Abstract
Background
Young children played a major role in pneumococcal nasopharyngeal carriage, acquisition, and transmission in the era before pneumococcal conjugate vaccine (PCV) use. Few studies document pneumococcal household dynamics in the routine-PCV7 era.
Methods
We investigated age-specific acquisition, household introduction, carriage clearance, and intra-household transmission in a prospective, longitudinal, observational cohort study of pneumococcal nasopharyngeal carriage in 300 American Indian households comprising 1,072 participants between March 2006 and March 2008.
Results
Pneumococcal acquisition rates were 2–6 times higher in children than adults. More household introductions of new pneumococcal strains were attributable to children <9 years than adults ≥17 years (p<0.001), and older children (2–8 years) than younger children (<2 years) (p<0.008). Compared to children <2 years, carriage clearance was more rapid in older children (2–4 years, HRclearance 1.53 [95% CI: 1.22, 1.91]; 5–8 years, HRclearance 1.71 [1.36, 2.15]) and adults (HRclearance 1.75 [1.16, 2.64]). Exposure to serotype-specific carriage in older children (2–8 years) most consistently increased the odds of subsequently acquiring that serotype for other household members.
Conclusions
In this community with a high burden of pneumococcal colonization and disease and routine PCV7 use, children (particularly older children 2–8 years) drive intra-household pneumococcal transmission: first, by acquiring, introducing, and harboring pneumococcus within the household, and then by transmitting acquired serotypes more efficiently than household members of other ages.
Introduction
Pneumococcal conjugate vaccines are being introduced into routine childhood immunization schedules around the world, including in low-resource countries. Routine infant vaccination with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar [Pfizer]) has substantially reduced pneumococcal disease in the United States [1]–[4] and Europe [5]. Still, in 2008, approximately 514,000 children under 5 years of age died from pneumococcal disease; over 90% of these deaths occurred in developing countries where PCV had not yet been introduced [6].
Nasopharyngeal (NP) colonization with Streptococcus pneumoniae plays a major epidemiologic role both as a disease precursor [7], [8] and the primary mode of horizontal transmission between individuals [9], with the household as a primary mixing pool [10]. Pre-PCV longitudinal NP colonization studies suggest that children introduce new serotypes into the household more frequently, acquire household serotypes more often, and carry serotypes longer than adults [10]–[14]. Serotype-specific NP carriage is highly temporally clustered within households [15], [16], and household exposure increases serotype-specific acquisition in other household members eight-fold [17]. Serotypes also differ intrinsically by carriage duration, intra-household transmissibility, and resistance to colonization by competing serotypes [11], [14].
Longitudinal studies of intra-household pneumococcal NP transmission have been conducted prior to PCV7 introduction [15], [16], [18] or in countries where PCV7 is not widely available [11], [17] but not in the setting of PCV use. PCV reduces vaccine-serotype (VT) carriage in both vaccinated and unvaccinated children [19], [20] and adults [21], but high carriage prevalence or suboptimal vaccine coverage may attenuate these indirect effects [22]. Widespread PCV use may substantially alter pneumococcal epidemiology within households, exerting differential effects by vaccination status and population under evaluation.
This longitudinal study aimed to characterize pneumococcal NP acquisition, carriage duration, and transmission in American Indian households during the routine PCV7 era. PCV7 was first introduced to these communities in 1997 during a group-randomized efficacy trial [23], then included in the routine infant immunization schedule immediately afterwards in 2000 [24]. PCV7 has virtually eliminated VT carriage [25] and dramatically reduced VT IPD (invasive pneumococcal disease), although residual IPD rates remain four-fold those of the general US population [24]. Since nearly all circulating pneumococci in these communities are now non-vaccine serotypes (NVT), we did not expect individual child vaccination status to influence household pneumococcal dynamics in this routine-PCV7 setting. We hypothesized that children would serve as the major route of pneumococcal serotype introduction into the household and the primary source of intra-household transmission, just as in the pre-PCV7 era. Furthermore, characterization of household transmission in this high-burden population may predict perturbations in pneumococcal ecology likely to occur in other high-burden populations worldwide that are introducing PCV.
Methods
Ethics Statement
The study was approved by the Navajo Nation, the White Mountain Apache tribe and the institutional review boards of the Johns Hopkins Bloomberg School of Public Health, the Navajo Nation, and the Phoenix Area Indian Health Service. Written informed consent was obtained from all adult participants and parents or guardians of enrolled children.
