Abstract
Environmental microbes harbor an enormous pool of antibiotic and biocide resistance genes that can impact the resistance profiles of animal and human pathogens via horizontal gene transfer. Pseudomonas putida strains are ubiquitous in soil and water but have been seldom isolated from humans. We have established a collection of P. putida strains isolated from in-patients in different hospitals in France. One of the isolated strains (HB3267) kills insects and is resistant to the majority of the antibiotics used in laboratories and hospitals, including aminoglycosides, ß-lactams, cationic peptides, chromoprotein enediyne antibiotics, dihydrofolate reductase inhibitors, fluoroquinolones and quinolones, glycopeptide antibiotics, macrolides, polyketides and sulfonamides. Similar to other P. putida clinical isolates the strain was sensitive to amikacin. To shed light on the broad pattern of antibiotic resistance, which is rarely found in clinical isolates of this species, the genome of this strain was sequenced and analysed. The study revealed that the determinants of multiple resistance are both chromosomally-borne as well as located on the pPC9 plasmid. Further analysis indicated that pPC9 has recruited antibiotic and biocide resistance genes from environmental microorganisms as well as from opportunistic and true human pathogens. The pPC9 plasmid is not self-transmissible, but can be mobilized by other bacterial plasmids making it capable of spreading antibiotic resistant determinants to new hosts.
Introduction
Human disease outbreaks are increasing at an alarming rate. One of the most recent and serious occurred in Germany, involving a Stx2a-producing Escherichia coli (STEC) strain of serotype O104:H4 that caused more than 4000 cases of illness and 50 deaths. This strain exhibited resistance to numerous antibiotics making it difficult to eradicate [1]. Horizontal gene transfer has been proposed as the most likely genetic event for the spread of multidrug resistant phenotypes in pathogens [2]; however, a question that still needs to be answered is ‘What is the origin of these acquired antibiotic resistant determinants?’
Pseudomonas putida strains are typically found in soil and water and members of this species have a broad metabolic versatility, which allows them to adapt to different habitats and nutritional environments [3], [4], [5], [6]. Strains of this species have occasionally been isolated from patients in hospitals in Japan, the USA, Italy and France. Infections by these microorganisms have been reported to be linked to insertion of catheters or drainage tubes [7], [8]. Hospital isolates of P. putida are often resistant to β-lactams [9], [10], [11], and instance Yomoda et al. [11] reported that of 32 P. putida strains isolated in hospitals in Japan twenty two of them harbored plasmids transferable to P. aeruginosa by conjugation or transformation. The same study also indicated that a number of plasmids from these clinical isolates were responsible for resistance to aminoglycosides. Apart from the fact that opportunistic microbes could become ‘specialized’ pathogens able to attack the most vulnerable immunocompromised patients [11], [12]; the ability to transfer antibiotic resistant determinants from non-pathogenic species to pathogens in hospital environments is a serious concern [11], [12].
The Hospital of Besançon in France has established a collection of P. putida isolates from in-patients, and in agreement with von Greenitz and Weinstein [7], it has been found that these strains have a low pathogenic potential when compared with P. aeruginosa PAO1 using virulence assays in a insect model (our unpublished results). We analyzed 15 of these isolates and found that one of them, P. putida HB3267 (Hospital of Bensançon 3267), was able to kill insects and exhibited resistant to a large number of antibiotics. To shed light on the unusual pattern of antibiotic resistance of the strain HB3267 we sequenced and analysed the genome. The analysis revealed that a number of genes involved in multi-drug resistant phenotypes are located in a non-self-transmissible plasmid that was shown to be an efficient vehicle for spreading antibiotic resistance between different Pseudomonas strains.
Materials and Methods
DNA analysis and identification of the HB3267 strain
Amplification of 16S rDNA using HB3267 chromosomal DNA was performed with the F8 and R798 primers and the complete sequence of the gene compared with 16S rDNA sequences in databases [13]. Aranda-Olmedo et al. [14] showed that P. putida strains are characterized by the presence of a highly conserved 35-mer REP sequence. A primer based on the KT2440 REP sequence was used to amplify HB3267 DNA. Positive (P. putida KT2442, [15]) and negative (Escherichia coli) DNA controls were included. The REPc method allows identification of P. putida strains [14] since this primer amplifies only DNA from this species, producing products of different sizes for each strain. For multilocus sequence typing (MLST) we used a set of primers to amplify RNA polymerase sigma factor rpoD, DNA gyrase subunit B gyrB, N-(5′-phosphoribosyl) anthranilate isomerase trpF, 6-phosphogluconate dehydratase edd, and recombinase A recA genes [16], [17]. The complete gene sequences were obtained, translated into the protein sequence and compared as described [17].
Antibiograms
For these assays 31 different antibiotics were used (Biomerieux commercial disk). Overnight cultures of HB3267 were spread on 240×240 mm LB plates, air dried in a laminar flow and then discs containing antibiotics were placed on the LB plates. Plates were incubated at 30°C for 16 h. Halos surrounding the discs were measured as an indication of inhibition of growth. This assay was repeated at least three times in duplicate.
Minimal inhibitory concentration (MIC) assay
These assays were performed in 96-well plates, using LB medium and the following stock antibiotic solutions: tetracycline (Tc), 10 mg/ml; kanamycin (Km), 25 mg/ml; gentamicin (Gm), 100 mg/ml; nalidixic acid (Nal), 10 mg/ml; spectinomycin (Sp), 100 mg/ml; rifampicin (Rif), 10 mg/ml; chloramphenicol (Cm), 30 mg/ml, ampicillin (Ap), 100 mg/ml, norfloxacin (Nor), 20 mg/ml and ceftriaxone (Cro), 25 ml. Serial 10-fold dilutions of the stock antibiotic solutions were prepared and 10 µl of each of these dilutions added to 190 µl of LB, minimal medium M9 and Mueller-Hinton broth medium. Optically standardized 18 hour cultures (10 µl) of P. putida strains were used as inoculum. The 96-well plates were incubated at 30°C and 200 rpm overnight and culture turbidity was measured as an indication of growth. The MIC value was established as the lowest concentration at which an antibiotic inhibits growth >90% [18]. The assays were repeated three times in duplicate.
Biofilm susceptibility testing
Biofilm assays were performed in 96-well flat-bottomed polystyrene microtitre plates. An aliquot of 100 µL of a bacterial suspension contains 105 CFU/ml was added to each well and incubated for 5–6 h at 30°C. Subsequently, liquid culture medium was removed; the wells of the plates washed twice to eliminate all planktonic cells and finally serially diluted antibiotics in LB medium added. These plates were incubated overnight at 30°C, and after removing planktonic cells as above 100 µL LB was added, and the biofilm cells released by 5 minutes low intensity sonication (Branson 1510 waterbath ultrasonicator). The minimal biofilm eradication concentration (MBEC) was defined as the minimal concentration of antibiotic required to eradicate the biofilm [19].
Conjugation experiments
Pseudomonas putida KT2440 (Tel), a tellurite resistant strain and P. putida HB3267 were grown overnight on LB medium.
