Abstract
Ochrobactrum intermedium is considered as an emerging human environmental opportunistic pathogen with mild virulence. The distribution of isolates and sequences described in literature and databases showed frequent association with human beings and polluted environments. As population structures are related to bacterial lifestyles, we investigated by multi-locus approach the genetic structure of a population of 65 isolates representative of the known natural distribution of O. intermedium. The population was further surveyed for genome dynamics using pulsed-field gel electrophoresis and genomics. The population displayed a clonal epidemic structure with events of recombination that occurred mainly in clonal complexes. Concerning biogeography, clones were shared by human and environments and were both cosmopolitan and local. The main cosmopolitan clone was genetically and genomically stable, and grouped isolates that all harbored an atypical insertion in the rrs. Ubiquitism and stability of this major clone suggested a clonal succes in a particular niche. Events of genomic reduction were detected in the population and the deleted genomic content was described for one isolate. O. intermedium displayed allopatric characters associated to a tendancy of genome reduction suggesting a specialization process. Considering its relatedness with Brucella, this specialization might be a commitment toward pathogenic life-style that could be driven by technological selective pressure related medical and industrial technologies.
Introduction
Experimental approaches on type or model strains of specific bacterial pathogens has led to fruitful results that revolutionized knowledge about the molecular mechanisms of infection [1]. By contrast, model-based approaches not fully succeeded for opportunistic pathogens, mostly because they formed heterogeneous populations, i.e., organised in species complexes rather than in true species [2]. It is now widely accepted that population structure and genome content depend on bacterial life-styles and niches [3]–[5]. Moreover, the population structure of environment-borne human opportunistic pathogens (EBOP) suggested that sub-groups of strains in a species could be associated with and perhaps adapted to human beings [6] or to human pathological niches [7], [8]. Therefore, exploring population structure is a prerequisite to the description of life-style, niche adaptation and evolution mechanisms originating in the emergence and pathogenesis of opportunistic pathogens.
The genus Ochrobactrum belongs to the family Brucellaceae in the class alphaproteobacteria and groups bacteria with versatile life-styles inhabiting various niches and displaying dynamic genomes [9], [10]. In most publications in the field of environmental sciences, Ochrobactrum strains are studied for their potential applications in bioremediation [11]–[14] or as plant growth-promoting rhizobacteria [11], [13], [15]. Ochrobactrum are considered as emerging human opportunistic pathogens, Ochrobactrum anthropi and Ochrobactrum intermedium being the two main species that cause infections, mostly in immunocompromised patients [16]. Multilocus sequence typing supported the hypothesis that O. anthropi displays a human-associated subpopulation but, as for many other EBOPs, the population structure as well as the reservoir(s) of O. intermedium are not precisely defined, impairing the understanding of its potential mechanisms of adaptation to human and thereby, its epidemiology and its pathogenesis. O. intermedium displays additional interest due to its phylogenetic relatedness to the genus Brucella grouping specific pathogens causing brucellosis, a worldwide anthropozoonosis [17]. Contrarily to Ochrobactrum, Brucella groups allopatric microorganisms that live in a narrow niche and display stable genomes with reduced size in comparison with Ochrobactrum genomes [18].
With the aims to explore the relationships of O. intermedium with human and to complete the story about the emergence of pathogenic life-styles among Brucellaceae, we studied here the population structure and the genome dynamics in a collection of strains encompassing the range of life-styles and habitats of this species.
Results
O. intermedium life-style and habitat deciphered to construct a representative collection
In order to learn about the habitat and lifestyle of O. intermedium, we undertook a literature review and we screened nucleotide databases in June 2013. We found thirty-nine publications presenting 115 strains or clones with confirmed affiliation to O. intermedium. In genetic databases, 56 sequences not associated with a publication matched with the reference sequences used for screening (Table S2). One study reported the isolation of O. intermedium whereas the strains belonged to the later described species O. pseudintermedium (NR_043756) on the basis of 16S rRNA gene sequencing [19]. Conversely, 13 strains or clones (Tables S1 and S2) that actually belonged to O. intermedium were affiliated to other Ochrobactrum species, mainly to O. anthropi. Misidentifications were especially linked to wrong nomenclature in databases. For instance, the partial 16S rRNA gene sequence of several collection strains (CCUG39736, LMG5446, LMG3306, CCUG1838, CCUG44770) are affiliated to the species O. anthropi although these strains were transferred to the species O. intermedium based on partial sequence of the recA gene [20]. Similarly, partial 16S rRNA gene sequence of the type strain of O. intermedium CNS 2–75 ( = LMG3301T) (NR_026039) is affiliated to O. anthropi.
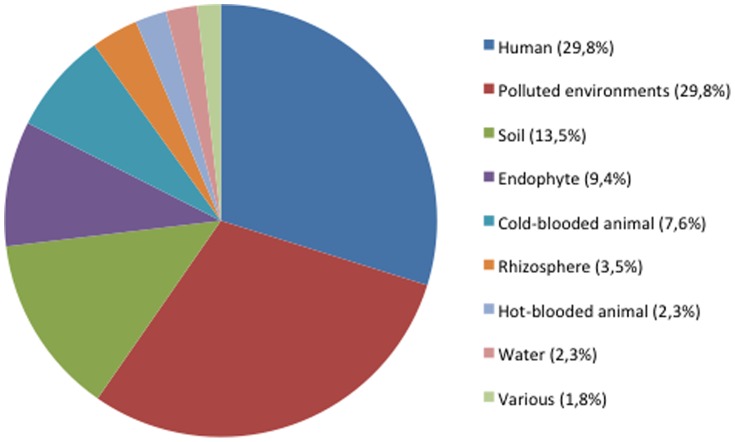
The databanks survey showed the affiliation of 171 strains and clones from publications (n = 115) and from genetic databases (n = 56) to the species O. intermedium on the basis of partial sequence of the 16S rRNA and/or recA genes, sometimes completed by phenotypic data. Figure 1 shows their distribution in a wide range of habitats and lifestyles. Strains were found as free-living bacteria or in more or less close associations with eukaryotes for half of the strains (n = 90). O. intermedium showed frequent association with human beings (n = 51) and environments polluted by a wide range of compounds (pesticides, herbicides, chromium, cadmium, lead, waste water, oil, petroleum, etc.) (n = 51). O. intermedium was also isolated from warm (n = 4) and cold-blooded (n = 13) animals, in association with plants, in the rhizosphere (n = 6) or as endophyte (n = 16) (Table S1). Some plant-associated strains were able to renodulate their host plant and stimulate their growth [15]. The 16S rRNA sequence of Ochrobactrum ciceri, a species isolated from plant nodules, displayed 99.8% of similarity with that of O. intermedium strain CCUG44770. This species has been described as a new taxon separated from O. intermedium mainly on the basis of plant nodules formation (Imran, 2010). Therefore, our database screening approach was not suitable for discriminating O. intermedium and O. ciceri.
Figure 1. Distribution of the 171 strains (n = 148) and clones (n = 23) of O. intermedium/O. ciceri identified from the litterature and databases according to the habitat.