Longitudinal Nasopharyngeal Colonization Study
The study protocol has been previously described [25]. Briefly, American Indian families with at least one child <9 years and living on or near the Navajo or White Mountain Apache reservations in the southwestern United States were enrolled between March 2006 and March 2008, then visited monthly for six months (seven total visits). Household carriage risk factors and family composition were ascertained at enrollment; individual carriage risk factors were collected at each visit. Pneumococcal immunization history and records of antibiotic use, hospitalizations, and (for children <2 years) outpatient illnesses during the study period were collected via medical chart review. Monthly NP specimen collection, pneumococcal isolation, and serotyping were conducted as previously described, allowing identification of multiple serotypes per swab when multiple morphologies were identified on a plate [25]–[27]. Among isolates originally classified as 6A, Quellung reaction [28], [29] or polymerase chain reaction [27] was used to identify serotype 6C isolates. Three isolates originally classified as 6A were not available for retesting and were assumed to be 6C based on the testing results of other isolates.
Definitions and Statistical Methods
Daycare attendance was defined as ≥4 hours per week outside of the home with ≥2 members of another household. PCV7 vaccination was defined as ≥3 doses. 23-valent pneumococcal polysaccharide (PS23; Pneumovax [Merck]) vaccination was defined as any previous receipt of PS23.
A NP specimen refers to a single sample from an individual; an isolate refers to a pneumococcal strain, specified by its serotype identified from a swab. A carriage episode refers to the isolation of a serotype from an individual along with as any subsequent isolation of that same serotype at consecutive visits. Carriage episodes were incident if preceded by ≥1 swab negative for that serotype and prevalent otherwise. Serotype acquisition occurred when an isolated serotype was immediately preceded by ≥1 swab negative for that serotype. Acquisitions were individual new acquisitions if the individual had never previously carried the serotype in the study, and individual reacquisitions otherwise. Acquisition of a serotype never previously observed among participating household members was a household introduction. Household introductions were unique if a single household member acquired the new serotype and concurrent if simultaneously acquired by >1 member. In order to investigate serotype-specific differences in household introduction, unadjusted categorical binomial regression was used to analyze the serotype-specific proportion of concurrent versus unique household introductions for the ten most-commonly-introduced serotypes (with all other serotypes as the reference category).
Statistical Methods
To analyze carriage duration, incident carriage episodes were aligned for survival analysis on a time metric of “days from first observed carriage”. Clearance was defined by two consecutive swabs negative for a carried serotype and assumed to occur at the midpoint between the last swab positive and the first swab negative for that serotype. Carriage episodes concluding with a missed swab or the last study visit were right-censored. Unadjusted median time to clearance was calculated using survival analysis. Cox regression with cluster robust standard errors was used to analyze the effect of age on clearance of a carried serotype, using only the first incident observation per individual and adjusting for time-varying (breastfeeding, tobacco or antibiotic use, daycare attendance, and visit month) and time-fixed (household running water, tobacco smoke, or indoor stove; vaccination status; number of household members [total and <6 years]; and persons per sleeping room) covariates. To examine whether clearance rate was affected by serotype in children, the above Cox regression was repeated for children <9 years, limiting to the first acquisition of one of the five most commonly carried serotypes. Serotype was included in the regression as a categorical variable and its inclusion tested for significance using a Wald test. Serotype values were carried forward for missed visits only if isolation of that same serotype both preceded and followed the missed visit.
Individual acquisition and household introduction rates were compared using Poisson regression with cluster robust standard errors. Exposure was defined as [number of swab specimens obtained–1] for all Poisson models, since acquisition could not occur at first visit by definition. All analyses were conducted in Stata 12 [30].
Multilevel analysis was used to investigate age-specific intra-household transmission patterns for the four serotypes with the greatest number of putative transmission events (6C, 19A, 22F, and 35B). For each serotype, separate three-level (visit, individual, household) mixed logistic regression models for children and adults were fitted [31], [32], specifying random intercepts for household and individual with an independent correlation structure. Visit-level adjustment for seasonality was performed using a truncated Fourier series [33] (including the first two pairs of terms as independent variables).
Results
Household and individual characteristics
A total of 1072 individuals in 300 households were enrolled in the study. Table 1 summarizes household and individual characteristics. Vaccination coverage was high (83.5%) among children <9 years, while daycare attendance was rare (4.7%). Of 299 households self-reporting household size, 69 (23%) had all persons ≥6 years enrolled, 77 (26%) were missing one person ≥6 years, 55 (18%) were missing two, and 98 (33%) were missing three or more. Enrollment was more complete among younger children: in 210 households (70%), all children <6 years were enrolled, in 74 (25%) one child <6 years was not enrolled and only 15 (5%) households had two or more children <6 years not enrolled.
Table 1. Characteristics of individuals and households enrolled in the study.
| Household characteristics (n = 300) | Median | IQR |
| # of members (by interview) | 6 | [??5, 7] |
| # of reported members not enrolled in study | 2 | [??1, 3] |
| # of members under age 6 (by interview) | 2 | [??1, 3] |
| # of reported members under age 6 | 0 | [??0, 1] |
| not enrolled in study | ||
| # rooms in house | 6 | [??4, 7] |
| # sleeping rooms in house | 3 | [??2, 3] |
a Daycare attendance was defined as ≥4 hours per week outside the home with 2 or more children from other households.
b Includes one child 10 years of age.
c Includes two parents <17 years.
d An additional 4 children <9 years of age reported receipt of PS23.