For biparental matings 1 ml cultures with a turbidity of around 1 at 660 nm were mixed, harvested by centrifugation, wasted with LB and resuspended in 50 µl LB that was laid on nitrocellulose filter disks placed on LB plates. After overnight incubation, transconjugants were selected on LB medium containing Tel, Sm and Tc. For triparental mating experiments, receptor and donor strains were used as previously described, but the pWW0 [20] and pRK600 plasmids were used as helper plasmids.
Sequencing
Genomic DNA containing both the chromosome and pPC9 plasmid was purified from strain P. putida HB3267, using the Wizard® Genomic DNA Purification Kit and sequenced using 454 technology by Macrogen (Seoul, Korea), and assembled into 278 contigs, providing 25× coverage. These contigs were ordered by comparison (BLASTN) with the genomic sequences from other P. putida available in the database (KT2440, NC_002947.3; F1, CP000712.1; GB-1, CP000926.1; W619, CP000949.1; BIRD1, CP002290.1), as well as with a close relative Pseudomonas entomophila L48 (NC_008027.1). Genomic gaps were closed by designing primers at the contig ends, followed by PCR and further sequencing of the junction sequences. Genomic DNA was automatically annotated using a program pipeline based on Glimmer 3.0 [21] for gene prediction, and BLAST and RPSBLAST for functional assignment of ORFs, based on sequence similarity to sequences deposited in the NR, Swissprot, COG, Pfam, Smart and Prk databases [22] Finally, automatic annotations were manually curated. The chromosome and plasmid sequences are available through Genbank under accession numbers CP003738 and CP003739, respectively.
Results and Discussion
Identification of strain HB3267
A collection of Pseudomonas putida strains isolated from humans has been established in the Bacteriology Laboratory of the University Hospital of Besançon (France). Among these, only one strain named HB3267 which was isolated from an in-patient who died from unknown causes, was able to kill insects (Porcel, M. and Duque, E., In preparation). Since P. putida strains are seldom isolated from humans a series of molecular analysis were carried out to unequivocally assign this strain to the putida species. Aranda-Olmedo et al. [14] showed that a conserved 35 bp repetitive extragenic palindromic (REP) sequence is specifically associated with P. putida strains. PCR analysis was performed using chromosomal DNA of HB3267 as a template and a primer based on the previously defined REP sequence [14]; and a positive amplicon was obtained. The same assay was performed with KT2440 as a positive control and E. coli as a negative control, with the expected results. The presence of the REP sequence in the genome of strain HB3267 suggested the original assignment of this strain to the species P. putida based on API classification was correct. To further confirm this result 16S rDNA amplification was carried out and the whole gene encoding the 16S sequence was obtained. Sequence comparison confirmed that HB3267 closest 16S genetic homologues all belong to the P. putida species. To establish a relationship with other P. putida strains, multi-locus sequence typing assays (MLST assays) were carried out using the housekeeping genes-rpoD, gyrB, trpF, edd and recA; these analyses confirmed that the gene products of HB3267 exhibited the highest identity with the gene products of several P. putida strains. Phylogenetically, strain HB3267 was closest to P. putida S16, a nicotine degrader isolated from a field under continuous tobacco cropping [23], [24] (Figure S1).
Antibiotic resistance profile of P. putida strain HB3267
A distinctive characteristic of the HB3267 strain was its apparent multidrug resistance compared to other P. putida from the same hospital collection. Disk inhibition and MIC assays were performed with the most frequently used laboratory/clinical antibiotics to obtain quantitative data; for comparison we used P. putida KT2440R, a well characterized strain as a control (Table 1). Antibiogram assays showed that P. putida HB3267 was resistant to most of the 31 antibiotics tested in this study (Table 1). The exceptions were the aminoglycoside amikacin, as well as rifampicin and nitrofurantoin. A remarkable discovery was that HB3267 was resistant to all fluoroquinolones tested (ciprofloxacin, norfloxacin, pefloxacin and ofloxacin), while KT2440R showed high sensitivity to these antibiotics. The same was true for most aminoglycoside antibiotics tested (gentamycin, kanamycin, neomycin, streptomycin and netilmicin), with the exception of amikacin as mentioned.. The two strains also differed in their resistance to polymyxin B, colistin, cefotaxime, amoxicillin, imipenem, cephalosporin and ceftazidime, being that HB3267 was resistant to all of them while KT2440 was sensitive. MIC assays revealed that HB3267 was highly resistant to aminoglycoside antibiotics such as gentamycin, kanamycin and spectinomycin being, depending of the medium employed, at least 220-fold, 75-fold and 333-fold more resistant than KT2440R, respectively. The HB3267 strain was also much more resistant to polyketide antibiotics such as tetracycline (23.-fold more resistant), quinolone antibiotics such as nalidixic acid (67-fold), β-lactams such as ampicillin (30-fold), and bacteriostatic antibiotics such as chloramphenicol (3-fold). Pseudomonas putida KT2440R is a spontaneous mutant obtained by exposure to rifampicin [25], which explains why the MIC concentration for this antibiotic was 32-fold higher for KT2440R than for HB3267 (Table 2). Compared to other pseudomonad clinical isolates, the HB3267 strain was more resistant (in terms of range of antibiotic and MICs) than P. aeruginosa PAO1. For example, HB3267 was 5000 times more resistant to gentamicin than P. aeruginosa PAO1 [26].
Table 1. Antibiogram assay.
| Antibiotic, concentration (mg) | Strain | Antibiotic group | |
| KT2440R* | HB3267* | ||
| Ciprofloxacin, 5 | 2.8 | 0 | fluoroquinolone |
| Norfloxacin, 10 | 2.5 | 0 | fluoroquinolone |
| Pefloxacin, 5 | 1.9 | 0 | fluoroquinolone |
| Ofloxacin, 5 | 1.8 | 0 | fluoroquinolone |
| Nalidixic acid, 30 | 0 | 0 | quinolone |
| Erithromycin, 15 | 0 | 0 | macrolide |
| Gentamycin, 10 | 1.8 | 0 | aminoglycoside c |
| Kanamycin, 30 | 2 | 0 | aminoglycoside |
| Neomycin, 30 | 1.8 | 0 | aminoglycoside |
| Streptomycin, 10 | 0.9 | 0 | aminoglycoside |
| Amikacin, 30 | 0 | 1.7 | aminoglycoside |
| Netilmicin, 30 | 1.4 | 0 | aminoglycoside |
| Tetracycline, 30 | 0 | 0 | polyketide |
| Polymyxin B, 300 | 1.3 | 0 | polymyxin |
| Colistin, 50 | 1.5 | 0 | polymyxin |
| Trimethoprim, 20 | 0 | 0 | dihydrofolate reductase inhibitors |
| Chloramphenicol, 30 | 0 | 0 | bacteriostatic |
| Amoxycillin, 25 | 1 | 0 | ß-lactam (penicillin) |
| Carbenicillin, 100 | 0 | 0 | ß-lactam (penicillin) |
| Ticarcillin, 70 | 0 | 0 | ß-lactam (penicillin) |
| Piperacillin, 10 | 0 | 0 | ß-lactam (penicillin) |
| Ampicillin, 10 | 0 | 0 | ß-lactam (penicillin) |
| Imipemen, 10 | 2.8 | 0 | ß-lactam (carbapenem) |
| Cefotaxime, 30 | 1.5 | 0 | ß-lactam (cephalosporin) |
| Ceftazidime, 30 | 1.6 | 0 | ß-lactam (cephalosporin) |
| Ceftriaxone, 30 | 0 | 0 | ß-lactam (cephalosporin) |
| Sulfonamide G, 20 | 0 | 0 | sulfonamides |
| Rifampicin, 30 | 0.6 | 1.6 | rifamycin |
| Vancomycin, 30 | 0 | 0 | glycopeptide |
| Esperamicin, 100 | 0 | 0 | chromoprotein enediyne |
| Nitrofurantoin, 300 | 1.8 | 2.4 | |
Numbers indicate the size of the inhibition halo surrounding the antibiotic disc in cm.