The figure has been constructed from data presented in Tables S1 and S2.
Metagenomic clones matching with the sequences of O. intermedium used for database screening were scarce considering the amount of metagenomic data currently deposited in databanks. Only 28 clones could be affiliated to O. intermedium/O. ciceri. They were detected in 8 different environments, most of them being technological and polluted environments, such as metal degreasing systems [21], aerosols and fluids in metalworking industry [22], biofouling biofilms, petroleum reservoir, polychlorinated bephenyl (PCB) polluted soil and polyacrylamide (PAM)-degrading consortium. Some clones were detected in velvetleaves seed and only one clone was found in the human skin microbiota (GQ115798) [23].
For available sequences, the presence of an atypical insertion previously described in the 16S rRNA gene [9] is reported in the Table S1. No relationships between insertion detection and habitat or lifestyle could be established.
In this study, we constituted the largest published collection of O. intermedium/O. ciceri strains (n = 65) (Tables 1 and 2) but mainly focused on clinical isolates. It contained 37 clinical isolates from French hospitals (this work and [9], [10], [16], [24]), 11 environmental isolates (this work and [25]) and to our knowledge all the 17 strains currently available in collections : 12 clinical including the type strains of O. intermedium and 5 environmental strains including the type strains of O. ciceri. Clinical strains were mainly isolated from the Montpellier Hospital in south of France from 1999 to 2011 but the clinical strain collection was completed with strains from other French towns, countries and continents isolated since 1972. Considering the reservoirs of O. intermedium/O. ciceri strains and clones in databases, our collection is globally representative of the species diversity except for an underrepresentation of strains from polluted environments. However, the only strain from polluted environment currently available in strain collections (CCUG57381) has been included in the present collection.
Table 1. Characteristics of the 49 O. intermedium strains of human origin included in this study presented according to MLST results.
| Strain | CC | ST | Allelic profilesa | 46-pb insertionb | Origin | Place (town, region or state, country) and date of isolation | ||||||
| dnaK | recA | rpoB | aroC | omp25 | trpE | gap | ||||||
| ADV1 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Sputum | Montpellier, Fr, jan 1999 |
| ADV107 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Rectum | Montpellier, Fr, feb 2008 |
| ADV32 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Rectum | Montpellier, Fr, jan 2003 |
| ADV9 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Ear | Montpellier, Fr, aug 1999 |
| LMG5425 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Urine | Sheffield, England, before 1983 |
| LMG5426 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Urine | Sheffield, England, before 1983 |
| Nim80 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Toe nail | Nîmes, Fr, 2006 |
| ADV10 | CC74 | 69 | 10 | 7 | 26 | 20 | 16 | 21 | 18 | − | Wound | Montpellier, Fr, nov 1999 |
| ADV101 | CC74 | 70 | 10 | 7 | 26 | 18 | 16 | 21 | 18 | − | Rectum | Montpellier, Fr, nov 2007 |
| ADV93 | CC74 | 70 | 10 | 7 | 26 | 18 | 16 | 21 | 18 | + | Rectum | Montpellier, Fr, jun 2007 |
| ADV11 | CC74 | 71 | 10 | 7 | 26 | 18 | 16 | 21 | 16 | − | Rectum | Montpellier, Fr, nov 1999 |
| ADV24 | CC74 | 71 | 10 | 7 | 26 | 18 | 16 | 21 | 16 | − | Axilla | Montpellier, Fr, feb 2002 |
| ADV14 | CC74 | 72 | 10 | 7 | 26 | 20 | 24 | 21 | 16 | − | Axilla | Montpellier, Fr, may 2000 |
| ADV56 | CC74 | 73 | 10 | 7 | 26 | 18 | 24 | 21 | 16 | − | Rectum | Montpellier, Fr, mar 2005 |
| ADV73A | CC74 | 74 | 10 | 7 | 26 | 21 | 16 | 21 | 18 | − | Rectum | Montpellier, Fr, may 2006 |
| Toul65 | CC74 | 74 | 10 | 7 | 26 | 21 | 16 | 21 | 18 | − | Sputum (CF) | Toulouse, Fr, aug 2005 |
| ADV89 | S | 75 | 12 | 7 | 26 | 18 | 24 | 22 | 19 | − | Rectum | Montpellier, Fr, apr 2007 |
| ADV124 | CC76 | 76 | 15 | 8 | 23 | 35 | 16 | 26 | 18 | − | Sputum | Montpellier, Fr, nov 2008 |
| CCUG39736 | CC76 | 77 | 15 | 8 | 23 | 31 | 25 | 26 | 18 | − | Blood | Umeå, Sweden, 1998 |
| ADV54 | CC68 | 78 | 11 | 13 | 24 | 27 | 27 | 18 | 15 | + | Rectum | Montpellier, Fr, sep 2004 |
| CCUG1838 | CC68 | 79 | 22 | 13 | 24 | 18 | 16 | 18 | 15 | + | Urine | Göteborg, Sweden, 1972 |
| CCUG44770 | CC68 | 80 | 12 | 13 | 24 | 21 | 16 | 21 | 15 | + | Sputum (CF) | Wien, Austria, 2000 |
| LMG379 | CC68 | 83 | 11 | 13 | 24 | 18 | 28 | 20 | 15 | + | Ear | Louisiana, USA, before 1988 |
| ADV143B | CC68 | 84 | 11 | 13 | 24 | 20 | 16 | 18 | 15 | + | Rectum | Montpellier, Fr, mar 2010 |
| ADV111 | CC68 | 85 | 11 | 13 | 24 | 21 | 16 | 18 | 15 | + | Sputum (CF) | Montpellier, Fr, apr 2008 |
| ADV126* | CC68 | 86 | 11 | 13 | 24 | 18 | 22 | 18 | 15 | + | Axilla | Montpellier, Fr, feb 2009 |
| ADV127* | CC68 | 86 | 11 | 13 | 24 | 18 | 22 | 18 | 15 | + | Axilla | Montpellier, Fr, feb 2009 |