NP specimens and carriage
Of the 7504 NP specimens anticipated from the 1072 participants, 6541 (87%) were successfully collected. Most (61%) participants provided all seven swabs, and 88% provided ≥5. A comprehensive description of prevalence and serotype distribution has been published [25]. Carriage prevalence was high: 35.8% overall and 55.5% in children <5 years.
The 2412 pneumococcal isolates represented 1655 distinct carriage episodes; 400 (24.2%) were prevalent and 1255 (75.8%) incident. Per-swab carriage probabilities are summarized in Table 2 . Among the 945 individuals with ≥5 swabs, 72% carried pneumococcus at least once during the study. The median number of unique serotypes carried by those with ≥5 swabs was 1 (IQR 0, 2), with carriage of ≥4 unique serotypes observed in 6.4%. Of those with ≥5 swabs, 92.5% of children <9 years carried pneumococcus at least once, compared to 41.9% of adults ≥17 years (p<0.0001). On average, 3.18 different serotypes were observed in a household over the study (SD 1.86), with carriage observed at least once in 94% of households. Circulation of >1 serotype over the course of the study was observed in 74% of households. Compared to households with carriage observed at least once, households without pneumococcal isolation had fewer self-reported members (median: 4.5 versus 6.0, Wilcoxon rank-sum p = 0.0076) and fewer children <6 years (median: 1 versus 2, Wilcoxon rank-sum p = 0.0009). Of 1922 household-visits with ≥1 NP swab obtained from the household, the number of serotypes detected in the household was zero at 32.1%, one at 45.4%, and two or more at 22.5% of household visits.
Table 2. Summary of pneumococcal acquisition events by age category.
| Age category | ||||||||||||||
| <2 years | 2–4 years | 5–8 years | 17–39 years | 40–64 years | ≥65 years | All ages | ||||||||
| Swabs obtained (#) | 1447 | 1379 | 1035 | 2233 | 376 | 58 | 6541 | |||||||
| Total # isolates a (#, per-swab probability) | 835 | 0.577 | 787 | 0.571 | 502 | 0.486 | 249 | 0.111 | 36 | 0.096 | 8 | 0.138 | 2417 | 0.37 |
| Individual acquisitions a (#,per-swab probability) | 369 | 0.301 | 426 | 0.367 | 285 | 0.326 | 151 | 0.078 | 21 | 0.065 | 5 | 0.098 | 1257 | 0.227 |
| New acquisitionsb (#, % individual acquisitions) | 282 | 76.4% | 357 | 83.8% | 229 | 80.4% | 133 | 88.1% | 21 | 100.0% | 4 | 80.0% | 1026 | 81.6% |
| Reacquisitionsb (#, % individual acquisitions) | 87 | 23.6% | 69 | 16.2% | 56 | 19.6% | 18 | 11.9% | 0 | 0.0% | 1 | 20.0% | 231 | 18.4% |
| Household introductions a , c (#,per-swab probability) | 175 | 0.143 | 249 | 0.215 | 173 | 0.198 | 70 | 0.036 | 9 | 0.028 | 3 | 0.059 | 679 | 0.123 |
| Unique HH introduction (#, % of HH introductions) | 102 | 58.3% | 165 | 66.3% | 125 | 72.3% | 36 | 51.4% | 5 | 55.6% | 1 | 33.3% | 434 | 63.9% |
| Concurrent HH introduction (#, % of HH introductions) | 73 | 41.7% | 84 | 33.7% | 48 | 27.7% | 34 | 48.6% | 4 | 44.4% | 2 | 66.7% | 245 | 36.1% |
a Including 73 swabs for which two serotypes were identified.
b New acquisitions defined as acquisition of serotype not previously carried; reacquisition defined as acquisition of a previously-carried serotype.
c Household introductions classified as “unique” if the participant was the only one in the family to acquire the new serotype; “concurrent” if more than one individual acquired the serotype simultaneously.
Concurrent serotype-specific carriage within households was also common: for 41.7% of isolates, ≥1 other household member simultaneously carried the same serotype. Among children <9 years, there was no difference between females and males in the proportion of isolates concurrently carried by other household members (42.1% of isolates from female children versus 39.4% from males, p = 0.204). Among adults ≥17 years, however, concurrent household carriage was significantly more common among adult females than males (53.1% versus 33.3% of isolates, p = 0.006).