Data are the average of 3 assays performed in duplicate with standard deviation below 5% of the given values.
Table 2. MIC and MBEC assays with different Pseudomonas putida strains.
| MIC (LB) | MIC (M9) | MIC (M-H) | MBEC | |||||
| Antibiotic | HB3267 | KT2440R | HB3267 | KT2440R | HB3267 | KT2440R | HB3267 | KT2440R |
| Tetracycline | 200 | 8 | 450 | 30 | 350 | 15 | >10000 | 2500 |
| Kanamycin | 3125 | 10 | 1500 | 10 | 1500 | 20 | >25000 | 1600 |
| Gentamicin | 10000 | 20 | 5000 | 2 | 5500 | 25 | >10000 | 1250 |
| Nalidixic acid | 2000 | 30 | >1800 | 25 | >1800 | 30 | >10000 | 625 |
| Spectinomycin | 10000 | 30 | >10000 | 10 | >10000 | 15 | >50000 | 1250 |
| Rifampicin | 62 | 2000 | 5 | 600 | 10 | 800 | 1250 | 5000 |
| Choramphenicol | 1000 | 376 | 1200 | 250 | 1000 | 300 | >30000 | 1800 |
| Ampicillin | 10000 | 625 | 10000 | 600 | 10000 | 600 | >100000 | 12500 |
| Fluoroantimicin | 1560 | >3000 | nt | nt | nt | nt | 25000 | nt |
| Amikacin | 16 | >100 | 1 | 2.5 | 1 | 2 | 50 | 625 |
| Ceftriaxone | 325 | 10 | 300 | 7.5 | 300 | 10 | 12500 | 1600 |
| Norfloxacin | 220 | 10 | 220 | 1 | 240 | 10 | 5000 | 75 |
Numbers indicate the MIC concentration (µg/ml) and MBEC concentrations (µg/ml) required to inhibit 90% growth and for biofilm eradication.
nt, not tested.
Horii et al. [27] analyzed the susceptibility of five clinical isolates of P. putida (from patients with acute, repetitive or chronic urinary tract infections) to fluoroquinolones. Similar to HB3267, four of the five isolates were resistant to fluoroquinolones, but in contrast with HB3267, all isolates were susceptible to aminoglycosides. MICs assays revealed that the resistance of HB3267 was at the same range than the described P. putida clinical isolates and at least 22-fold more than KT2440 [27]. Treviño et al. [28] also isolated two P. putida strains from immunocompromised patients. These two strains, as for the five isolates of Horii et al. [27], and the HB3267 strain we describe here, are susceptible to amikacin; these results indicate that this antibiotic could be used for treatment of multidrug-resistant P. putida strains. Resistance of P. putida clinical isolates to β-lactams [26], [27], [28], [29], aminoglycosides [26], [27], [30], and fluoroquinolones has been described [26], [27], [31], [32]; however, it should be noted that the minimal inhibitory concentrations found for HB3267 are much higher than what has been reported for other clinical isolates. HB3267 is the only P. putida clinical isolate reported to be resistant to tetracycline. The HB3267 strain is also the only P. putida clinical isolate described to be resistant to the biocide sulfonamide, trimethoprim/sulfamethoxazole and colistin, which is a characteristic often associated to P. aeruginosa [33], [34], [35].
It is known that P. putida strains are able to form biofilms on biotic and abiotic surfaces [25], [36]. Bacterial cells in biofilms are often more resistant to antibiotics than planktonic cells. We used the O'Toole and Kolter approach to produce biofilms of P. putida HB3267 which were flat and dense and similar to those produced by the KT2440 strain [36]. We then determined the Minimal Biofilm Eradication Concentration (MBEC) as described by Ceri et al. [19]. We found that both strains, KT2440 and HB3267 were much more resistant forming biofilms than living as planktonic cells, but most of the antibiotic tested, such as gentamicin, ampicillin, tetracycline, kanamycin, nalidixic acid, spectinomicyn and chloramphenicol, that were able to eradicate at high concentrations the KT2440 biofilms, were unable to eradicate those formed by HB3267. Antibiotics that were effective (rifampicin, fluoroantimicin, amikacin, ceftriaxone, and norfloxacin were required in 3- to 40-fold higher concentrations than those required to inhibit more than 90% growth of planktonic cells (Table 2).
Gaining insight into antibiotic resistance through whole genome sequencing of HB3267
The total genome size of the P. putida HB3267 strain is 5908671 bp with a G+C content of 63.61%, which is similar to the published genomes of other P. putida strains. The HB3267 genome comprises a chromosome of 5829171 bp (Gene Bank CP003738) and a plasmid of 80360 bp named pPC9 (Gene Bank CP003739). The genome of HB3267 contains 5261 ORFs of which 5196 encode proteins. The other ORFs code for 61 tRNAs, 4 5S rRNAs, 4 16S rRNAs and 4 23S rRNAs. All essential conditional genes identified in KT2440 [4], [37] are present in the genome of HB3267, and functions were assigned to almost 75% of the total genes which encode proteins.
Analysis of the sequence of the pPC9 plasmid revealed that this plasmid has a modular structure with 90 open reading frames (ORFs) that are distributed across several domains (Figure 1). The pPC9 backbone (38 kb) primarily contains genes for plasmid-related functions such as those required for replication and partitioning [38]. (Figure 1), and it exhibits high similarity to the backbone of pCT14 from Pseudomonas sp. CT14, a strain isolated from activated sewage sludge, while the “insert” region is made of several transposon-like elements bearing genes related to transposition and resistance to multiple antibiotics (Figure 2).
Figure 1. The pPC9 map.
Genetic organization of pPC9, in white, genes forming the backbone of pPC9, in grey genes from the insert with homology to genes related to transposition, in black genes from the insert with antibiotic resistance function.
Figure 2. Genetic organization of the pPC9 “insert.”.
Black arrows represent genes with functions related to antibiotic resistance. In grey are genes with functions related to transposition and insertion machinery. Non-colored genes are those that encode hypothetical proteins, those with unknown function, or those with functions unrelated to antibiotic resistance, transposition or integration. Horizontal lines over genes represent DNA homology to different microorganisms, and percentages indicate the degree of homology.