| CRBIP17.121 | CC68 | 86 | 11 | 13 | 24 | 18 | 22 | 18 | 15 | + | Peritoneal fluid | Montélimar, Fr, 2005 |
| ADV109 | S | 87 | 14 | 15 | 18 | 28 | 19 | 27 | 23 | + | Blood | Montpellier, Fr, mar 2008 |
| ADV78 | CC74 | 88 | 10 | 15 | 26 | 18 | 16 | 21 | 16 | − | Axilla | Montpellier, Fr, oct 2006 |
| ADV42 | S | 89 | 19 | 15 | 27 | 23 | 20 | 28 | 20 | − | Rectum | Montpellier, Fr, nov 2003 |
| ADV67** | CC91 | 90 | 18 | 16 | 20 | 19 | 17 | 31 | 25 | − | Pancreas | Montpellier, Fr, sep 2005 |
| ADV69** | CC91 | 90 | 18 | 16 | 20 | 19 | 17 | 31 | 25 | − | Rectum | Montpellier, Fr, oct 2005 |
| ADV147 | CC91 | 91 | 18 | 16 | 20 | 18 | 16 | 31 | 25 | − | Sputum | Montpellier, Fr, sep 2010 |
| LMG3301T | CC91 | 91 | 18 | 16 | 20 | 18 | 16 | 31 | 25 | − | Blood | France, before 1988 |
| ADV85 | S | 92 | 13 | 16 | 22 | 26 | 24 | 32 | 26 | − | Rectum | Montpellier, Fr, jan 2007 |
| ADV44 | CC93 | 93 | 12 | 14 | 25 | 24 | 23 | 22 | 19 | − | Rectum | Montpellier, Fr, feb 2004 |
| Nim125 | CC93 | 94 | 12 | 14 | 25 | 18 | 16 | 22 | 19 | − | Broncho-alveolar lavage fluid | Nîmes, Fr, dec 2008 |
| ADV21 | CC96 | 95 | 16 | 17 | 21 | 21 | 16 | 25 | 21 | − | Rectum | Montpellier, Fr, feb 2001 |
| CIP105839 | CC96 | 96 | 16 | 17 | 21 | 29 | 21 | 25 | 21 | − | Blood | Pamplona, Spain, before 1998 |
| CIP105840 | CC96 | 96 | 16 | 17 | 21 | 29 | 21 | 25 | 21 | − | Blood | Pamplona, Spain, before 1998 |
| ADV35 | S | 97 | 17 | 18 | 17 | 22 | 23 | 23 | 16 | − | Blood | Montpellier, Fr, jun 2003 |
| LMG5446 | S | 98 | 21 | 9 | 28 | 32 | 16 | 24 | 18 | − | Bladder | Georgia, USA, before 1986 |
| Tou55 | S | 100 | 19 | 12 | 16 | 34 | 23 | 30 | 17 | + | Sputum (CF) | Toulouse, Fr, nov 2004 |
| LMG5443 | CC68 | 101 | 11 | 13 | 24 | 30 | 16 | 18 | 15 | + | Urine | North Carolina, USA, before 1988 |
| ADV152 | S | 104 | 24 | 19 | 29 | 18 | 16 | 33 | 27 | − | Rectum | Montpellier, Fr, dec 2010 |
| ADV46 | S | 105 | 23 | 10 | 19 | 25 | 26 | 29 | 24 | − | Bladder drain liquid | Montpellier, Fr, may 2004 |
| NAN157 | CC74 | 135 | 12 | 7 | 26 | 21 | 16 | 21 | 28 | − | Broncho-alveolar lavage fluid | Nancy, Fr, dec 2010 |
| ADV158 | CC74 | 136 | 15 | 7 | 26 | 33 | 16 | 21 | 28 | − | Sinus | Montpellier, Fr, apr 2011 |
CC, clonal complex; ST, sequence type; Fr, France; USA, United States of America; CF, Cystic Fibrosis. Strains marked by * or ** were isolated in the same patient.
a For each locus, each different allele was assigned an arbitrary number;
b 46-bp atypical insertion described in Teyssier et al. 2003 [9].
Table 2. Characteristics of the 15 O. intermedium strains and O. ciceri type strain of environmental origin presented according to MLST results.
| Straina | CC | ST | Allelic profilesb | 46-pb insertionc | Origin | Place (town, region or state, country) and date of isolatione | ||||||
| dnaK | recA | rpoB | aroC | omp25 | trpE | gap | ||||||
| RT148-2 | CC68 | 68 | 11 | 13 | 24 | 18 | 16 | 18 | 15 | + | Water (lake) | Liausson, Fr, 2010 |
| RT148-1P | CC74 | 70 | 10 | 7 | 26 | 18 | 16 | 21 | 18 | − | Water (lake) | Liausson, Fr, 2010 |
| RT168-1 | CC74 | 71 | 10 | 7 | 26 | 18 | 16 | 21 | 16 | − | Water (river) | Montpellier, Fr, 2010 |
| FRG10/sat | CC74 | 74 | 10 | 7 | 26 | 21 | 16 | 21 | 18 | − | Nematode (Heterorhabditis indica) | Guadeloupe, Fr, 2005 |
| LMG18956 ( = OiC8-6) | CC74 | 74 | 10 | 7 | 26 | 21 | 16 | 21 | 18 | − | Agricultural soil | Grignon, Fr, before 1996 |
| LMG3306 | CC74 | 74 | 10 | 7 | 26 | 21 | 16 | 21 | 18 | − | Soil | France, before 1988 |
| RT172 | CC76 | 76 | 15 | 8 | 23 | 35 | 16 | 26 | 18 | − | Sand (beach) | Saint Pierre, La Réunion, Fr, 2011 |
| RT190-1 | CC76 | 76 | 15 | 8 | 23 | 35 | 16 | 26 | 18 | − | Water (river) | Montpellier, Fr, 2011 |
| CCUG57381 | CC76 | 77 | 15 | 8 | 23 | 31 | 25 | 26 | 18 | − | Water from antibiotic production mixed with sewage | Hyderabad, India, 2007 |
| FRG14/sat | CC68 | 81 | 11 | 13 | 24 | 18 | 15 | 18 | 15 | + | Nematode (Heterorhabditis indica) | Guadeloupe, Fr, 2005 |
| JLJ57 | CC68 | 82 | 11 | 13 | 24 | 18 | 24 | 18 | 15 | + | Pharmaceutical water | Montpellier, Fr, 2005 |
| DO07/sat | CC68 | 84 | 11 | 13 | 24 | 20 | 16 | 18 | 15 | + | Nematode (Heterorhabditis indica) | Dominican Republic, 1996 |
| PR17/sat | CC68 | 84 | 11 | 13 | 24 | 20 | 16 | 18 | 15 | + | Nematode (Heterorhabditis indica) | Puerto Rico, 1996 |
| CCM7036 | S | 99 | 20 | 11 | 15 | 29 | 18 | 19 | 22 | + | Insect (Phlebotomus duboscqi) | Czech Republic, before 2002 |
| RT23-4 | CC93 | 102 | 12 | 14 | 25 | 33 | 24 | 22 | 19 | − | Water and sediments (river) | Blois, Fr, 2009 |
| O. ciceri DSM22292T | CC68 | 113 | 11 | 13 | 24 | 18 | 48 | 18 | 15 | + | Root nodules of Cicer arietinum | Faisalabad, Pakistan, 1996 |
CC, clonal complex; ST, sequence type; Fr, France.
a Strains noted RT were isolated from systematic searching of O. intermedium in 200 soil and water samples randomly collected worldwide.
b For each locus, each different allele was assigned an arbitrary number.
c 46-bp atypical insertion in rrs described by Teyssier et al., 2003 [9].
