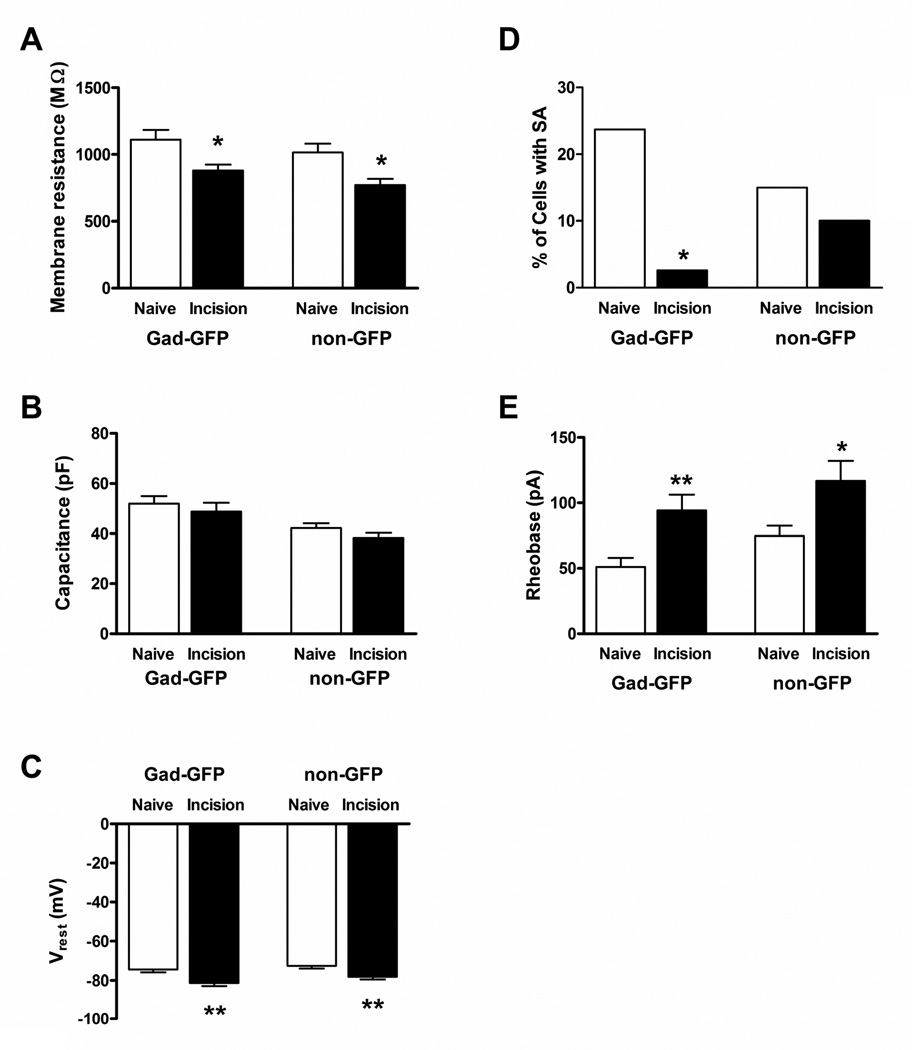
P3 hindpaw incision evoked a significant reduction in membrane resistance (Rm) in both Gad-GFP (n = 37 in the Naive group; n = 38 in Incision group; p = 0.021; Mann-Whitney test; Fig. 1A, left) and adjacent non-GFP neurons (n = 40 in Naïve; n = 39 in Incision; p = 0.011; Fig. 1A, right) within the adult SDH compared to naïve littermate controls. Membrane capacitance (Cm) was significantly higher in the Gad-GFP population compared to adjacent, non-GFP neurons in naïve spinal cord slices (p = 0.033; Mann-Whitney test; Fig. 1B), which could reflect the sampling of GABAergic islet neurons which are known to have extensive dendritic arbors in the rostrocaudal direction (Grudt and Perl, 2002; Yasaka et al., 2010). However, more importantly, early tissue damage failed to affect Cm in both the GABAergic (p = 0.26; Mann-Whitney) and presumed glutamatergic (p = 0.14) populations (Fig. 1B), suggesting that the above decrease in Rm is unlikely to be explained by alterations in cell size following neonatal incision. Both subtypes of adult lamina II neurons also displayed more negative resting membrane potentials (Vrest) after early tissue damage (p = 0.002 for Gad-GFP; p = 0.007 for non-GFP; Mann-Whitney test; Fig. 1C). Interestingly, this was accompanied by a significant reduction in the prevalence of spontaneous action potential (AP) discharge in Gad-GFP (p = 0.014; Fisher’s exact test; Fig. 1D, left) but not in adjacent non-GFP neurons (p = 0.737; Fig. 1D, right) within the mature SDH. In both the GABAergic and presumed glutamatergic groups, spontaneously active neurons exhibited irregular AP discharge and we observed neither tonic nor bursting patterns of spontaneous firing within lamina II, as previously reported in this lamina during the neonatal period (Li and Baccei, 2011).