The backbone of pPC9 (Figure 1), bears only two putative conjugation genes (traJ,I), lacking most of the genes that would be necessary for self-mobilization. In agreement with this is that in biparental conjugation experiments this plasmid was unable to be transferred from HB3267 to other P. putida strains. Triparental mating experiments using the Escherichia coli pRK600 plasmid or the P. putida pWW0 plasmid as a helper also showed the inability of pPC9 to be transferred to P. putida strains. However, in experiments with pWW0, recombination events between pPC9 and pWW0 were detected; with transconjugants bearing a plasmid that conferred the ability to grow on toluene from pWW0, and streptomycin and tetracycline resistance from pPC9. This event occurred at a rate of 10−4 transconjugants per donor. This result confirms the presumption made by Yomoda et al. [11] that the existence of P. putida resident species in in-patients can facilitate the spread of drug resistance genes via horizontal gene transfer.
The “insert” region of the pPC9 plasmid is a mosaic of recruited DNA from different microorganisms. The antibiotic resistant determinants of pPC9 are grouped within three subregions as illustrated in Figure 2, surrounded by genes related to transposition and/or integration. A potential source of this DNA were closely related Pseudomonas strains such as the nonhuman pathogen Pseudomonas sp. ND6, isolated from industrial wastewater [39]; Pseudomonas syringae pv. actinidiae, which is the causal agent of bacterial canker of green-fleshed kiwifruit [40]; and P. fluorescens HK44, which colonizes plant roots and degrades phenolic compounds [41]. Within this “insert” region, DNA fragments could have also been acquired from human opportunistic pathogens or pathogens, such as P. aeruginosa, Aeromonas hydrophila, Acinetobacter baumannii, Corynebacterium resistens and Enterobacteriaceae (Klebsiella pneumoniae, Salmonella enterica) (Figure 2). This mosaic suggests that HB3267 may have been a resident of different environments, such as water, soil, plant rhizospheres and the human body, or that it has exchanged DNA with these potential microorganisms in different habitats.
Antibiotic resistance determinants encoded only on the HB3267 chromosome
Quinolones and fluoroquinolones
Quinolones and fluoroquinolones are chemotherapeutic bactericidal drugs that eradicate bacteria by interfering with DNA replication. Quinolones enter into cells through porins and their targets are DNA gyrases and topoisomerases [42]. Point mutations in gyrA and gyrB (DNA gyrase subunits), and parC and parE (topoisomerase IV subunits) are associated with resistance to fluoroquinolone antibiotics [43]. In the HB3267 strain, GyrA showed a series of amino acid replacements with respect to the GyrA protein of fluoroquinolone-sensitive strains; HB3267 shows a Thr83Iso mutation in GyrA, which is known to lead to fluoroquinolone resistance in other clinic isolates of P. putida as HU2001-412 strain [27], that is present also in P. aeruginosa LESB58; only a unique difference in the case of HB3267 was a Val to Gly change at residue 894 (Figure S2A). In the case of GyrB, a polymorphism unique to HB3267 and the resistant HU2001-412 strain was Glu468Asp, also known to lead fluoroguinolone resistance [27] (Figure S2B). For ParC, the substitution Ser87Leu was present in HB3267 and P. aeruginosa PA7; however, for the other topoisomerase IV subunit ParE no substitution was found. The unique polymorphism in GyrA and/or GyrB and/or ParC may be responsible for the resistance of HB3267 against these antibiotics, but this hypothesis requires further testing using site specific mutants and gene complementation experiments.
Cationic antimicrobial peptides
Strain HB3267 is more resistant to polymyxin B than KT2440R (Tables 1 and 2) and other strains [44]. LPS is one of the surface elements that influence resistance to cationic peptides in P. aeruginosa [45]. Vaara (1993) described that the firA gene product, involved in lipid A biosynthesis was relevant in the resistance of E. coli and S. typhimurium to polymyxin B [46]. Strain HB3267 harbours two copies of this gene which encodes for a UDP-3-O-3-hydroxymyristoyl glucosamine N-acyltransferase that is 70% identical to the FirA of Klebsiella pneumoniae. The higher copy number of firA may provide an explanation for the HB3267 strain's higher resistance to polymyxin B than in other strains of P. putida.
Antibiotic resistance determinants encoded on the chromosome of HB3267 and its resident pPC9 plasmid.
Aminoglycosides
Aminoglycosides bind to the A-site of the 30S subunit of bacterial ribosomes disturbing elongation of the peptide chain. Wei et al. (2011) demonstrated using data obtained from phenomics, transcriptomics and proteomic analysis that resistance to aminoglycosides in the P. aeruginosa PAO1 strain is multifactorial including the presence of mutations in chromosomal genes such as the phoP and phoQ, as well as in the mexZ gene encoding a repressor of the mexXY genes encoding an efflux pump [47]. When all the sequences of phoQ from aminoglycoside sensitive Pseudomonas strains were compared to the resistant HB3267 strain, the Gly365Arg polymorphism was exclusive to HB3267(Figure S2C). In the case of PhoP, Ser21Gly (exception P. putida S16 and P. entomophila L48), and Arg204His (exception P. putida S16) changes were found but none were exclusive of HB3267. No clear homolog to mexZ was present in the chromosome of HB3267.
HB3267 sensitivity and KT2440 resistance to amikacin may be explained by multifactorial differences in expression of chromosomal genes involved in cell permeability, LPS synthesis, efflux pumps and chemical modification [48]. Vaziri et al. (2011) described the existence of aminoglycoside modifying enzymes that are encoded by plasmids as the primary resistance mechanism employed by P. aeruginosa against these antibiotics [49]. The pPC9 plasmid carries genes that encode 6 aminoglycoside modifying enzymes that are not present in the genome of P putida strains sensitive to aminoglycosides. Therefore, these aminoglycosidases likely contribute to the resistance phenotype of HB3267. One of these aminoglycosidases was 100% identical to the aadB gene product of P. aeruginosa (Figure 2 subunit 1). This protein has 2″-aminoglycoside nucleotidyltransferase activity, and has been proposed to be responsible for bacterial resistance to the aminoglycosides dibekacin, gentamicin, kanamycin, sisomicin and tobramycin [50]. Another protein was 100% identical to StrA from Salmonella enterica that has aminoglycoside 3′-phosphotransferase activity and a third protein was 100% identical to StrB from Acinetobacter baumannii, which has aminoglycoside 6′-phosphotransferase activity (Figure 2 subunit 2). Both of these proteins have been traditionally associated with resistance to the aminoglycoside streptomycin [51]. We also found a gene that encodes for a protein that is 100% identical to AphA1-IAB (Figure 2 subunit 2) from Corynebacterium striatum, which is an aminoglycoside 3′-phosphotransferase involved in the inactivation of aminoglycoside antibiotics such as kanamycin, neomycin, neamine, and ribostamycin [52]. Another gene coding for a protein with 99% identity to the aadA1 gene product from Escherichia coli (Figure 2 subunit 3) is also present. AadA1 is an aminoglycoside-3′-adenylyltransferase that confers resistance to streptomycin and spectinomycin [53]. Finally, there is also a gene that codes a protein which is 98% identical to Aac6 from Enterobacter cloacae, an aminoglycoside phosphotransferase that confers resistance to netilmicin and tobramycin [54]. It therefore appears that the pPC9 plasmid has recruited a number of aminoglycoside modifying enzymes from different origins.