Multilocus genetics
Among the 65 strains studied, MLSA showed a total of 227 single nucleotide polymorphisms (SNPs) in the 7 loci corresponding to 3.6% and 9.5% of polymorphic sites depending on the gene (Table 3). The mean genetic diversity (H) among strains was 0.7795+/−0.0253 and the genetic diversity at each locus (h) is given in Table 3. H in the clinical strain population (0.7936+/−0.0271) did not differ significantly from H in the environmental population (0.7452+/−0.0259), P = 0.456. Genes had equivalent mol% G+C contents from 56.6% to 61.7% with a mean value of 59.7% that was similar to the mean mol% G+C contents of the O. intermedium chromosomes (57.7%) (http://www.ncbi.nlm.nih.gov/genome/2167?project_id=55963). The number of alleles ranged from 13 (recA) to 18 (aroC) (Table 3) and did not depend on the size of the sequence studied. The locus omp25 that codes for an antigenic surface protein displayed a number of alleles (15 against 15.1 on average) and a percentage of polymorphic sites (6.9 against 6.5 on average) similar to other loci. However, this locus had the lowest genetic diversity (0.6322). Indeed, 60% (39/65) of strains shared the allele 16 for this locus and belonged to different CCs or were singleton (Tables 1 and 2). The same particularity was observed for the allele 18 of aroC shared by 44% of the strains (Tables 1 and 2). No relationship was observed between these major alleles and the sample type, lifestyle or geographical origin of the strains. The majority of SNPs were predominantly (rpoB, aroC and gap) or exclusively (recA and dnaK) synonymous (Table 3). Loci omp25 et trpE included the majority of nonsynonymous mutations observed (9 and 8, respectively) with a high ratio of non-synonymous to synonymous SNPs. The non-synonymous mutations did not correspond to any premature stop codon.
Table 3. Sequence analysis of the seven loci.
| Locus (sequence length) | Number of alleles | Number of polymorphic sites (%) | Genetic diversity (h) | Number of non-synonymous codons | dNa | dSb | dN/dS |
| dnaK (534 pb) | 15 | 19 (3.6%) | 0.8293 | 0 | 0.000 | - | - |
| recA (490 pb) | 13 | 28 (5,7%) | 0.7990 | 0 | 0.000 | - | - |
| rpoB (501 pb) | 15 | 37 (7,4%) | 0.7942 | 1 | 0.0026 | 0.0677 | 0.0384 |
| aroC (433 pb) | 18 | 41 (9.5%) | 0.7784 | 2 | 0.0031 | 0.1259 | 0.0246 |
| omp25 (390 pb) | 15 | 27 (6.9%) | 0.6322 | 9 | 0.0118 | 0.0775 | 0.1522 |
| trpE (564 pb) | 16 | 40 (7.1%) | 0.8135 | 8 | 0.0060 | 0.0560 | 0.1071 |
| gap (578 pb) | 14 | 35 (6.0%) | 0.8101 | 2 | 0.0034 | 0.0575 | 0.0591 |
a dN = non-synonymous substitutions per non-synonymous site.
b dS = synonymous substitutions per synonymous site.
Population structure
The 65 strains studied grouped in 40 sequences types (STs) (Tables 1 and 2). Twenty-nine of them (72%) were identified only once suggesting an overall high level of genetic diversity among the studied population (Tables 1 and 2). The largest STs were ST68 and ST74 (8 and 5 isolates, respectively), grouping 20% of the studied strains. In each of these STs, most strains appeared epidemiologically unrelated, i.e., they were isolated over large periods of time and from geographically distant places. Nine other STs contained 2 or 3 strains. All the strains belonging to ST86, ST90, ST91 and ST96 were clinical isolates whereas ST70, ST71, ST76, ST77, ST84 grouped strains from man and environment. With the exception of ST86 (3 clinical strains, 2 of them being from the same patient), the STs comprising more than 2 strains included strains of clinical and environmental origins. The remaining 29 singleton STs corresponded to clinical (n = 24) and environmental (n = 5) strains.
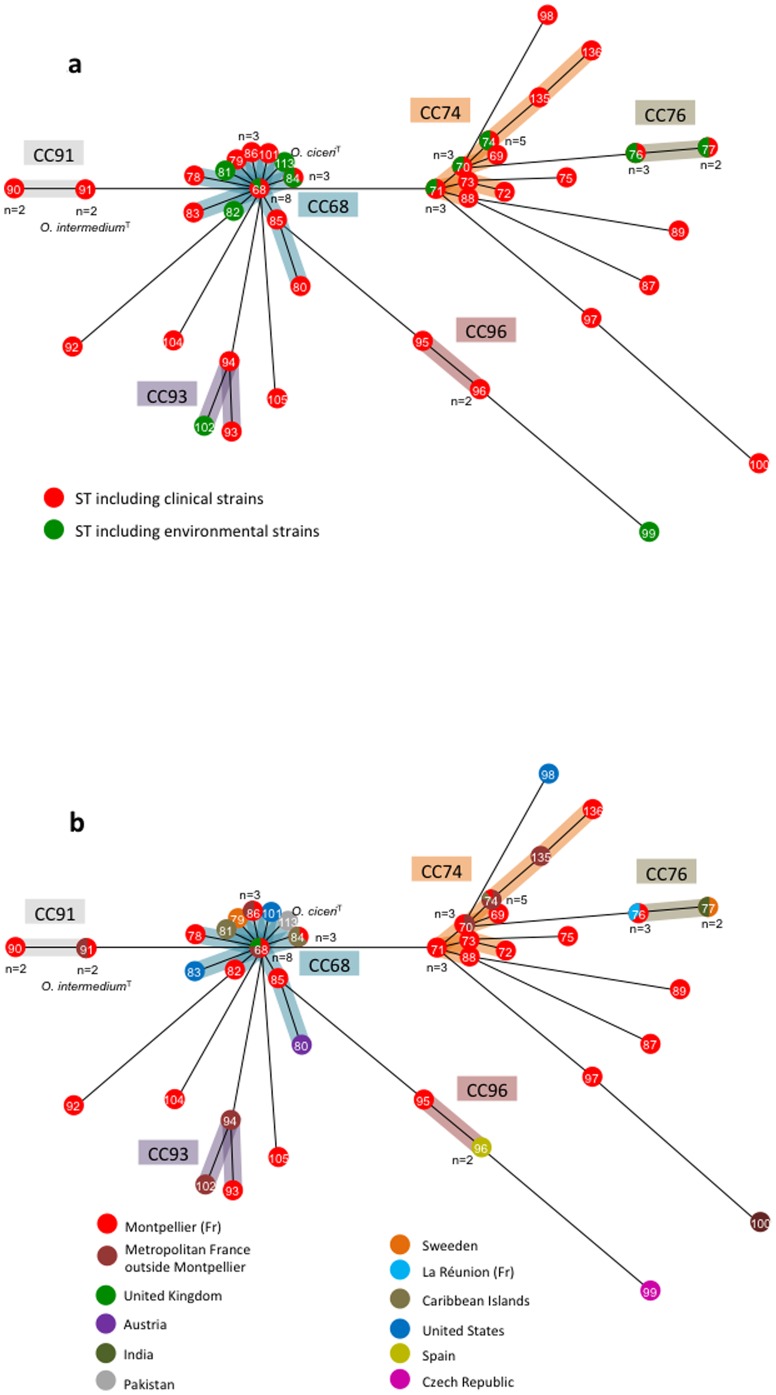
We constructed a minimum-spanning (MS) tree based on clustering of the MLST profiles as a graphic representation of the population structure (Fig. 2a and 2b). In the MS tree, strains formed two major clonal complexes CC68 (23 strains, 12 STs) and CC74 (17 strains, 9 STs), as well as four minor complexes, CC76 (5 strains, 2 STs), CC91 (4 strains, 2 STs), CC93 (3 strains, 3 STs), CC96 (3 strains, 2 STs) and 10 singletons. The type strain of O. ciceri, which was the sole representative of the ST113, belonged to the major complex CC68. The locus rpoB was the sole to be shared by all strains of the same CC. CC68 strains also shared the same alleles of loci recA and gap whereas the same allele of trpE was found in the CC74. The 4 minor CCs were variable only for loci aroC and omp25.