Figure 1. Neonatal surgical incision modulates the passive and active membrane properties of mature SDH neurons.
A: Hindpaw incision at P3 evoked a significant reduction in membrane resistance in both Gad-GFP (left; *p = 0.021; Mann-Whitney test) and adjacent non-GFP (right; *p = 0.011) neurons within lamina II of the adult mouse SDH. B: P3 injury failed to affect membrane capacitance in either population during adulthood (p = 0.26 for Gad-GFP; p = 0.14 for non-GFP; Mann-Whitney test). C: Neonatal tissue injury led to a hyperpolarizing shift in resting membrane potential in both mature Gad-GFP (left, **p = 0.002; Mann-Whitney) and non-GFP neurons (right, **p = 0.007). D: The prevalence of spontaneous action potential discharge (SA) was significantly reduced by P3 injury in the GABAergic (left, *p = 0.014; Fisher’s exact test), but not presumed glutamatergic (right, p = 0.737), population of adult SDH neurons. E: Rheobase amplitude was significantly elevated by P3 hindpaw incision in both mature GABAergic (left; **p = 0.002; unpaired t-test) and presumed excitatory neurons (right; *p = 0.022) within lamina II.
To further examine the consequences of neonatal incision on the intrinsic membrane excitability of adult SDH neurons, brief (50 ms) depolarizing current steps of increasing intensity were applied from Vrest via the patch electrode in order to measure rheobase (i.e. the minimum current amplitude which evokes AP discharge). As illustrated in Fig. 1E, early tissue injury resulted in a marked elevation in rheobase levels in both Gad-GFP (n = 38 in Naïve; n = 38 in Incision; p = 0.002; unpaired t-test) and non-GFP lamina II neurons (n = 40 in each group; p = 0.022; unpaired t-test) during adulthood. Meanwhile, AP threshold was not significantly altered by P3 incision in either Gad-GFP (Naïve: −49.2 ± 0.7 mV; Incision: −47.3 ± 0.8 mV; p = 0.058, unpaired t-test) or non-GFP neurons (Naïve: −44.0 ± 0.8 mV; Incision: −44.3 ± 1.0 mV; p = 0.837). Neonatal injury did significantly reduce AP duration in the non-GFP population during adulthood (Naïve: 1.40 ± 0.06 ms; Incision: 1.23 ± 0.03 ms; p = 0.030; unpaired t-test), but no such alteration was observed in GABAergic neurons within the same slices (Naïve: 1.25 ± 0.05 ms; Incision: 1.21 ± 0.04 ms; p = 0.557; Mann-Whitney test).
Persistent reduction in repetitive firing across the mature SDH following tissue damage during the early postnatal period
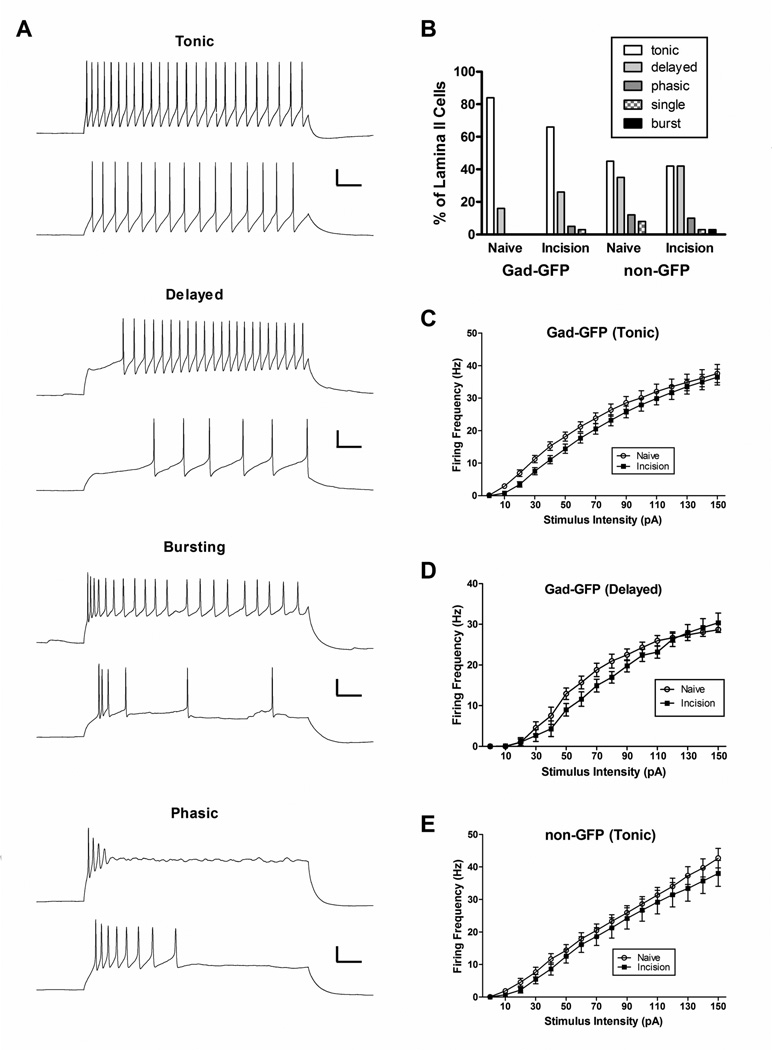
Intracellular current injections of longer duration (800 ms) revealed five distinct patterns of AP discharge (tonic, delayed, phasic, single-spiking and bursting; Fig. 2A), which is similar to previous studies in lamina II of the adult rodent SDH (Ruscheweyh and Sandkuhler, 2002; Heinke et al., 2004; Graham et al., 2008; Yasaka et al., 2010). While we did observe “gap-firing” as described previously (Heinke et al., 2004), this seemed to occur at higher stimulus intensities in neurons which also displayed a delayed firing pattern in response to weaker stimulation, and thus these neurons were classified as delayed-firing in the present study. Notably, P3 injury did not change the overall distribution of evoked firing patterns in either the GABAergic or glutamatergic populations in the mature SDH (Fig. 2B), as there were no statistically significant differences between the Naïve and Incision groups in terms of the prevalence of tonic (p = 0.111 for Gad-GFP; p = 0.823 for non-GFP; Fisher’s exact test) or delayed (p = 0.399 for Gad-GFP; p = 0.642 for non-GFP) patterns of AP firing.