Tetracyclines
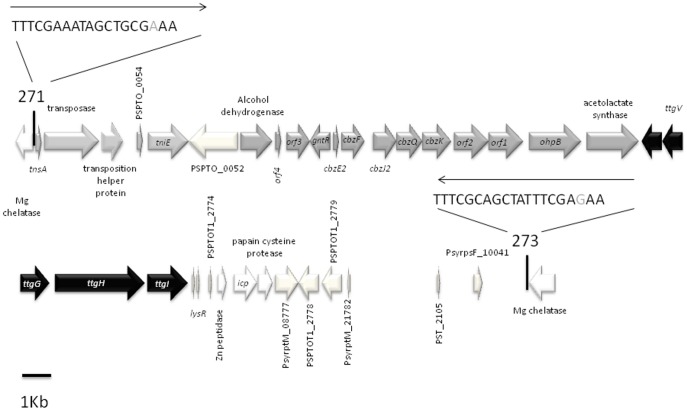
Tetracyclines bind to the 30S subunit of microbial ribosomes. They inhibit protein synthesis by blocking the attachment of charged aminoacyl-tRNAs to the A site on the ribosome. Thus, they prevent introduction of new amino acids to the nascent peptide chain [55]. Several mechanisms have been described by which bacteria gain resistance to tetracycline, namely, extrusion of tetracycline via efflux pumps, changes in ribosome proteins so that tetracycline no longer binds, and chemical inactivation of tetracyclines. Tetracycline efflux is the most efficient mechanism of resistance to this antibiotic for Gram-negative bacteria [56]. The resistance of P. putida KT2440 to tetracycline is linked to the RND TtgABC efflux pump [57]. The TtgABC pump is also responsible for resistance to a broad range of antibiotics such as β-lactams, nalidixic acid, and chloramphenicol [57], [58]. The genes which encode the TtgABC pump are located on the chromosome of HB3267. A secondary role in tetracycline resistance was assigned to the TtgGHI efflux pump, which is located on the pGRT1 plasmid in P. putida DOT-T1E [59], although the primary role of this pump in P. putida DOT-T1E appears to be solvent extrusion [58], [59], [60]. The ttgGHI genes are present in the chromosome of HB3267 and the operon is located in a 44 Kb genomic island (Figure 3) with no homology to the chromosomal sequences of other P. putida strains or with the rest of pGRT1 plasmid [60]. The total G+C content of this island is 55%, a value lower than that of the rest of chromosome. The gene that encodes the repressor of this ttgGHI operon, the TtgV protein is 89% identical to that of DOT-T1E.
Figure 3. Location of the antibiotic and solvent efflux ttgGHI pumps within a genomic island in the chromosome of strain HB3267.
ttgGHI efflux genes are indicated in black; genes involved in transposition events are in light grey; the cbz operon, which is involved in chlorobenzene degradation, is in medium grey. Vertical lines indicate the insertion point; arrows above the sequence indicate the inverted repeat sequences of the Tn552-like transposon, which are within the magnesium chelatase.
It should be noted that the pPC9 plasmid also encodes another tetracycline efflux pump of the TetA type, which is 100% identical to the Acinetobacter baumannii Tn1721-like transposon (Figure 2 subunit 2) [61]. This gene is not present in the genome of KT2440R; the presence of multiple tetracycline efflux pumps supports the data which shows that HB3267 exhibits higher levels of resistance to tetracycline than other strains.
β-lactams
RND efflux pumps and β-lactamases are key players in the resistance of Pseudomonads to β-lactam antibiotics. The HB3267 chromosome carries 10 β-lactamase genes that are also present in KT2440, explaining the resistance of both strains to penicillin-derived antibiotics such as ampicillin, carbenicillin, ticarcillin and piperacillin, and the cephalosporin ceftriaxone. Livermore suggested that the phenotype of resistance to the cephalosporin cefotaxime and ceftazidime of some Pseudomonas clinical isolates was mediated by the action of the chromosomal ampC gene that codes for a β-lactamase [62]. This gene is present in KT2440, which is sensitive to these antibiotics. Two single nucleotide polymorphisms were found in the ampC gene of HB3267 when compared to the sensitive KT2440 strain, namely Pro148Ala and Gly263Arg, which may explain the HB3267 resistance to these antibiotics.
We found that the pPC9 plasmid bears 2 additional β-lactamase genes, one that codes for an enzyme that is 95% identical to AER-1 from Aeromonas hydrophila (Figure 2 subunit 1)—a protein that can efficiently hydrolyze carbenicillin [63]; as well as a gene that codes for a protein that is 100% identical to VIM-1 from Klebsiella pneumonia (Figure 2 subunit 3), which is involved in the carbapenem-resistant phenotype of that microorganism [64].
Chloramphenicol
Chloramphenicol is a bacteriostatic antimicrobial that functions by inhibiting bacterial protein synthesis. P. putida KT2440 is a chloramphenicol-resistant bacterium that is able to grow in the presence of this antibiotic at a concentration of up to 25 µg/ml. Genomic analysis revealed that the TtgABC efflux pump and biosynthesis of pyrroloquinoline (PQQ) were involved in chloramphenicol resistance [65]. These genes are present in the chromosome of the HB3267 strain, which also shows high resistance to this antibiotic. An additional pqqC (coenzyme PQQ synthesis protein C) gene is present in the chromosome of the HB3267 strain, which is also present in the close relatives P. putida S16 and Pseudomonas sp. TJI-51, but not in KT2440 (Figure 4A). The AgmR regulator (PP2665) controls the expression of the pqq genes and the operon encoding the ABC extrusion pump [65]. Up to three polymorphisms were present in PqqC of HB3267 when compared to other P. putida strains, namely HisGln44 (Figure 4B), His142Leu and Ala116Gly. Per se, these mutations and the presence of an additional copy of the pqqC gene could explain in part the high resistance of HB3267 to chloramphenicol. In addition plasmid pPC9 encodes a protein that is 99% identical to CmlA from Aeromonas caviae (Figure 1 subunit 3), an efflux pump that expels chloramphenicol and ethidium from the cells [66].
Figure 4. Potential chromosomal determinants for chloramphenicol resistance of HB3267.
(A) In black, chromosomal location of the additional pqqC gene of HB3267.; in grey, the region of the HB3267 chromosome that is not present in KT2440; in white, genes in synteny with KT2440. (B) Protein alignment of AgmR from P. putida HB3267 (HB3267, Locus B479_11475), P. putida S16 (S16, PPS_2213), P. putida KT2440 (PPS_2213, PP_2665), P. putida BIRD-1 (BIRD1, PPUBIRD1_3011), P. putida GB-1 (GB1, PputGB1_3138), P. aeruginosa PA7 (PA7, PSPA7_3317), and P. aeruginosa PAO1 (PAO1, PA1978) strains. Amino acid mutations referred to in the text are indicated in bold.