Duration of carriage
Most carriage episodes (70.9%, 1170/1651) were observed at a single visit. Four individuals <5 years carried the same serotype at all seven study visits (two 6C and one each 19A and 33F). Compared to children <2 years, older children and adults cleared carried serotypes at a greater rate (2–4 year-olds, adjusted hazard ratio [HR] of clearance 1.53 [95% CI: 1.22, 1.91]; 5–8 year-olds, HR 1.71 [1.36, 2.15]; adults ≥17 years, HR 1.75 [1.16, 2.64]). Of the five most-commonly carried serotypes, serotype did not significantly affect the hazard of clearance among children <9 years (p = 0.13) or adults ≥17 years (p = 0.37).
Individual acquisitions
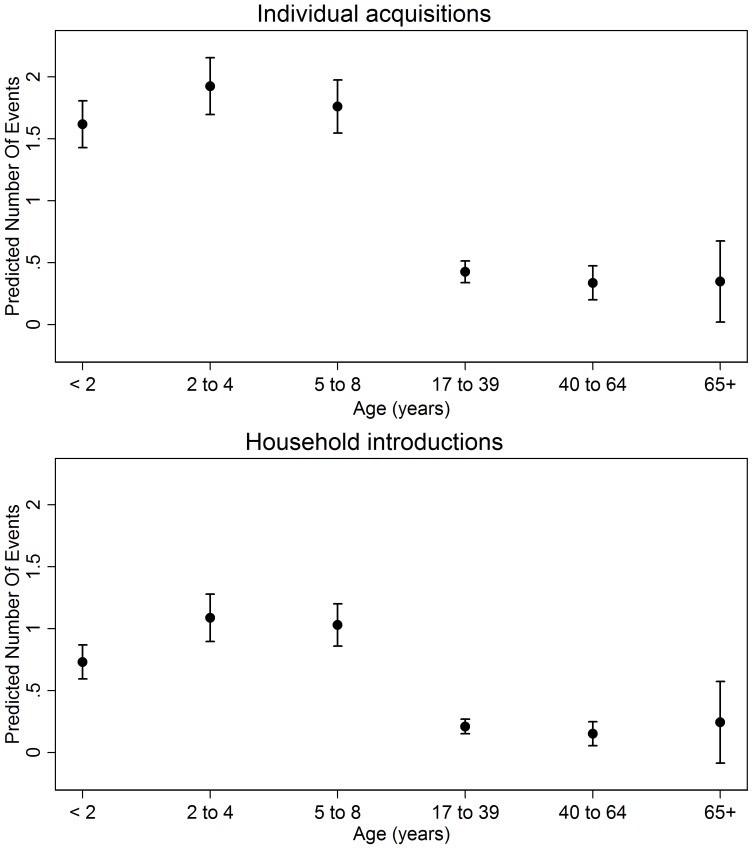
Table 2 summarizes per-swab acquisition probabilities by age, while Figure 1 shows the adjusted mean number of events by age. At least one individual acquisition was observed in 66.8% (632/945) of individuals who contributed ≥5 swabs and 89.3% (268/300) of all households. After adjusting for individual and household characteristics, the rate of individual acquisition was 3–4 times higher in children <9 years compared to adults 17–39 years ( Table 3 ). No significant differences were seen between adult age groups. Month of study enrollment was significantly associated with individual acquisition (p<0.001), with adjusted acquisition rates twice as high in those who enrolled in September compared to February (Figure S1). Of other risk factors, only the association between rate of acquisition and reported number of children <6 years in the household reached significance (incidence rate ratio [IRR] 1.09 for each additional child <6 years, 95% CI: 1.02, 1.16).
Figure 1. Adjusted number of pneumococcal acquisition events, by age category.
Each point represents the mean number of individual acquisitions or household introduction for an individual in a given age category (with 95% confidence interval) over the course of the study, adjusted by all other covariates in the Poisson models set to their means.
Table 3. Risk factors for pneumococcal acquisition (Poisson regression).