Figure 2. Decreased repetitive firing in adult lamina II neurons following early tissue damage.
A: Direct current injection through the patch electrode at increasing intensities (bottom to top) revealed firing patterns that were classified as tonic, delayed, bursting, phasic or single-spiking (not shown). Scale bar = 20 mV, 100 ms. B: Neonatal incision failed to significantly affect the percentage of lamina II GABAergic neurons (left) which exhibited tonic or delayed patterns of action potential discharge (p>0.05; Fisher’s exact test). Similarly, early tissue damage had no significant effect on the distribution of firing patterns in the non-GFP population of adult SDH neurons (right). Total number of neurons sampled in each group: 38 from Naïve and 38 from Incision for the Gad-GFP population; 40 Naïve and 40 Incision for the non-GFP population. C: Plot of instantaneous firing frequency as a function of stimulus intensity in adult GABAergic neurons exhibiting tonic-firing, illustrating a significant decrease in firing rate after neonatal incision compared to naïve littermate controls (n = 25–31 in each group; p<0.0001; two-way ANOVA). D: Early injury also decreased firing frequency in mature Gad-GFP neurons showing a delayed pattern of action potential discharge (n = 6–10 for each group; p<0.01; two-way ANOVA). E: Similar plot for tonic-firing, non-GFP cells from the same slices. Incision also significantly reduced firing frequency within this population of adult SDH neurons (n = 16–18 in each group; p<0.001; two-way ANOVA).
However, P3 hindpaw incision did significantly reduce the repetitive firing frequency in adult GABAergic neurons showing a tonic pattern of AP discharge, as evidenced by a rightward shift in the stimulus-response curve (n = 31 for Naïve; n = 25 for Incision; p<0.0001; two-way ANOVA; Fig. 2C). This was accompanied by a hyperpolarizing shift in Vrest and an elevation in rheobase within this subset of mature SDH neurons (Table 1). GABAergic neurons with delayed firing patterns also exhibited a significantly lower rate of AP discharge following early tissue damage (n = 6 for Naïve; n = 10 for Incision; p<0.01; two-way ANOVA; Fig. 2D). Tonic, non-GFP neurons from the same spinal cord slices also displayed a more hyperpolarized Vrest (Table 1) and a significantly lower repetitive firing frequency following neonatal hindpaw incision (n = 18 for Naïve; n = 16 for Incision; p = 0.007; two-way ANOVA; Fig. 2E). Meanwhile, non-GFP neurons with delayed firing patterns showed no such change in repetitive firing (n = 12–16 in each group; p = 0.346; two-way ANOVA; data not shown), although rheobase in this population was significantly higher after early incision compared to naïve controls (Table 1).
Table 1.
Effects of neonatal incision on the electrophysiological properties of adult mouse SDH neurons as a function of cell type.