Figure 2. Minimum-Spanning (MS) tree for 64 strains of O. intermedium and type strain of O. ciceri based on MLST data.
The tree was based on the allelic profiles. Each circle corresponds to a sequence type (ST). The number given in the circle corresponds to the ST designation. The number given near the circle corresponds to the number of isolates forming the ST. Shading areas indicate the clonal complexes (CC68, CC74, CC76, CC91, CC93 and CC96). (A) MS tree depending on the clinical (red circles) or environmental origin (green circles) of the strains. (B) MS tree depending on the geographical origin of the strains.
No grouping depending on the clinical or environmental origin of the strains was observed (Fig. 2a). The 2 major CCs and the 2 minor CCs CC76 and CC93 included strains isolated from both humans and environment. In contrast, the 2 minor CCs CC91 and CC96 contained only strains of clinical origin (4 and 3 strains, respectively). The population structure did not reflect the strain origin and habitat. For instance, the 4 strains isolated from nematode belonged to 3 STs and 2 CCs.
The geographical origin of strains appeared unrelated to the population structure (Fig. 2b). A high genetic diversity was observed among strains from Montpellier, each CC being represented and 7 strains corresponding to singleton STs. Nearly 90% (16/18) of strains isolated outside Metropolitan France belong to CCs also containing strains from Montpellier, suggesting that the collection studied is representative of the diversity of the species. The CC68 appeared cosmopolitan and included strains isolated from 8 different countries on 3 continents. The CC74 contained only strains isolated in France but in very different regions including Guadeloupe, a Carribean island. The minor CCs were cosmopolitan (CC76 and CC96) or exclusively French (CC91 and CC93).
Two pairs of strains (ADV67-ADV69 and ADV126-127) isolated from the same patient shared the same ST (ST90 and ST86, respectively). By contrast, strains ADV85 and ADV89, isolated from rectal carriage in a same patient more than three months apart and the environmental strains RT148-1P and RT148-2, isolated from the same sample, belonged respectively to different STs.
For clinical strains, there were no relationships between STs and/or CCs and the origin of clinical specimen from which the strain was isolated. Consequently, CCs and STs could not be related to particular body sites and their associated microbiota. Clinical isolates corresponded either to bacterial carriage without related local infection (mainly digestive and axillary carriage) (n = 18) or to the isolation of O. intermedium from a body site of infection (n = 31) (Table 1). The “carriage” or “infection” status was unrelated to the strain genetic population structure. Some strains were recovered from sites and fluids that are normaly sterile, such as blood, urine and peritoneal fluid, and again, these strains did not belong to a particular genetic cluster. Similarly, the 4 strains involved in the colonization of the respiratory tract of cystic fibrosis patients belonged to 4 different STs, one being a singleton and the 3 others being distributed in 2 CCs (Tables 1 and 2).
The atypical 16S rDNA insertion was present in all CC68 strains, including O. ciceri (Tables 1 and 2). Outside the cosmopolitan CC68, only three singletons (ADV109, Toul55 and CCM7036) and a strain of the CC74 (ADV93) were positive for this insertion. Apart from O. intermedium, a database search showed the insertion to be found in Ochrobactrum daejeonense (HQ171203) and Ochrobactrum pituitosum (AM490609), and outside the genus Ochrobactrum, in Brucella strains isolated from frogs (HE608873 and HE603360), Rhizobium sp. (FM173842), Paenochrobactrum sp. (JF804769) and Phyllobacterium sp. (GQ183850). The sequences of the insertion were identical for strains belonging to Ochrobactrum and Brucella sp. but differed by 1 to 6 bp in the 3 other genera.
Phylogeny and recombination
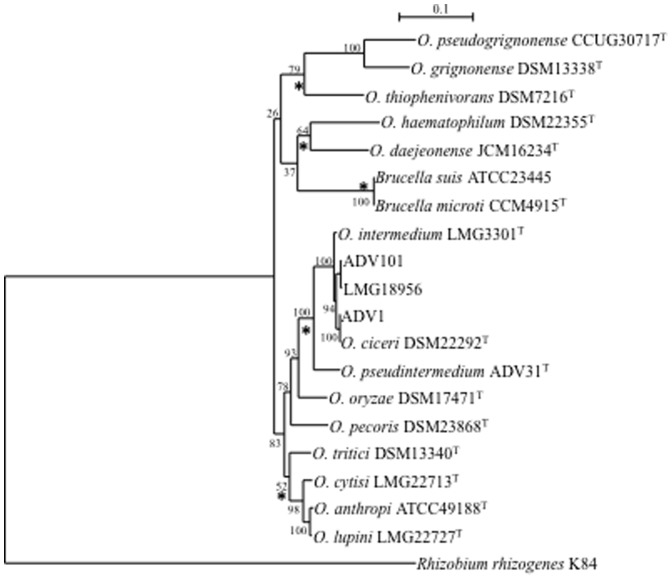
Distance and ML phylogenies were reconstructed from concatenated sequences (3480 bp) of the seven loci for 14 type strains of Ochrobactrum spp., representatives of major O. intermedium clones and 2 strains of Brucella spp. In both phylogenies, O. intermedium belonged to a robust clade together with O. anthropi and 7 other species (Fig. 3). This clade was clearly separated from Ochrobactrum haematophilum, O. daejeonense, Ochrobactrum thiophenivorans, Ochrobactrum grignonense and Ochrobactrum pseudogrignonense that formed a weak phylogenetic group with Brucella spp. in ML phylogeny whereas distance tree placed Brucella in outgroup position. Whatever the tree considered, the tree structure did not suggest an ancestral position of Ochrobactrum regarding Brucella but rather a parallel speciation.
Figure 3. Maximum-Likelihood tree based on concatenated sequences of the seven housekeeping gene fragments of the MSLT scheme indicating the relative placement of type strains of Ochrobactrum spp. and 2 strains of Brucella spp.