| Individual acquisition | Household introduction | ||||||
| Individual characteristics | IRR | [95% CI] | p-value | IRR | [95% CI] | p-value | |
| Age (years) | <2 | 3.79 | [2.92,4.93] | <0.001 | 3.47 | [2.36,5.11] | <0.001 |
| 2–4 | 4.51 | [3.47,5.88] | <0.001 | 5.16 | [3.54,7.53] | <0.001 | |
| 5–8 | 4.13 | [3.20,5.34] | <0.001 | 4.89 | [3.42,6.99] | <0.001 | |
| 17–40 | REF | REF | |||||
| 40–65 | 0.79 | [0.51,1.22] | 0.289 | 0.73 | [0.37,1.41] | 0.343 | |
| ≥65 | 0.82 | [0.31,2.12] | 0.676 | 1.17 | [0.31,4.34] | 0.819 | |
| Sex (male) | 1.01 | [0.91,1.11] | 0.889 | 1.07 | [0.92,1.25] | 0.368 | |
| During studya: | Breastfed | 1.07 | [0.92,1.23] | 0.392 | 1.01 | [0.81,1.27] | 0.926 |
| Attended daycare | 0.85 | [0.70,1.03] | 0.093 | 0.96 | [0.75,1.23] | 0.728 | |
| Smoked tobacco | 0.78 | [0.50,1.20] | 0.252 | 0.78 | [0.42,1.44] | 0.43 | |
| Chewed tobacco | 1.45 | [0.84,2.49] | 0.183 | 1.01 | [0.39,2.66] | 0.977 | |
| Vaccination statusb | PCV7 (>3 doses) | 0.94 | [0.79,1.12] | 0.493 | 1.1 | [0.85,1.42] | 0.452 |
| PS23 (any) | 1.12 | [0.70,1.78] | 0.636 | 1.17 | [0.53,2.57] | 0.702 | |
| Month of Study Entryc | <0.001 | <0.001 | |||||
| Household characteristics | # children <6 | 1.09 | [1.02,1.16] | 0.009 | 0.93 | [0.85,1.00] | 0.063 |
| All other household covariatesd | NS | NS | |||||
a Reported at least once during the study. Daycare defined as ≥4 hours/week outside of home with ≥2 members of other families.
b From chart review; all other covariates ascertained via interview.
c All models adjusted for month of study entry as a categorical variable.
d Estimates additionally adjusted for # persons in household, crowding (number of persons per sleeping room), presence of smoker, presence of wood burning stove, and presence of running water in house. None of the other variables examined had a significant association with either individual or household carriage.
Household introductions
Five hundred fifty-one (551) household introductions were observed; 78.8% (434/551) were unique and 21.2% (117/551) were concurrent. Of the concurrent introductions, two household members simultaneously acquired the serotype on 105 occasions, three on 11 occasions, and four on one occasion. Of individuals with ≥5 swabs, 48.3% (457/945) had at least one household introduction. For the ten most commonly introduced serotypes in this study, there was no difference in the proportion of concurrent versus unique household introductions by serotype – except for serotype 15A, which was more commonly concurrently introduced (44.0% of introductions, p = 0.005) than other serotypes.
In multivariable Poisson analysis, the rate of new household introduction was 3–5 times higher in children <9 years compared to adults 17–39 years and varied significantly with month of study entry ( Table 3 ; Figure S1). No other risk factor reached statistical significance.
In a secondary analysis allowing the effect of gender to differ between children <9 years and adults ≥17 years, adult males had a higher rate of household introduction than adult females (IRR 1.72, 95% CI: 1.03, 2.87), while the impact of gender among children was non-significant (IRR 1.02 for males versus females, 95% CI: 0.87, 1.19).
Intra-household transmission
Considering serotypes 6C, 19A, 35B, and 22F, exposure to carriage in a child 2–8 years was most consistently associated with a significant increase in the odds of other household members acquiring that serotype at the next visit ( Table 4 ). This association was observed for serotypes 19A and 22F considering children <9 years as potential recipients and for 19A, 22F and 35B considering adults ≥17 years. Exposure to carriage in children 2–8 years appeared to have a greater effect on acquisition for adults than children, although wide, overlapping confidence intervals and differences in model structure precluded direct comparison. The only other pair to reach statistical significance was exposure of adults ≥17 years to serotype 6C carriage in children <2 years (OR 19.55, 95% CI: 4.36, 87.69).
Table 4. Intra-household pneumococcal transmission (multilevel logistic regression.
| OR for acquisition [95% CI] with p-value, by serotypeb | |||||||||||||
| 6C | 19A | 35B | 22F | ||||||||||
| Recipient | Exposurea | OR | [95% CI] | p-value | OR | [95% CI] | p-value | OR | [95% CI] | p-value | OR | [95% CI] | p-value |
| Child (<9 y) | No HH carriage | REF | REF | REF | REF | ||||||||
| Child (<2 y) | 1.08 | [0.37,3.13] | 0.89 | 1.04 | [0.34,3.15] | 0.95 | 1.98 | [0.49,8.06] | 0.34 | 2.43 | [0.71,8.25] | 0.156 | |
| Child (2–8 y) | 2.30 | [0.90,5.86] | 0.08 | 3.80 | [1.66,8.70] | 0.002 | 3.00 | [0.93,9.65] | 0.065 | 6.93 | [2.16,22.19] | 0.001 | |
| Adult (≥17 y) | 0.29 | [0.03,2.82] | 0.28 | 0.88 | [0.13,5.86] | 0.89 | 2.53 | [0.12,51.98] | 0.55 | 1.48 | [0.24,9.11] | 0.673 | |
| Adult (≥17 y) | No HH carriage | REF | REF | REF | REF | ||||||||
| Child (<2 y) | 19.55 | [4.36,87.69] | <0.001 | 5.63 | [0.86,36.63] | 0.071 | 1.72 | [0.17,17.93] | 0.65 | 5.59 | [1.05,29.87] | 0.044 | |
| Child (2–8 y) | 3.69 | [0.83,16.33] | 0.085 | 10.36 | [1.80,59.45] | 0.009 | 43.68 | [7.41,257.6] | <0.001 | 20.99 | [4.90,89.95] | <0.001 | |
a Exposure defined as carriage of the target serotype in at least one household member in the specified age range at the previous visit.