Antibiotic resistance determinants encoded only on the pPC9 plasmid –
Sulfonamides
Sulfonamides were the first compounds used as chemotherapy drugs and have been used as antibacterial agents since the 1930's. In bacteria, sulfonamides act as competitive inhibitors of the dihydropteroate synthetase (DHPS), an enzyme involved in folate synthesis. As such, the compounds cause microorganisms to become “starved” of folate and die [67]. Plasmid-mediated sulfonamide resistance in Gram-negative bacteria is very frequently found in clinical isolates and often in combination with other antibiotic resistance traits. The plasmids generally express alternative dihydropteroate synthases, the Sul proteins, which confer resistance to the drug [68]. In pPC9, three genes homologous to those encoding Sul proteins were found: one with 97% identity to the SulI protein of P. aeruginosa (Figure 2. subunit 2), another with a 100% identity with SulII of diverse enteric bacteria (Figure 2. subunit 2), and finally a gene coding a protein with 99% identity to SulI of E. coli (Figure 2. subunit 3).
Our results show that strain P. putida HB3267, isolated from a deceased in-patient in a French hospital, is resistant to the majority of antibiotics and biocides used in laboratories and hospitals (aminoglycosides, ß-lactam antibiotics, cationic peptides dihydrofolate reductase inhibitors, fluoroquinolones, quinolones, glycopeptide antibiotics, macrolides, polyketide, and sulfonamides). This broad range of resistance is rarely found in clinical isolates. Another relevant finding is that MICs for these antibiotics in planktonic cells were much higher for HB3267 than that of multidrug-resistant strains of Pseudomonas aeruginosa. Sequencing of the genome of HB3267 revealed that the determinants of multiple resistances are located chromosomally and on the plasmid pPC9. Regions of the plasmid bearing multidrug resistant genes show high homology with DNA from environmental microorganisms as well as from human opportunistic and true human pathogens indicating both contact and DNA exchange between the HB3267 strain and these environmental and clinically relevant microorganisms. The pPC9 plasmid carries integrons and transposons where the antibiotic resistant determinants are grouped. We have shown that pPC9 is not self-transmissible but transfer of the antibiotic resistant genes from pPC9 to other microbes can be mediated by shuttle vectors, such as the TOL plasmid pWW0. The results presented in this work support the notion that the acquisition of new antibiotic and biocide resistant traits by opportunistic human pathogens, may arise from the cohabitation in the human body of pathogens with new multidrug-resistant “residents”, such as the HB3267 strain.
Supporting Information
Phylogenetic tree comparing gyrB genes of Pseudomonas strains . Phylogram constructed using the platform Phylogeny.fr. which is a combination of a predefined pipeline using leading programs that include MUSCLE, Gblocks, PhyML and TreeDyn [23]. P. aeruginosa PAO-1 (NC_018080), P. fluorescens F113 (NC_016830), P. monteilii BCRC 17520 (FJ418641), P. putida BIRD-1 (NC_017530), P putida GB-1 (NC_010322), P. putida KT2440 (NC_002947), P. putida HB3267 (CP003738), P. putida S16 (NC_015733).
(TIF)
Protein alignment of GyrA (A), GyrB (B) and PhoQ (C) from P. putida HB3267 (HB3267, Locus B479_00265, B479_06830, B479_20445, respectively), P. putida S16 (S16, PPS_1408, PPS_0012, PPS_4028), P. putida KT2440 (KT2440, PP_1767, PP_0013, PP_1187), P. putida BIRD-1 (BIRD1, PPUBIRD1_3846…., PPUBIRD1_1228), P. putida GB-1 (GB1, PputGB1_1358, PputGB1_0006, PputGB1_4229) strains and P. aeruginosa LESB58 (LESB PLES_19001, PLES_00031, PLES_41411), P. aeruginosa PAO1 (PAO1, PA3168, PA0004, PA1180) strains. Amino acid changes referred to in the text are indicated in bold; “*”Identical residues, “:” conservative substitutions and “.” semiconservative substitutions.
(TIF)
RND efflux pumps in P. putida KT2440 and their orthologs in the HB3267 strains.
(TIF)
Acknowledgments
We thank M. Mar Fandila for secretarial help and Ben Pakuts for critical reading of the MS.
Funding Statement
Funds were recieved from ERANET BIO2008-04419-E and BIO2010-17227. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, et al. (2011) Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis 11: 671–676. [DOI] [PubMed] [Google Scholar]
- 2. Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, et al. (2011) Origins of the Escherichia coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 365: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernández M, Duque E, Pizarro-Tobías P, Van Dillewijn P, Wittich RM, et al. (2009) Microbial responses to xenobiotic compounds. Identification of genes that allow Pseudomonas putida KT2440 to cope with 2,4,6-trinitrotoluene. Microb Biotechnol 2: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu X, Monchy S, Taghavi S, Zhu W, Ramos J, et al. (2011) Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida . FEMS Microbiol Rev 35: 299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dogan NM, Kantar C, Gulcan S, Dodge CJ, Yilmaz BC, et al. (2011) Chromium(VI) bioremoval by Pseudomonas bacteria: role of microbial exudates for natural attenuation and biotreatment of Cr(VI) contamination. Environ Sci Technol 45: 2278–2285. [DOI] [PubMed] [Google Scholar]
- 6. Tang H, Yu H, Li Q, Wang X, Gai Z, et al. (2011) Genome sequence of Pseudomonas putida strain B6-2, a superdegrader of polycyclic aromatic hydrocarbons and dioxin-like compounds. J Bacteriol 193: 6789–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Graevenitz A, Weinstein J (1971) Pathogenic significance of Pseudomonas fluorescens and Pseudomonas putida . Yale J Biol Med 44: 265–273. [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshino Y, Kitazawa T, Kamimura M, Tatsuno K, Ota Y, et al. (2011) Pseudomonas putida bacteremia in adult patients: five case reports and a review of the literature. J Infect Chemother 17: 278–282. [DOI] [PubMed] [Google Scholar]
- 9. Loiseau-Marolleau ML, Malarre N (1977) Pseudomonas putida: identification, antibiotic sensitivity and pathogenicity. Pathol Biol (Paris) 25: 637–645. [PubMed] [Google Scholar]
- 10. Docquier JD, Riccio ML, Mugnaioli C, Luzzaro F, Endimiani A, et al. (2003) IMP-12, a new plasmid-encoded metallo-beta-lactamase from a Pseudomonas putida clinical isolate. Antimicrob Agents Chemother 47: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yomoda S, Okubo T, Takahashi A, Murakami M, Iyobe S (2003) Presence of Pseudomonas putida strains harboring plasmids bearing the metallo-beta-lactamase gene bla(IMP) in a hospital in Japan. J Clin Microbiol 41: 4246–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy SB (2002 a) The 2000 Garrod lecture. Factors impacting on the problem of antibiotic resistance. J Antimicrob Chemother 49: 25–30. [DOI] [PubMed] [Google Scholar]