The scale bar indicates the number of substitutions per nucleotide position. The numbers at the nodes are support values estimated with 100 bootstrap replicates. Rhizobium rhizogenes K84 was used as the outgroup organism. The sequences of O. intermedium strains ADV1, ADV101 and LMG18956 that represented the major CC in O. intermedium were also included. Asterisks indicate common nodes in Maximum-Likelihood and Neighbor-Joining trees.
Phylogenetic relationships among O. intermedium strains were shown in Figure S1. ML phylogeny confirmed the genetic structure since all CCs corresponded to robust clades. Inside each clade, the relationships between strains reflected their distribution in STs. Of note, the type strain of O. ciceri belonged to a O. intermedium clade in multilocus phylogeny. ML phylogenies were also reconstructed for each locus (data not shown). The recA, rpoB, gap, dnaK and trpE-based phylogenies were globally congruent with multilocus phylogeny. In aroC and omp25-based phylogenies, clade structuration and relationships among strains were not robust due to low phylogenetic signal in the corresponding sequences. Some incongruences between reconstructed phylogenies probably corresponded to genetic exchanges between clades.
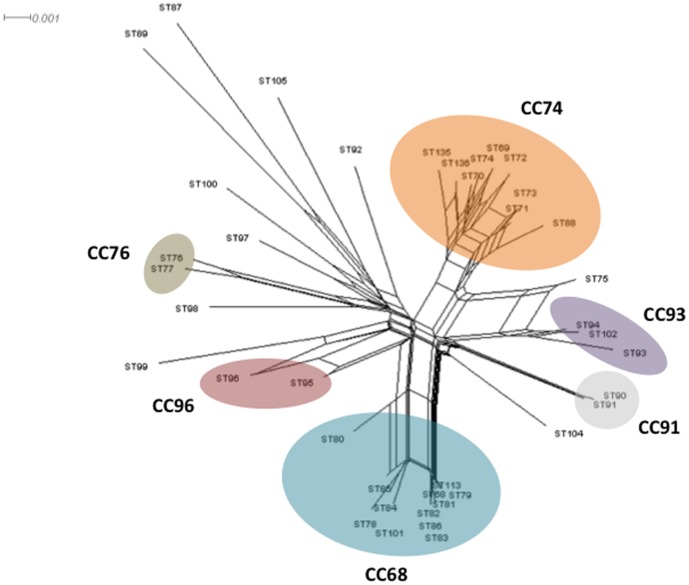
Evidence in favor of clonal or recombining population structure can be obtained by assessing the levels of linkage between alleles at different loci by sIA determination. The sIA value is expected to be zero when a population is at linkage equilibrium, i.e., when free recombination occurs. Analyses were carried out using one isolate from each ST in order to minimize bias due to a possible epidemic population structure. sIA was significantly different from zero (sIA = 0.3594; P value <10E3), suggesting that the recombination rates were low. In contrast, the homoplasy index φw test, which discriminates between recurrent mutation and recombination, found statistically significant evidence for recombination (P value = 0.0). Evidence of recombination was also supported by the Neighbor-Net analysis, which revealed an interconnected network (Fig. 4). Clusters observed were consistent with the population structure determined by MS tree. Recombination events appeared more frequent inside the CCs but also occurred between singletons and CCs, and between singletons. For example, several parallel paths were observed between the singleton ST75 and the CCs CC74 and CC93. All these data suggested that O. intermedium displayed a clonal epidemic population structure with recombination events.
Figure 4. Neighbor-net graph constructed from the concatened sequences of the 40 STs of O. intermedium and O. ciceri using Splits Tree 4.0.
STs are indicated at the branch tips. STs belonging to the same CC are enclosed in an ellipse. A network-like graph indicates recombination events.
Survey of genome dynamics in O. intermedium
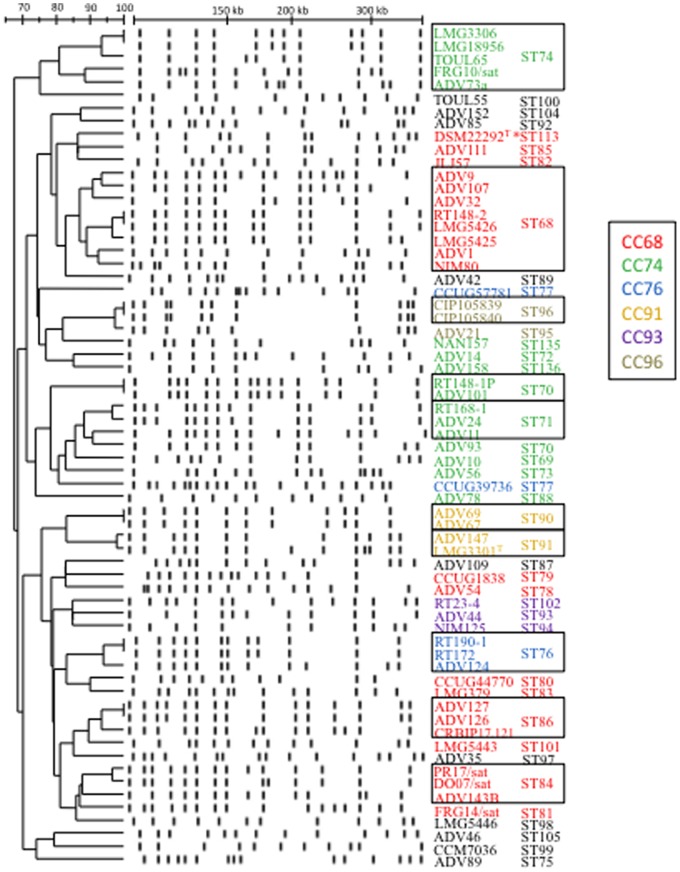
The genomic DNA of strains was analysed by PFGE after macro-restriction with the endonuclease SpeI. The 65 strains showed 58 pulsotypes and nearly 80% (51/65) of strains had a unique pulsotype, indicating the global non-redondancy of the collection (Fig. 5). Seven pairs of strains displaying identical ST shared a pulsotype. Among them, two pairs of strains were isolated from the same patients (ADV67/ADV69 in ST90 and ADV127/ADV126 in ST86). Two other pairs (CIP105839-CIP105840 in ST96 and LMG3306-LMG18956 in ST74) consisted of strains isolated from the same environmental habitat or body site, in the same country but no data on the link between these strains was available from bacterial collections. The three remaining pairs of strains appeared epidemiologically unrelated: LMG5426 and RT148-2 in ST68 were isolated from human urine (UK, before 1983) and water lake (France 2010), respectively; strains ADV101/RT148-1P in ST70 and RT172/RT190-1 in ST76 were respectively isolated from distinct environmental habitats at different dates.
Figure 5. Dendrogram derived from UPGMA cluster analysis of SpeI-restricted DNA PFGE patterns of O. intermedium/O. ciceri.
Dice coefficients and approximate fragment sizes are shown at the top of the dendrogram. Clonal complexes (CC) are depicted by a colour code. Sequence types (ST) of the strains were also reported. Frames indicated PFGE clusters grouping strains with the same ST. *; O. ciceri.