| Vrest (mV) | Rheobase (pA) | Rm (MΩ) | Threshold (mV) | AP50 (ms) | |||
|---|---|---|---|---|---|---|---|
| Naïve | GFP | Tonic (n=31) | −73.4 ± 1.6 | 40.8 ± 5.1 | 1130 ± 85.8 | −50.0 ± 0.6 | 1.3 ± 0.1 |
| Delayed (n=6) | −81.7 ± 3.0 | 110.8 ± 22.8 | 1021 ± 107.0 | −44.7 ± 1.5 | 1.2 ± 0.1 | ||
| nonGFP | Tonic (n=18) | −69.1 ± 1.9 | 46.5 ± 7.5 | 1001 ± 116.0 | −46.1 ± 0.8 | 1.3 ± 0.1 | |
| Delayed (n=14) | −74.7 ± 1.8 | 108.8 ± 15.4 | 1013 ± 79.1 | −42.4 ± 1.7 | 1.5 ± 0.1 | ||
| Phasic (n=5) | −72.8 ± 2.9 | 55.5 ± 6.0 | 861.4 ± 137.2 | −42.4 ± 2.9 | 1.5 ± 0.2 | ||
| Single (n=3) | −69.8 ± 5.2 | 116.7 ± 17.4 | 1357 ± 396.3 | −41.8 ± 1.5 | 1.3 ± 0.1 | ||
| Incision | GFP | Tonic (n=25) | −80.5 ± 1.4** | 67.3 ± 7.7## | 910.7 ± 57.6 | −49.2 ± 0.8 | 1.2 ± 0.04 |
| Delayed (n=10) | −87.4 ±1.9 | 177.0 ± 28.4 | 869.9 ± 74.6 | −43.3 ± 1.2 | 1.3 ± 0.1 | ||
| Phasic (n=2) | −55.3 ± 2.5 | 8.8 ± 3.8 | 700.9 ± 165.8 | −46.0 ± 1.8 | 1.8 ± 0.2 | ||
| Single (n=1) | −82.3 | 107.5 | 633.9 | −41.2 | 0.8 | ||
| nonGFP | Tonic (n=16) | −76.7 ±2.3* | 64.7 ± 10.1 | 720.1 ± 74.3 | −48.0 ± 0.7 | 1.1 ± 0.1 | |
| Delayed (n=16) | −79.6 ± 2.1 | 188.3 ± 26.7* | 799.3 ± 73.5 | −41.2 ± 1.4 | 1.3 ± 0.03* | ||
| Phasic (n=4) | −81.2 ±5.7 | 73.8 ± 29.7 | 766.4 ± 125.8 | −44.8 ± 2.0 | 1.3 ± 0.1 | ||
| Single (n=2) | −72.5 ± 7.3 | 125.0 ± 5.0 | 1151 ± 123.2 | −33.8 ± 2.0 | 1.4 ± 0.1 | ||
| Bursting (n=1) | −79.7 | 50.0 | 436.4 | −52.4 | 1.4 | ||
Cells were classified based on the presence or absence of Gad-GFP expression and the pattern of repetitive firing (see Fig. 2A–B).
p<0.05,
p<0.01, unpaired t-test;
p<0.01, Mann-Whitney test compared to the same cell type in naïve animals.
Data are expressed as means ± SEM.
Neonatal injury up-regulates inward-rectifying potassium (Kir) currents in GABAergic neurons within adult spinal nociceptive circuits
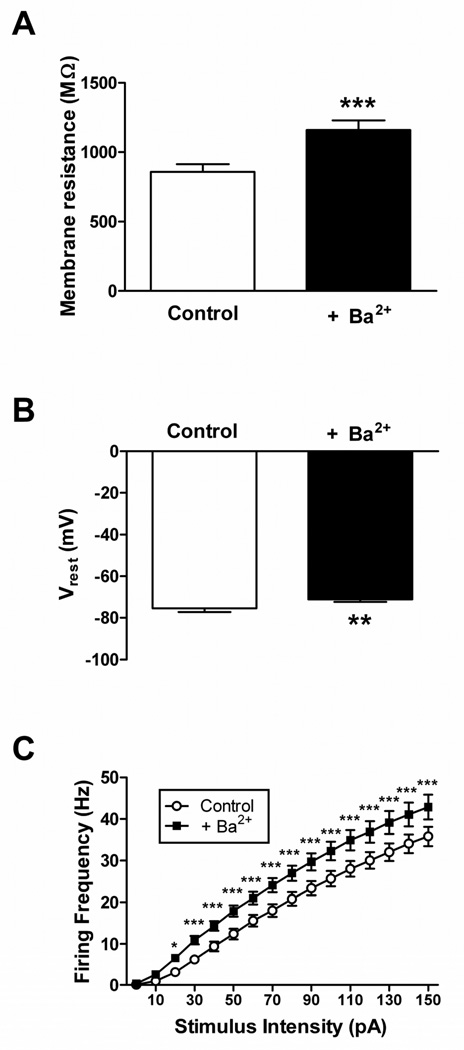
Our recent work has provided evidence that inward-rectifying potassium (Kir) currents make a significant contribution to membrane resistance (Rm) in developing lamina I neurons and can strongly regulate action potential discharge in these cells (Li et al., 2013b). Kir currents also appear to be important determinants of neuronal excitability within the SDH of the mature spinal cord, as blocking Kir channels with extracellular Ba2+ (Coetzee et al., 1999) significantly increased Rm (n = 24; p<0.0001; paired t-test; Fig. 3A), depolarized Vrest (p = 0.007; paired t-test; Fig. 3B) and enhanced the rate of repetitive firing (p<0.0001; Repeated Measure two-way ANOVA; Fig. 3C) across the general population of adult lamina II neurons. Therefore, we next examined the hypothesis that the observed long-term reduction in the intrinsic membrane excitability of mature SDH neurons (Figs. 1, 2) reflects, at least in part, an injury-evoked increase in the levels of Kir conductance.