- 13. Janda JM, Abbott SL (2007) 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45: 2761–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aranda-Olmedo I, Tobes R, Manzanera M, Ramos JL, Marques S (2002) Species-specific repetitive extragenic palindromic (REP) sequences in Pseudomonas putida . Nucleic Acids Res 30: 1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bagdasarian M, Lurz R, Rückert B, Franklin FC, Bagdasarian MM, et al. (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas . Gene 16: 237–247. [DOI] [PubMed] [Google Scholar]
- 16. Frapolli M, Défago G, Moënne-Loccoz Y (2007) Multilocus sequence analysis of biocontrol fluorescent Pseudomonas spp. producing the antifungal compound 2,4-diacetylphloroglucinol. Environ Microbiol 9: 1939–1955. [DOI] [PubMed] [Google Scholar]
- 17. Khan NH, Ahsan M, Yoshizawa S, Hosoya S, Yokota A, et al. (2008) Multilocus sequence typing and phylogenetic analyses of Pseudomonas aeruginosa isolates from the ocean. Appl Environ Microbiol 74: 6194–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrews JM (2001) Determination of minimum inhibitory concentrations. J. Antimicrob Chemother 48 Suppl. 1: 5–16. [DOI] [PubMed] [Google Scholar]
- 19. Ceri H, Olson M, Morck D, Storey D, Read R, et al. (2001) The MBEC assay system; Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol 337: 377–385. [DOI] [PubMed] [Google Scholar]
- 20. Williams PA, Murray K (1974) Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol 120: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delcher AL, Harmon D, Kasif S, White O, Salzberg SL (1999) Improved microbial gene identification with GLIMMER. Nucleic Acids Res 27: 4636–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang SN, Liu Z, Tang HZ, Meng J, Xu P (2007) Characterization of environmentally friendly nicotine degradation by Pseudomonas putida biotype A strain S16. Microbiology 153: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 24. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Espinosa-Urgel M, Kolter R, Ramos JL (2002) Root colonization by Pseudomonas putida; love at first sight. Microbiology 148: 341–343. [DOI] [PubMed] [Google Scholar]
- 26. Muller C, Plésiat P, Jeannot K (2011) A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and β-lactams in Pseudomonas aeruginosa . Antimicrob Agents Chemother 55: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horii T, Muramatsu H, Iinuma Y (2005) Mechanisms of resistance to fluoroquinolones and carbapenems in Pseudomonas putida . J Antimicrob Chemother 56: 643–647. [DOI] [PubMed] [Google Scholar]
- 28. Treviño M, Moldes L, Hernández M, Martínez-Lamas L, García-Riestra C, et al. (2010) Nosocomial infection by VIM-2 metallo-beta-lactamase-producing Pseudomonas putida . J Med Microbiol 59: 853–855. [DOI] [PubMed] [Google Scholar]
- 29. Saha R, Jain S, Kaur IR (2010) Metallo beta-lactamase producing Pseudomonas species–a major cause of concern among hospital associated urinary tract infection. J Indian Med Assoc 108: 344–348. [PubMed] [Google Scholar]
- 30. Mendes RE, Castanheira M, Toleman MA, Sader HS, Jones RN, et al. (2007) Characterization of an integron carrying blaIMP-1 and a new aminoglycoside resistance gene, aac(6′)-31, and its dissemination among genetically unrelated clinical isolates in a Brazilian hospital. Antimicrob Agents Chemother 51: 2611–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumita W, Saito R, Sato K, Ode T, Moriya K, et al. (2009) Molecular characterizations of carbapenem and ciprofloxacin resistance in clinical isolates of Pseudomonas putida . J Infect Chemother 15: 6–12. [DOI] [PubMed] [Google Scholar]
- 32. Rolston KV, Kontoyiannis DP, Yadegarynia D, Raad II (2005) Nonfermentative gram-negative bacilli in cancer patients: increasing frequency of infection and antimicrobial susceptibility of clinical isolates to fluoroquinolones. Diagn Microbiol Infect Dis 51: 215–218. [DOI] [PubMed] [Google Scholar]
- 33. Poirel L, Naas T, Nicolas D, Collet L, Bellais S, et al. (2000) Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother 44: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iyer PG, Murphy TF (2009) Chronic obstructive pulmonary disease: role of bacteria and updated guide to antibacterial selection in the older patient. Drugs Aging 26: 985–995. [DOI] [PubMed] [Google Scholar]
- 35. Moskowitz SM, Brannon MK, Dasgupta N, Pier M, Sgambati N, et al. (2012) PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 56: 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duque E, de la Torre J, Bernal P, Molina-Henares MA, Alaminos M, et al. (2012) Identication of reciprocal adhesión genes in pathogenic and nonpathogenic Pseudomnonas . Environ Microbiol and EMI R In press. [DOI] [PubMed] [Google Scholar]
- 37. Molina-Henares MA, de la Torre J, García-Salamanca A, Molina-Henares AJ, Herrera MC, et al. (2010) Identification of conditionally essential genes for growth of Pseudomonas putida KT2440 on minimal medium through the screening of a genome-wide mutant library. Environ Microbiol 12: 1468–1485. [DOI] [PubMed] [Google Scholar]
- 38. Bramucci M, Chen M, Nagarajan V (2006) Genetic organization of a plasmid from an industrial wastewater bioreactor. Appl Microbiol Biotechnol 71: 67–74. [DOI] [PubMed] [Google Scholar]
- 39. Zhao H, Chen D, Li Y, Cai B (2005) Overexpression, purification and characterization of a new salicylate hydroxylase from naphthalene-degrading Pseudomonas sp. strain ND6. Microbiol Res 160: 307–313. [DOI] [PubMed] [Google Scholar]
- 40. Marcelletti S, Ferrante P, Petriccione M, Firrao G, Scortichini M (2011) Pseudomonas syringae pv. actinidiae draft genomes comparison reveal strain-specific features involved in adaptation and virulence to Actinidia species. PLoS One 6: e27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamath R, Schnoor JL, Alvarez PJ (2004) Effect of root-derived substrates on the expression of nah-lux genes in Pseudomonas fluorescens HK44: implications for PAH biodegradation in the rhizosphere. Environ Sci Technol 38: 1740–1745. [DOI] [PubMed] [Google Scholar]
- 42. Hernández A, Sánchez MB, Martínez JL (2011) Quinolone Resistance: Much More than Predicted. Front Microbiol 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eaves DJ, Randall L, Gray DT, Buckley A, Woodward MJ, et al. (2004) Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica . Antimicrob Agents Chemother 48: 4012–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McPhee JB, Lewenza S, Hancock REW (2003) Cationic antimicrobial peptides activate a two component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa . Mol Microbiol 50: 205–217. [DOI] [PubMed] [Google Scholar]