Numerical analysis of the PFGE fingerprints showed an overall conservation of the genomic structure with 67% of pattern similarity in the population. The dendrogram revealed clusters consistent with the multilocus population structure and phylogenies (Fig. 5). Except for ST70 and ST77, strains sharing a ST were grouped into the same PFGE cluster and exhibited more than 80% of pattern similarity. PFGE clustering of strains of the same CC was also observed for CC91 and CC96 (>95% of pattern similarity), and CC93 (>80% of pattern similarity). The two major CCs contained a limited number of PFGE clusters (>80% of pattern similarity): 21 of the 23 strains belonging to CC68 were grouped in 5 PFGE clusters and 15 of the 17 strains belonging to CC74 were grouped in 4 PFGE clusters.
The pulsotypes of the two strains of ST91 differed by only two bands present for strain LMG3301T and absent in strain ADV147. The sizes and sequences were determined from the complete sequence of strain LMG3301T (PRJNA55963) for these two fragments. They corresponded to fragments of 206 and 289 kbp, respectively present on the large and the small chromosome of the bipartite genome of LMG3301T. The chromosome size of the two strains was compared by PFGE after macrorestriction with the intronic enzyme I-CeuI. Each of the two chromosomes were cut in two parts by hydrolysis in the 23S rRNA genes. The I-CeuI fragments could be placed on each chromosomes by reference to the complete sequence of strain LMG3301T. The fragments corresponding to the small chromosomes had the same size, suggesting that the 289-kbp fragment was not deleted but that chromosomal rearrangement occurred in strain ADV147 (data not show). In contrast, a decrease in size consistent with the loss of a fragment of approximately 200 kbp was observed for the large I-CeuI fragment (2.36 Mbp for LMG3301T) of the large chromosome of strain ADV147 without increase in size of the other I-CeuI fragment (240 kb). These results suggested that a genomic deletion occurred during the evolution of this clone. The DNA G+C content of the deleted fragment (59.07%) was similar to the DNA G+C content of the O. intermedium LMG3301T genome (57.7%). The deleted fragment corresponded to positions 1734814 to 1941167 of the complete sequence of strain LMG3301T (PRJNA55963) and contained 206 genes. The entire genomic fragment with 206 genes was syntenic in the genome of O. intermedium strain M86, the other strain of O. intermedium with completely sequenced genome [26]. We considered that these 206 genes were potentially deleted in strain ADV147, even if sporadic translocation of some genes on other genomic contigs could not be excluded. Among them, 32% (65/206) encoded for hypothetical proteins while other genes were mainly involved in transport (16%), metabolism (14%) and regulation (8%). Two percents of genes were pseudogenes and 4% were phage-related sequences. Other minor gene functions were biosynthesis, stress response, chitinase activity, tRNAs, etc. The fonction could not be determined for 16% of these genes. Among the potentially deleted genes, 23% were specific of the O. intermedium genome with no significant similarity with any other genomes. Some of the 206 genes were housekeeping genes, such as trpE. However, MLSA demonstrated that the trpE gene was not deleted in strain ADV147. This result confirmed that some genes, particularly essential ones, might have been translocated to other parts of the genome during a complex genomic rearrangement that included the large deletion.
The gene content of the deleted fragment was further compared with complete genome sequences of related species O. anthropi (ATCC 49188T) and Brucella spp. (B. microti CCM4915T and B. suis ATCC23445), 77% and 60% of the genes deleted in O. intermedium strain ADV147 were present in O. anthropi and Brucella genomes, respectively. The corresponding genes were distributed in two blocks on the two Brucella chromosomes (37% on the small chromosome and 23% on the large one) whereas they were only located on the large chromosome of O. anthropi, with a conserved synteny.
The strain ADV1 (ST68) was previously described as having encountered a large deletion of 150 kb and loss of one rrn operon during the chronic colonisation of human respiratory tract [9]. The 150-kb deleted fragment was to the smaller band observed in I-CeuI patterns and corresponded to the smaller I-CeuI fragment of the small chromosome. All the 7 other strains belonging to ST68 displayed the 150-kb fragment after I-CeuI restriction and PFGE (Figure S2). However, the strain ADV107 presented a deletion of approximately 100 kb on the 1.8 Mb I-CeuI-restricted fragment corresponding to the large fragment of the small chromosome. Out of the ST68, 6 other CC68 strains displayed large variations in genome size corresponding to 3 indel events of approximately 100 to 400 kb on the small chromosome and 2 indels of approximately 100 and 140 kb on the large chromosome (Figure S2). Genome dynamics was not studied in other CCs such as CC74 and CC76.
In conclusion, the survey of genome size and organisation among the population showed the presence of large deletion events, which appeared as a common mechanism of genomic evolution in the species O. intermedium.
Discussion
Considered as environmental bacteria and human opportunistic pathogens with mild virulence, members of the genus Ochrobactrum are increasingly described in human infections (26 publications between January 2005 and October 2013). This genus presented two major traits that worth to be considered for a better understanding of human pathogen emergence : i) its high level of resistance to a large panel of antimicrobial agents and xenobiotics [27]–[29], ii) its membership of a genetically tight clade with Brucella spp., a strict and specific anthropozoonotic pathogen [20]. Knowledge about Ochrobactrum is therefore of great interest to describe and then to predict the evolution of environmental bacteria towards host pathogenic behaviors.
As observed for numerous EBOPs, the reservoir of Ochrobactrum remains unclear and consequently, epidemiology of infections is difficult to assess. The lack of data about Ochrobactrum reservoir is mainly due to rare environmental investigations when clinical cases are described but also to difficulties for species identification in the genus. The database survey performed in this study showed that strains of O. intermedium/O. ciceri were affiliated to O. anthropi in 7.6% of cases including recent publications probably because this species remains the only representative of the genus in several identification system databases. Consequently, the role of O. intermedium in human infection remains probably underestimated, as previously suggested [16].
Despite the increasing number of infections due to O. intermedium, clones affiliated to this species were not found in human microbiotae [30], except one sequence from normal skin microbiota [23]. Moreover, environmental metagenomics detected scarce O. intermedium clones, mainly in polluted or technological environments. Finally, the databanks survey suggests that O. intermedium is mostly found in hospitalized patients and in environments modified by human activities suggesting that this minority bacterium is selected by human practices involving the use of xenobiotics such as medicine, agriculture and industry. O. intermedium displayed a high level of resistance to several antimicrobial agents, notably to beta lactam antibiotics [16], [31], which represent the most commonly prescribed antimicrobial agents, and antibiotic resistance is frequently associated with resistance to environmental xenobiotics [32], [33]. Altogether, the niche of O. intermedium could be described as a human-associated technological niche.