b Both child recipient and adult recipient models adjusted for seasonality (using the first two pairs of a truncated Fourier series), recipient characteristics (age, sex) and household characteristics (number of children <6 y, wood- or coal-burning indoor stove, and running water). Child recipient models also adjusted for vaccination status (defined as at least 3 doses of PCV7), and breastfeeding status (since last visit). Low PS23 coverage in adult age groups and the rarity of serotype-specific adult-to-adult exposure precluded the addition of PS23 vaccination status and exposure to carriage in adults ≥17 years to the adult model. Visits in which adults 17 years were exposed to carriage in another adult ≥17 years at the previous visit were excluded from the adult model.
Discussion
This study is the largest longitudinal household analysis of pneumococcal carriage and transmission to date and the first conducted in the setting of routine PCV use. Concordant with pre-PCV7 household studies, our findings support a major role for children in pneumococcal acquisition and transmission in the household, but we have now shown that this holds true even for settings of PCV use and high pneumococcal transmission.
Numerous studies indicate that duration of carriage decreases with increasing age [7], [15], [11], [13], [34]. Our findings suggest that trend is unchanged in the presence of routine PCV use. Overall carriage duration was generally similar to that observed in this population prior to the PCV7 introduction [26], despite changes in serotype distribution. These results suggest that older children continue to serve as important reservoirs of pneumococcal carriage within households when PCV is routinely used. Unlike other household studies [11], [34], we did not detect a significant difference in carriage duration by serotype. This observation may be an artifact of our monthly sampling frame, which is biased toward detection of serotypes with longer durations of carriage and limited our ability to resolve finer distinctions in carriage duration.
Children have previously been observed to be the major route of introduction of pneumococcus into the household, both in developing [11] and developed [14] settings. Our findings indicate that this role continues after routine PCV use, across all of the most commonly carried serotypes in this study. Moreover, older children appear to play a larger role in household introductions than children <2 years, likely due to increased exposure in the community and school. In contrast, children <2 years were the primary source of household introduction in a pre-PCV cohort from the United Kingdom [35], perhaps reflecting differences in serotype distribution or age-specific exposure and acquisition patterns between study populations. Other household introduction analyses have not compared children by age subgroup [11], [14].
Among adults in this community, men may introduce serotypes into the household at a higher rate than women; conversely, women may be more involved in intra-household transmission (given their higher rates of concurrent carriage). These findings may reflect higher rates of community exposure in men and effective household contact in women or a bias in the subset of men who participated in the study compared with women.
By isolating the effect of age on transmission – independent of age-specific carriage prevalence – multilevel analysis further confirmed the importance of older children in pneumococcal household dynamics, especially as transmitters to adults. Interestingly, for serotype 6C, exposure to carriage in children <2 years was significantly associated with adult acquisition, while exposure to carriage in older children was not. This observation may be due to imprecision alone or a true difference in serotype-specific transmissibility patterns. In a recent pre-PCV7 Kenyan study, mothers and siblings of all ages transmitted to neonates with similar efficiency [36]. While our study did not specifically examine this pairing, exposure to carriage in children 2–8 years here tended to be more strongly associated with acquisition in children <9 years than carriage in other age groups.
Our study has several limitations. Risk factor data was collected via interview, potentially introducing reporting bias. Most households had members who declined to participate, especially older adults and especially men. Our analyses adjusted for reported household composition and accounted for the number of swabs obtained by age category. Still, we cannot exclude the possibility that observed acquisitions may have been transmitted from missing household members. Our inferences assume that consecutively carried or transmitted serotypes represented the same pneumococcal strain. Multi-locus sequence typing or whole genome sequencing may provide stronger strain-specific inferences than serotyping alone [11]. Although our methods were capable of detecting multiple serotypes per swab when more than one morphology was present, our methods were unable to evaluate the impact of strain-strain competition within the nasopharynx. Our analysis does not distinguish between immune-mediated clearance and displacement of one serotype by another, two biologically distinct processes which are likely to have differential impacts on our observed clearance rate by age group and risk factor under study. Even in the absence of complete displacement, if a serotype were reduced below our threshold of detection by proliferation of a second, co-colonizing serotype, our analysis would register clearance of the former and an acquisition of the latter, rather than continued co-colonization. Finally, our monthly sampling frame limited our ability to observe events occurring on a shorter time scale.