- 45. Pescaretti ML, López FE, Morero RD, Delgado MA (2011) The PmrA/PmrB regulatory system controls the expression of the wzzfepE gene involved in the O-antigen synthesis of Salmonella enterica serovar Typhimurium. Microbiology 157: 2515–2521. [DOI] [PubMed] [Google Scholar]
- 46. Vaara M (1993) Outer membrane permeability barrier to azithromycin, clarithromycin, and roxithromycin in gram-negative enteric bacteria. Antimicrob Agents Chemother 37: 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei Q, Tarighi S, Dötsch A, Häussler S, Müsken M, et al. (2011) Phenotypic and genome-wide analysis of an antibiotic-resistant small colony variant (SCV) of Pseudomonas aeruginosa . PLoS One 6: e29276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Struble JM, Gill RT (2006) Reverse engineering antibiotic sensitivity in a multidrug-resistant Pseudomonas aeruginosa isolate. Antimicrob Agents Chemother 50: 2506–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaziri F, Peerayeh SN, Nejad QB, Farhadian A (2011) The prevalence of aminoglycoside-modifying enzyme genes (aac (6′)-I, aac (6′)-II, ant (2″)-I, aph (3′)-VI) in Pseudomonas aeruginosa . Clinics (Sao Paulo) 66: 1519–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt FR, Nücken EJ, Henschke RB (1988) Nucleotide sequence analysis of 2″-aminoglycoside nucleotidyl-transferase ANT(2″) from Tn4000: its relationship with AAD(3″) and impact on Tn21 evolution. Mol Microbiol 2: 709–717. [DOI] [PubMed] [Google Scholar]
- 51. Han HS, Koh YJ, Hur JS, Jung JS (2004) Occurrence of the strA-strB streptomycin resistance genes in Pseudomonas species isolated from kiwifruit plants. J Microbiol 42: 365–368. [PubMed] [Google Scholar]
- 52. Hainrichson M, Yaniv O, Cherniavsky M, Nudelman I, Shallom-Shezifi D, et al. (2007) Overexpression and initial characterization of the chromosomal aminoglycoside 3′-O-phosphotransferase APH(3′)-IIb from Pseudomonas aeruginosa . Antimicrob Agents Chemother 51: 774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dahshan H, Abd-El-Kader MA, Chuma T, Moriki H, Okamoto K (2011) Re-emergence of multi-drug resistant Salmonella enterica serovar Stanley from cattle. Vet Res Commun 35: 55–60. [DOI] [PubMed] [Google Scholar]
- 54. Santos C, Caetano T, Ferreira S, Mendo S (2010) Tn5090-like class 1 integron carrying bla(VIM-2) in a Pseudomonas putida strain from Portugal. Clin Microbiol Infect 16: 1558–1561. [DOI] [PubMed] [Google Scholar]
- 55. Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS (1983) Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry 22: 359–368. [DOI] [PubMed] [Google Scholar]
- 56. Levy SB (2002 b) Active efflux, a common mechanism for biocide and antibiotic resistance. J Appl Microbiol 92: 65S–71S. [PubMed] [Google Scholar]
- 57. Godoy P, Molina-Henares AJ, de la Torre J, Duque E, Ramos JL (2010) Characterization of the RND family of multidrug efflux pumps: in silico to in vivo confirmation of four functionally distinct subgroups. Microb Biotech 3: 601–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rojas A, Duque E, Mosqueda G, Golden G, Hurtado A, et al. (2001) Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J Bacteriol 183: 3967–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodríguez-Herva JJ, García V, Hurtado A, Segura A, Ramos JL (2007) The ttgGHI solvent efflux pump operon of Pseudomonas putida DOT-T1E is located on a large self-transmissible plasmid. Environ Microbiol 9: 1550–1561. [DOI] [PubMed] [Google Scholar]
- 60. Molina L, Duque E, Gómez MJ, Krell T, Lacal J, et al. (2011) The pGRT1 plasmid of Pseudomonas putida DOT-T1E encodes functions relevant for survival under harsh conditions in the environment. Environ Microbiol 13: 2315–2327. [DOI] [PubMed] [Google Scholar]
- 61. McMurry LM, Petrucci R, Levy SB (1980) Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli . Proc Natl Acad Sci (USA) 77: 3974–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Livermore DM (1995) beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev 8: 557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hedges RW, Medeiros AA, Cohenford M, Jacoby GA (1985) Genetic and biochemical properties of AER-1, a novel carbenicillin-hydrolyzing beta-lactamase from Aeromonas hydrophila . Antimicrob Agents Chemother 1985 27: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steinmann J, Kaase M, Gatermann S, Popp W, Steinmann E, et al. (2011) Outbreak due to a Klebsiella pneumoniae strain harbouring KPC-2 and VIM-1 in a German university hospital, July 2010 to January. Euro Surveill 16 pii: 19944. [PubMed] [Google Scholar]
- 65. Fernández M, Conde S, de la Torre J, Molina-Santiago C, Ramos JL, et al. (2012) Mechanisms of resistance to chloramphenicol in Pseudomonas putida KT2440. Antimicrob Agents Chemother 56: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Minek Tl, Morita Y, Kataoka A, Mizushima T, Tsuchiya T (1998) Evidence for chloramphenicol/H+ antiport in Cmr (MdfA) system of Escherichia coli and properties of the antiporter. J Biochem 124: 187–193. [DOI] [PubMed] [Google Scholar]
- 67.Kent M (2000) Advanced Biology, Oxford University Press, p. 46 ISBN 9780199141951.
- 68. Rådström P, Swedberg G (1988) RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob Agents Chemother 32: 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree comparing gyrB genes of Pseudomonas strains . Phylogram constructed using the platform Phylogeny.fr. which is a combination of a predefined pipeline using leading programs that include MUSCLE, Gblocks, PhyML and TreeDyn [23]. P. aeruginosa PAO-1 (NC_018080), P. fluorescens F113 (NC_016830), P. monteilii BCRC 17520 (FJ418641), P. putida BIRD-1 (NC_017530), P putida GB-1 (NC_010322), P. putida KT2440 (NC_002947), P. putida HB3267 (CP003738), P. putida S16 (NC_015733).
(TIF)
Protein alignment of GyrA (A), GyrB (B) and PhoQ (C) from P. putida HB3267 (HB3267, Locus B479_00265, B479_06830, B479_20445, respectively), P. putida S16 (S16, PPS_1408, PPS_0012, PPS_4028), P. putida KT2440 (KT2440, PP_1767, PP_0013, PP_1187), P. putida BIRD-1 (BIRD1, PPUBIRD1_3846…., PPUBIRD1_1228), P. putida GB-1 (GB1, PputGB1_1358, PputGB1_0006, PputGB1_4229) strains and P. aeruginosa LESB58 (LESB PLES_19001, PLES_00031, PLES_41411), P. aeruginosa PAO1 (PAO1, PA3168, PA0004, PA1180) strains. Amino acid changes referred to in the text are indicated in bold; “*”Identical residues, “:” conservative substitutions and “.” semiconservative substitutions.
(TIF)
RND efflux pumps in P. putida KT2440 and their orthologs in the HB3267 strains.
(TIF)