Figure 3. Barium-sensitive conductances regulate intrinsic membrane excitability in adult mouse SDH neurons.
Bath application of BaCl2 (200 µM) significantly increased membrane resistance (A; ***p<0.0001; paired t-test), depolarized the resting membrane potential (B; **p = 0.007; paired t-test) and elevated instantaneous firing frequency (C; *p<0.05, ***p<0.001; two-way ANOVA with Bonferroni correction for multiple comparisons) in mature lamina II neurons exhibiting tonic firing. Similar results were observed for the Gad-GFP and non-GFP populations, therefore data from the two groups were pooled for analysis.
Kir currents were isolated in SDH neurons as described previously (Derjean et al., 2003; Li et al., 2013b). Briefly, negative voltage ramps (from −55 to −155 mV at 0.2 mV/ms) were applied to the SDH cell in the presence of antagonists to block fast synaptic transmission in the slice (Fig. 4A, black trace). After obtaining baseline recordings, BaCl2 (200 µM) was subsequently added to the bath (Fig. 4A, gray trace). Electronic subtraction of the Ba2+-sensitive component (Fig. 4B) revealed a current with a mean reversal potential (Erev) of −94.2 ± 1.9 mV (n = 48), which was close to the predicted equilibrium potential for K+ ions under our experimental conditions (−101.4 mV). Measurements of conductance (see Methods) at two potentials equidistant from Erev (Fig. 4B) consistently demonstrated inward rectification, as evidenced by a higher conductance at more negative membrane potentials (Vm), which suggests that the above protocol effectively isolates Kir currents in lamina II neurons.
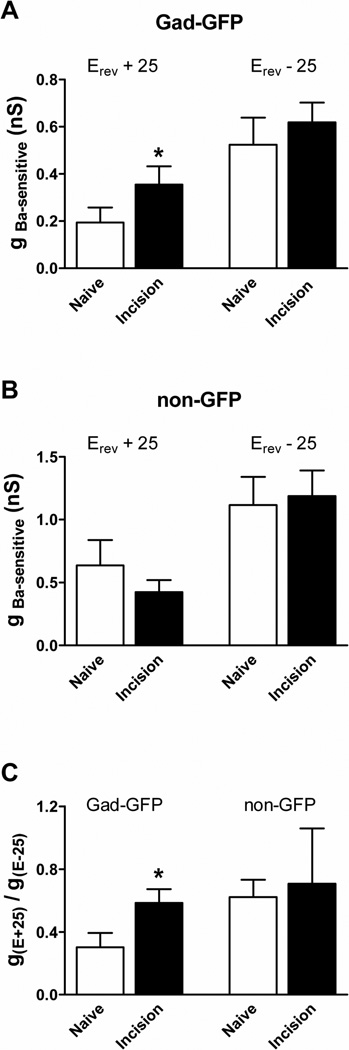
Neonatal tissue injury significantly enhanced the Ba2+-sensitive conductance (gBa-sensitive) within mature GABAergic SDH neurons at Vm positive to the reversal potential (i.e. Erev + 25; n = 11 in Naïve; n = 12 in Incision; p = 0.02; unpaired t-test; Fig. 5A, left), but not at more negative membrane potentials (i.e. Erev - 25; p = 0.213; unpaired t-test; Fig. 5A, right). In addition, Kir currents in adult Gad-GFP cells exhibited a lower degree of inward rectification following P3 tissue damage, as the ratio of gBa-sensitive measured at the more positive Vm relative to that measured at the negative Vm was significantly elevated in the Incision group (p = 0.018; Mann-Whitney test; Fig. 5C, left). In contrast, P3 incision had no significant effects on gBa-sensitive in non-GFP lamina II neurons at either membrane potential (p = 0.842 at Erev + 25; p = 0.828 at Erev - 25; n = 12 in Naïve; n = 13 in Incision; unpaired t-test; Fig. 5B). Neonatal tissue damage also failed to significantly influence the degree of Kir inward rectification in presumed glutamatergic neurons of the adult SDH (p = 0.173; Mann-Whitney test; Fig. 5C, right).
Figure 5. Neonatal tissue damage enhances Kir conductance in GABAergic neurons of the adult SDH.
A: P3 injury evoked a significant increase in gBa-sensitive in Gad-GFP neurons compared to naïve littermate controls at membrane potentials positive to Erev (*p = 0.020; unpaired t-test; left) but not at potentials more negative than Erev (p = 0.213; right). B: In contrast, no such up-regulation of Ba2+-sensitive K+ conductance was observed at either membrane potential (p = 0.842 at Erev + 25; p = 0.828 at Erev – 25; unpaired t-test) within adjacent, non-GFP neurons after neonatal hindpaw incision. C: The degree of inward rectification of Ba2+-sensitive K+ currents (inversely related to g(Erev +25)/g(Erev −25)) was significantly lower after early injury in GABAergic (*p = 0.020 ; Mann-Whitney test; left), but not presumed glutamatergic (p = 0.173; right), neurons of the mature SDH.