This study provides evidence that, in the setting of PCV, children remain the major route of pneumococcal entry into the household, a reservoir for maintaining carriage within households, and both the source and recipient of the majority of intra-household transmission. The 13-valent conjugate vaccine (Prevnar13, [Pfizer]) now in routine use in the United States contains capsular polysaccharide to serotype 6A, which will likely confer cross-protection to serotype 6C [37], [38], and 19A; 19A and 6C are the two most frequently acquired and transmitted serotypes in this cohort. The CDC currently recommends a single PCV13 catch-up dose for children ages 2–5 years previously vaccinated with PCV7 [39]. Given the important role of older children in household pneumococcal dynamics, this catch-up schedule likely accelerates PCV indirect effects by limiting household introduction and transmission of vaccine serotypes. Finally, while our results provide insight into pneumococcal transmission in a high-burden population, transmission dynamics may differ in other settings according to risk factor distribution and prevalence of carriage. Longitudinal household studies will continue to be an important tool, defining pneumococcal carriage dynamics as new conjugate vaccines are introduced around the world.
Supporting Information
Seasonal trends in individual acquisition (⧫) and household introduction (•). Incidence rate ratios and 95% confidence intervals obtained from Poisson regression analysis (with January as reference month of study entry).
(TIF)
Acknowledgments
The authors express their appreciation to the children and adults from the Navajo and White Mountain Apache communities who participated actively in the studies. We also gratefully acknowledge the dedicated efforts of the Center for American Indian Health field staff who collected these data over many years – particularly Stella Cly, Stevie Cosay, Virgina Crocker, Beverly Gorman, Lora Lavender, Lori Samuel, and Roxy Thompson – and the Indian Health Service, which provided clinic space as well as clinical and laboratory support. We also gratefully acknowledge the work by Jennifer Scott on preceding analyses and by Mindy Perilla on data management, as well as the statistical guidance provided by Lawrence Moulton.
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
Funding Statement
This work was supported by the PneumoCarr Consortium funded by the Grand Challenges in Global Health initiative which is supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health (US), the Wellcome Trust and the Canadian Institutes of Health Research. This study was additionally supported by The Native American Research Centers for Health [U26IHS300013/03], a joint initiative between the National Institutes of Health and Indian Health Service to reduce Native health disparities and build tribal autonomy in conducting health research. This study was also funded by the Centers for Disease Control and Prevention National Vaccine Program Office and the Thrasher Research Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shah SS, Ratner AJ (2006) Trends in invasive pneumococcal disease-associated hospitalizations. Clin Infect Dis 42 (1) e1–5. [DOI] [PubMed] [Google Scholar]
- 2. Lexau C, Lynfield R, Danila R, Pilishvili T, Facklam R, et al. (2005) Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294 (16) 2043–51. [DOI] [PubMed] [Google Scholar]
- 3. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, et al. (2010) Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201 (1) 32–41. [DOI] [PubMed] [Google Scholar]
- 4. Lacapa R, Bliss SJ, Larzelere-Hinton F, Eagle KJ, McGinty DJ, et al. (2008) Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis 47 (4) 476–84. [DOI] [PubMed] [Google Scholar]
- 5. Isaacman DJ, McIntosh ED, Reinert RR (2010) Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 14 (3) e197–209. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (2008) Estimated Hib and pneumococcal deaths for children under 5 years of age Available: http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/en/index.html. ccessed 6 December 2012.