Discussion
This study demonstrates, for the first time, that the intrinsic membrane properties of adult SDH neurons are shaped by sensory experience during early postnatal development. Surgical injury during the neonatal period evoked a reduction in neuronal excitability across multiple subpopulations within lamina II of the mature spinal cord. These results also confirm that inward-rectifying K+ (Kir) channels are potent regulators of membrane excitability in adult SDH neurons and further suggest that injury-evoked changes in the expression of Kir channels may contribute to the persistent alterations in intrinsic firing seen in GABAergic interneurons within adult spinal nociceptive circuits.
Long-term changes in nociceptive processing in the SDH following neonatal tissue damage
The enlarged receptive fields, elevated spontaneous firing and greater response to mechanical input observed in mature dorsal horn cells after neonatal injury (Torsney and Fitzgerald, 2003; Peng et al., 2003) would seemingly predict that early tissue damage causes a persistent increase in the intrinsic excitability of developing SDH neurons. However, the present results clearly show that hindpaw incision during the first days of life leads to a prolonged reduction in membrane excitability in both GABAergic and presumed glutamatergic neurons within lamina II of the adult spinal cord (Figs. 1, 2), which likely reflects the injury-evoked decrease in Rm (Fig. 1A) and hyperpolarization in Vrest (Fig. 1C). Interestingly, a previous study has demonstrated that carrageenan inflammation in P7–12 mice causes a biphasic change in intrinsic excitability within immature dorsal horn neurons, with an initial enhancement of excitability observed at 1 hour post-injury followed by a subsequent decrease in action potential (AP) firing at 20 hours (Rivera-Arconada and Lopez-Garcia, 2010). Collectively, these findings suggest that the delayed reduction in intrinsic neuronal excitability following early injury may persist throughout development.
Studies in rodents have demonstrated that tissue damage occurring during a critical neonatal period leads to a delayed (~4 weeks) reduction in the baseline sensitivity of the nociceptive withdrawal reflex which lasts into adulthood (Ren et al., 2004; LaPrairie and Murphy, 2007). The delayed emergence of the persistent hypoalgesia could be explained by the gradual maturation of descending modulatory pathways from the rodent brainstem (Hathway et al., 2009), and neonatal hindpaw inflammation has been reported to enhance descending inhibition originating from the adult rostroventral medulla (Zhang et al., 2010). It is inherently difficult to unambiguously link changes in SDH neuronal firing to behavioral assays of pain sensitivity in rodents, as the latter rely on measuring the motor output of the spinal cord network. Nonetheless, the persistent decrease in the intrinsic membrane excitability of adult SDH neurons following early incision would be expected to limit AP discharge in these cells during acute noxious stimulation, which might also contribute to an elevated threshold of the nociceptive withdrawal reflex.
Our findings also suggest that the hyperexcitability of adult dorsal horn neurons after early tissue injury, previously documented using in vivo recordings from anesthetized animals, likely results from long-term changes in synaptic signaling within spinal nociceptive networks. Notably, we have recently shown a persistent reduction in the efficacy of glycinergic transmission onto both GABAergic and presumed excitatory lamina II neurons of adult Gad-GFP mice following hindpaw incision at P3 (Li et al., 2013a). Given that decreasing synaptic inhibition within the dorsal horn is sufficient to enhance receptive field size and facilitate AP discharge in response to sensory input (Reeve et al., 1998; Bremner et al., 2006; Koch et al., 2012), reduced glycinergic tone within the mature SDH circuit could explain the persistent changes in dorsal horn neuronal firing observed in vivo.
Potential contribution of homeostatic plasticity to altered nociceptive signaling within the spinal cord
It remains to be determined if the decreased glycinergic inhibition and the reduced intrinsic membrane excitability in the adult SDH following early injury are causally related. However, it is now clear that central neurons, including those in the spinal cord (Gonzalez-Islas and Wenner, 2006), can globally adjust the strength of their synaptic inputs (referred to as synaptic scaling) in response to chronic alterations in their firing rate, in order to maintain overall activity levels within a stable range (Turrigiano and Nelson, 2004). This “homeostatic plasticity” can occur in a cell-autonomous manner and the resultant effects on synaptic efficacy depend on the developmental stage of the neuronal network (Burrone et al., 2002). While the majority of studies involving synaptic scaling have focused on the regulation of glutamatergic synaptic transmission following perturbations in firing rates (Turrigiano et al., 1998; O'Brien et al., 1998; Watt et al., 2000), shifts in the strength of inhibitory signaling can also serve to counteract changes in activity levels (Kilman et al., 2002; Peng et al., 2010; Rannals and Kapur, 2011) via mechanisms involving intracellular Ca2+ signaling (Kilman et al., 2002; Saliba et al., 2009). Therefore, although neonatal hindpaw incision failed to significantly affect excitatory synapses in the mature SDH (Li et al., 2013a), the long-term reduction in glycinergic signaling could reflect a homeostatic compensation in response to an injury-evoked decrease in the intrinsic membrane excitability of adult lamina II neurons. Conversely, the loss of normal glycinergic function in the SDH could occur first following neonatal tissue damage, and subsequently trigger the dampening of intrinsic excitability in SDH neurons in order to minimize the shifts in their overall firing rates. Indeed, blocking inhibitory hippocampal synapses with bicuculline evokes a significant reduction in repetitive firing frequency in CA1 neurons (Karmarkar and Buonomano, 2006). Further experiments identifying the precise developmental time points at which these two changes emerge in the SDH following neonatal injury may yield insight into this issue.