- 7. Gray BM, Converse GM, Dillon HC (1980) Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142 (6) 923–33. [DOI] [PubMed] [Google Scholar]
- 8. Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, et al. (1997) Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis 175 (6) 1440–1445. [DOI] [PubMed] [Google Scholar]
- 9. Bogaert D, Groot RD, Hermans PWM (2004) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet 4 (March) 144–154. [DOI] [PubMed] [Google Scholar]
- 10. Leino T, Hoti F, Syrjänen R, Tanskanen A, Auranen K (2008) Clustering of serotypes in a longitudinal study of Streptococcus pneumoniae carriage in three day care centres. BMC Infect Dis 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill PC, Townend J, Antonio M, Akisanya B, Ebruke C, et al. (2010) Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis 50 (11) 1468–76. [DOI] [PubMed] [Google Scholar]
- 12. Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, et al. (2005) Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis 192 (3) 387–93. [DOI] [PubMed] [Google Scholar]
- 13. Melegaro A, Gay NJ, Medley GF (2004) Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol Infect 132 (3) 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melegaro A, Choi Y, Pebody R, Gay N (2007) Pneumococcal carriage in United Kingdom families: estimating serotype-specific transmission parameters from longitudinal data. Am J Epidemiol 166 (2) 228–35. [DOI] [PubMed] [Google Scholar]
- 15. Auranen K, Arjas E, Leino T, Takala AK (2000) Transmission of pneumococcal carriage in families: a latent Markov process model for binary longitudinal data. J Am Stat Assoc 95 (452) 1044. [Google Scholar]
- 16. Leino T, Auranen K, Jokinen J, Leinonen M, Tervonen P, et al. (2001) Pneumococcal carriage in children during their first two years: important role of family exposure. Pediatr Infect Dis J 20 (11) 1022–7. [DOI] [PubMed] [Google Scholar]
- 17. Granat SM, Ollgren J, Herva E, Mia Z, Auranen K, et al. (2009) Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J Infect Dis 200 (1) 99–106. [DOI] [PubMed] [Google Scholar]
- 18. Hussain M, Melegaro A, Pebody RG, George R, Edmunds WJ, et al. (2005) A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect 133 (5) 891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Millar EV, O'Brien KL, Watt JP, Bronsdon MA, Dallas J, et al. (2006) Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis 43 (1) 8–15. [DOI] [PubMed] [Google Scholar]
- 20. Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, et al. (2008) Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis 47 (8) 989–96. [DOI] [PubMed] [Google Scholar]
- 21. Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, et al. (2006) Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis 193 (11) 1487–94. [DOI] [PubMed] [Google Scholar]
- 22. Cheung YB, Zaman SM, Nsekpong ED, Van Beneden CA, Adegbola RA, et al. (2009) Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr Infect Dis J 28 (11) 990–5. [DOI] [PubMed] [Google Scholar]
- 23. O'Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, et al. (2007) Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 196 (8) 1211–20. [DOI] [PubMed] [Google Scholar]
- 24. Weatherholtz R, Millar EV, Moulton LH, Reid R, Rudolph K, et al. (2010) Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis 50 (9) 1238–46. [DOI] [PubMed] [Google Scholar]
- 25. Scott JR, Millar EV, Lipsitch M, Moulton LH, Weatherholtz R, et al. (2012) Impact of more than a decade of pneumococcal conjugate vaccine use on carriage and invasive potential in Native American communities. J Infect Dis 205 (2) 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Millar EV, O'Brien KL, Zell ER, Bronsdon MA, Reid R, et al. (2009) Nasopharyngeal carriage of Streptococcus pneumoniae in Navajo and White Mountain Apache children before the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 28 (8) 711–6. [DOI] [PubMed] [Google Scholar]
- 27. Millar EV, Pimenta FC, Roundtree A, Jackson D, Carvalho Mda G, et al. (2010) Pre- and post-conjugate vaccine epidemiology of pneumococcal serotype 6C invasive disease and carriage within Navajo and White Mountain Apache communities. Clin Infect Dis 51 (11) 1258–65. [DOI] [PubMed] [Google Scholar]
- 28. Mercado E, Srinivasan V, Hawkins P, Chochua S, Ochoa T, et al. (2011) First report of Streptococcus pneumoniae serotype 6D in South America. J Clin Microbiol 49 (5) 2080–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melnick N, Thompson TA, Beall BW (2010) Serotype-specific typing antisera for pneumococcal serogroup 6 serotypes 6A, 6B, and 6C. J Clin Microbiol 48 (6) 2311–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011. [Google Scholar]
- 31. Pinheiro JC, Bates DM (1995) Approximations to the log-likelihood function in the nonlinear mixed-effects model. J Comput Graph Stat 4: 12–35. [Google Scholar]
- 32. Pinheiro JC, Chao EC (2006) Efficient Laplacian and adaptive Gaussian quadrature algorithms for multilevel generalized linear mixed models . J Comput Graph Stat 15: 58–81. [Google Scholar]
- 33. Darboe MK, Fulford AJ, Secka O, Prentice AM (2010) The dynamics of nasopharyngeal Streptococcus pneumoniae carriage among rural Gambian mother-infant pairs. BMC Infect Dis 10: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Högberg L, Geli P, Ringberg H, Melander E, Lipsitch M, et al. (2007) Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J Clin Microbiol 45 (3) 948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussain M, Melegaro A, Pebody RG, George R, Edmunds WJ, et al. (2005) A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect 133 (5) 891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tigoi CC, Gatakaa H, Karani A, Mugo D, Kungu S, et al. (2012) Rates of acquisition of pneumococcal colonization and transmission probabilities, by serotype, among newborn infants in Kilifi District, Kenya. Clin Infect Dis 55 (2) 180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grant LR, O'Brien SE, Burbidge P, Haston M, Zancolli M, et al. (2013) Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One 8: e74906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, et al. (2011) The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 29 (41) 7207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nuorti JP, Whitney CG (2010) Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 59 (RR-11) 1–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seasonal trends in individual acquisition (⧫) and household introduction (•). Incidence rate ratios and 95% confidence intervals obtained from Poisson regression analysis (with January as reference month of study entry).
(TIF)