Persistent, cell type-specific changes in Ba2+-sensitive Kir conductance within the mature SDH after early injury
Neurons with larger conductance through inward-rectifying K+ (Kir) channels generally possess more negative resting potentials, lower membrane resistance (Rm) and reduced spontaneous firing (Hibino et al., 2010). Consistent with the notion that Kir currents function to limit neuronal excitability in the adult SDH via their contribution to the resting leak conductance, blocking Kir channels with Ba2+ significantly increased Rm and facilitated repetitive action potential discharge in mature lamina II neurons (Fig. 3). Therefore, it seems probable that the up-regulation of Kir conductance in GABAergic neurons near physiological membrane potentials (Fig. 5A) contributes to the observed decrease in intrinsic membrane excitability within this population after neonatal injury (Figs. 1, 2). Although the mechanisms underlying this enhanced Kir conductance remain to be determined, the altered degree of Kir inward rectification after neonatal incision (Fig. 5C) argues against an increased number of Kir channels as the sole explanation for this effect, and instead suggests that mature GABAergic neurons express functionally distinct Kir currents in the aftermath of neonatal tissue damage. Given that Kir channels can be composed of heteromultimers (Fink et al., 1996; Preisig-Muller et al., 2002) and that different Kir subunits vary in their degree of inward rectification (Dhamoon et al., 2004; Ishihara and Yan, 2007), it is possible that neonatal injury evokes persistent changes in the stoichiometry of Kir channels expressed by inhibitory interneurons within developing spinal pain networks.
However, such changes in Kir expression are clearly not required for the persistent reduction in intrinsic neuronal excitability after early injury, as adjacent non-GFP neurons exhibited many of the same alterations in passive and active membrane properties without measurable changes in their levels of Kir conductance (Fig. 5B). The lower Rm and negative shift in Vrest seen in this population after early tissue damage may instead reflect the elevated expression of other K+ channels known to contribute to leak membrane conductance in central neurons, including (but not limited to) two-pore domain K+ channels (Goldstein et al., 2001) and some subtypes of voltage-gated K+ channels, including members of the Kv3.x and Kv7.x (KCNQ) families, which can be open near Vrest (Schroeder et al., 2000; Abbott et al., 2001; Rivera-Arconada and Lopez-Garcia, 2010). In addition, long-term changes in the density of sustained voltage-gated K+ currents may play an important role in the reduced membrane excitability seen during adulthood, as a significant up-regulation of delayed-rectifier K+ currents has been reported in developing dorsal horn neurons following neonatal inflammation (Rivera-Arconada and Lopez-Garcia, 2010). Although a more thorough characterization of the voltage-dependent and voltage-independent ionic conductances of developing SDH neurons is clearly required, it would not be surprising if distinct subpopulations of lamina II neurons relied on different underlying mechanisms to achieve a reduction in intrinsic membrane excitability following neonatal tissue damage. In fact, it is now well-established that neurons with identical firing patterns can nonetheless express very different combinations of ion channels (Marder and Goaillard, 2006).
Future Directions
It will ultimately be important to elucidate the somatotopy of these changes in intrinsic excitability within the mature superficial dorsal horn (SDH) following early tissue damage. In other words, are these alterations in membrane properties restricted to the regions of the SDH which receive direct projections from primary afferents innervating the injury site, or do similar shifts in excitability occur throughout the rostrocaudal axis of the spinal cord? Notably, the long-term hypoalgesia seen in rodents following neonatal tissue injury occurs in a global manner (Ren et al., 2004; Laprairie and Murphy, 2007). These behavioral studies also identified a clear critical period of postnatal development in which the injury must occur in order to evoke these persistent changes in pain sensitivity. Therefore, it also remains to be determined if the documented shifts in intrinsic membrane excitability share this same critical period or also occur in response to tissue injury at later ages.
Conclusions
The present findings demonstrate that the intrinsic firing properties of neurons within adult spinal pain circuits are highly sensitive to noxious sensory experience during the neonatal period. As a result, this study adds to the growing body of work illustrating that early trauma can have lifelong consequences for nociceptive processing in the CNS.
Highlights.
• Neonatal injury alters the passive membrane properties of adult dorsal horn neurons
• Early incision reduces intrinsic membrane excitability in the mature dorsal horn
• Kir channels regulate membrane excitability in adult mouse dorsal horn cells
• Neonatal incision up-regulates Kir currents selectively in adult GABAergic neurons
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NS072202 to MLB). The authors would also like to thank Elizabeth Kritzer for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jie Li, Email: li2je@uc.edu.
Mark L. Baccei, Email: mark.baccei@uc.edu.
References
- Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SA. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- Blankenship ML, Coyle DE, Baccei ML. Transcriptional expression of voltage-gated Na(+) and voltage-independent K(+) channels in the developing rat superficial dorsal horn. Neuroscience. 2013;231:305–314. doi: 10.1016/j.neuroscience.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner L, Fitzgerald M, Baccei M. Functional GABA(A)-receptor-mediated inhibition in the neonatal dorsal horn. J Neurophysiol. 2006;95:3893–3897. doi: 10.1152/jn.00123.2006. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de ME, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le MG, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Sawchuk MA, Hochman S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience. 2009;163:909–919. doi: 10.1016/j.neuroscience.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Heurteaux C, Lesage F, Romey G, Barhanin J, Lazdunski M. Dominant negative chimeras provide evidence for homo and heteromultimeric assembly of inward rectifier K+ channel proteins via their N-terminal end. FEBS Lett. 1996;378:64–68. doi: 10.1016/0014-5793(95)01388-1. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. Recording temperature affects the excitability of mouse superficial dorsal horn neurons, in vitro. J Neurophysiol. 2008;99:2048–2059. doi: 10.1152/jn.01176.2007. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587:2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkϋhler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Huang M, Huang T, Xiang Y, Xie Z, Chen Y, Yan R, Xu J, Cheng L. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev Biol. 2008;322:394–405. doi: 10.1016/j.ydbio.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yan DH. Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels. J Physiol. 2007;583:891–908. doi: 10.1113/jphysiol.2007.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur J Neurosci. 2006;23:1575–1584. doi: 10.1111/j.1460-9568.2006.04692.x. [DOI] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Tochiki KK, Hirschberg S, Fitzgerald M. C-fiber activity-dependent maturation of glycinergic inhibition in the spinal dorsal horn of the postnatal rat. Proc Natl Acad Sci U S A. 2012;109:12201–12206. doi: 10.1073/pnas.1118960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. 2007;132(Suppl 1):S124–S133. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML. Pacemaker neurons within newborn spinal pain circuits. J Neurosci. 2011;31:9010–9022. doi: 10.1523/JNEUROSCI.6555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML. Developmental regulation of membrane excitability in rat spinal lamina I projection neurons. J Neurophysiol. 2012;107:2604–2614. doi: 10.1152/jn.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Blankenship ML, Baccei ML. Deficits in glycinergic inhibition within adult spinal nociceptive circuits after neonatal tissue damage. Pain. 2013a;154:1129–1139. doi: 10.1016/j.pain.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Blankenship ML, Baccei ML. Inward-rectifying potassium (Kir) channels regulate pacemaker activity in spinal nociceptive circuits during early life. J Neurosci. 2013b;33:3352–3362. doi: 10.1523/JNEUROSCI.4365-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YB, Ling QD, Ruda MA, Kenshalo DR. Electrophysiological changes in adult rat dorsal horn neurons after neonatal peripheral inflammation. J Neurophysiol. 2003;90:73–80. doi: 10.1152/jn.01019.2002. [DOI] [PubMed] [Google Scholar]
- Peng YR, Zeng SY, Song HL, Li MY, Yamada MK, Yu X. Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J Neurosci. 2010;30:16220–16231. doi: 10.1523/JNEUROSCI.3085-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Preisig-Muller R, Schlichthorl G, Goerge T, Heinen S, Bruggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci U S A. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. J Neurophysiol. 1998;79:1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Lopez-Garcia JA. Changes in membrane excitability and potassium currents in sensitized dorsal horn neurons of mice pups. J Neurosci. 2010;30:5376–5383. doi: 10.1523/JNEUROSCI.4359-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkϋhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Gu Z, Yan Z, Moss SJ. Blocking L-type voltage-gated Ca2+ channels with dihydropyridines reduces gamma-aminobutyric acid type A receptor expression and synaptic inhibition. J Biol Chem. 2009;284:32544–32550. doi: 10.1074/jbc.M109.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol. 2003;550:255–261. doi: 10.1113/jphysiol.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Walsh MA, Graham BA, Brichta AM, Callister RJ. Evidence for a critical period in the development of excitability and potassium currents in mouse lumbar superficial dorsal horn neurons. J Neurophysiol. 2009;101:1800–1812. doi: 10.1152/jn.90755.2008. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Wang XM, Ennis M. Effects of neonatal inflammation on descending modulation from the rostroventromedial medulla. Brain Res Bull. 2010;83:16–22. doi: 10.1016/j.brainresbull.2010.07.007. [DOI] [PubMed] [Google Scholar]