Due to the general lack of specific virulence factors in opportunistic pathogens, population studies gave insights about adaptation to human and/or pathogenic behavior by the detection of specialized clones [7], [34]–[36]. The other human EBOP in the genus Ochrobactrum, O. anthropi, displayed a human-associated clone suggesting the emergence of human-adapted bacteria from the environmental population [37]. Similar conclusions have been reported for Agrobacterium tumefaciens, another EBOP in Alphaproteobacteria [38]. The representativeness of the studied population is a pre-requisite to draw such conclusions. Despite a bias in representativeness of O. intermedium strains from polluted environments, the genetic diversity of the population studied was high and displayed low redondancy by PFGE typing as well as by examining date and site of strain isolation. We showed that O. intermedium displayed a clonal epidemic population structure with recombination events which is a classical structure for EBOP [7], [37], [39]–[41]. CC68 appeared as a cosmopolitan clone for which the local diversity in Montpellier (1999–2010) reflected the mondial diversity (<1983–2005). In contrast, CC74 appeared as a local South French clone including few isolates from other French regions and no isolate from other countries. Whether clonal complexes are cosmopolitan or local, they group human and environmental clones. The clustering of both human and environmental isolates has been observed in CCs and in STs determinated by MLST but also in PFGE fingerprint clusters. The low genetic and genomic diversity in O. intermedium cosmopolitan population suggests a clonal success driven by global selective pressures.
Low PFGE polymorphism also evokes genomic synteny that is generally observed in bacteria associated with narrow niches, particularly to eukaryotic host cells, the higher genome stability being reported for intracellular bacteria. For instance, the genetic relatedness assessed by PFGE in the alpha-proteobacteria Bartonella henselae is over 80% [42]. In Brucella, each species or pathovar displayed a specific PFGE fingerprint [43], [44] suggesting that stable genomes are selected in particular narrow niches represented by the preferential host in the case of Brucella. Association with narrow habitat niches is often associated with loss in genomic content compared to free-living relative bacteria [45]. In O. intermedium population, we observed indel events leading to decreased genomic size. The indel sequence detailed herein presented a GC% similar to that of the overall genome suggesting that this region was not recently aquired by lateral transfer in LMG3301T but more probably deleted in strain ADV147. Moreover, the presence of most of the homologous genes in the sequence of relative bacteria such as O. anthropi and Brucella spp. was also in favor of a deletion in strain ADV147. A large deletion event has been previously described in O. intermedium [9] and could be suspected for several isolates in our collection particularly in clinical strains of CC91 and CC68. Finally, a previous study showed that megaplasmids, which are frequently described in Rhizobiales, were less represented in O. intermedium than in O. anthropi [10]. Therefore, genome reduction could be considered as a general tendancy in the species O. intermedium even if further studies on other CC and environmental strains are required. The bipartite Brucella genome appeared very similar to that of O. intermedium but showed a genomic size reduced by 1.3 Mb as awaited for a bacterium that lives quasi-exclusively in a narrow cellular niche [46]. Since, phylogeny of Brucellaceae did not place O. intermedium in an ancestral position regarding Brucella, we could consider that genomic reduction is a common theme in Brucellaceae.
Genomic deletion in O. intermedium ADV1 has been related to the presence of a 46-bp atypical insertion in 16S rDNA [9]. Fourty-seven percent and 41% of environmental and clinical strains of O. intermedium, respectively displayed this insertion that could be involved in the tendancy of genomic reduction observed in the population. In addition to O. intermedium and its very related phylogenetic neighbour O. ciceri [47], identical or similar insertion was also present in O. daejeonense [48], O. pituitosum [49] and very sporadically in diverse related proteobacteria rrs. Of note, atypical mobile and fast-growing Brucella strains isolated from frogs [50], [51] displayed similar insertion than O. intermedium. These atypical Brucella isolates could represent a third evolutionary lineage from the common ancestor of Brucellaceae. Among the set of genes used in MLST, omp25 encodes an outer membrane protein involved in Brucella virulence, i.e., invasiveness and intracellular survival [52], and it is noteworthy that a common omp25 allele was found in 60% of the O. intermedium strains, suggesting a particular selective pressure applied on this gene involved in critical envelope properties, like selective permeability. Barquero-Calvo et al. (2009) hypothetized that Brucellaceae ancestors carried molecules not readily recognized by innate immunity leading to the emergence of stealthy intracellular pathogens such as Brucella [53]. Such hypothesis together with population and genomic data presented here, completes the story of the emergence of pathogenic life-styles among Brucellaceae and stresses to study behaviour of O. intermedium against eukaryotic cells.
The presence of a cosmopolitan and local clones that presented a genomic stability in a general tendancy of genome reduction suggested that O. intermedium is involved in a specialisation process with niche adaptation. This niche is not the eukaryotic cell as observed for Brucella because of the large reservoir of O. intermedium in man and in various environments but could be a technological niches in which evolution processes are driven by xenobiotics related to human activities in medecine and industry. If such selective pressure is worldwide distributed, it leads to cosmopolitan clone whereas particular pressure could lead to the local emergence of distinct clones. The industrial revolution that opened the anthropocene era [54] could be considered as a global change driving bacterial evolution and emergence of new pathogens as the neolithic revolution did it [55].
Materials and Methods
Bacterial strains
A total of 64 strains of O. intermedium including 49 clinical and 15 environmental isolates was analyzed (Tables 1 and 2). Clinical strains were sampled over a 39-year period in 6 countries in Europe and North America. Thirty-seven strains were obtained from patients hospitalized in 4 French hospitals in Montpellier, Nîmes (Southern France), Toulouse (Southwestern France) and Nancy (Northeastern France) from 1999 to 2011. Eleven collection strains isolated from man in Europe and the USA were also included, as well as O. intermedium LMG 3301T. Non-human strains were from diverse origins, including water, soil and invertebrates. They were collected in 6 countries and 4 continents over a 23-year period. The type strain of the species O. ciceri was also included. The affiliation of the isolates to O. intermedium was assessed by 16S rRNA gene sequencing as previously described [16], [56].
Ethic statements
No primary human sample materials were used in this study but bacterial isolates from routine clinical diagnostic procedures. This in vitro study required neither the agreement of the ethical committee of our institution nor the patient informed consent because it involved only bacterial strains, as stated by the French reglementation.
Bibliography and BLAST research strategy
The literature search was conducted in March 2013 using Pubmed and the keywords “Ochrobactrum intermedium”, cross-references were also considered. When sequence accession numbers were reported in a publication, the affiliation of the sequence(s) to the species O. intermedium has been verified. Selection of other O. intermedium sequences deposited in databases was performed using the nearly complete 16S rRNA gene of O. intermedium strains LMG3301T (AM490623), CCUG44770 (AM114410), LMG18956 (AJ242582), CCUG39736 (AM114408), CCM7036 (AM490631) and of O. ciceri strain Ca-34T (DQ647056) using the Megablast program optimized for highly similar sequences for Genbank [57] and the Simrank program for Greengene [58] databases. The sequences having a similarity level of at least 99% with those of previous strains were selected.
Restriction Fragment Length Polymorphism in Pulsed-Field Gel Electrophoresis (RFLP-PFGE)
Genomic DNA was prepared in agarose plugs as previously described [10] and digested at 37°C with 40 U of SpeI (New England Biolabs) or with 1 U of the intronic endonuclease I-CeuI (New England Biolabs). Restriction fragments were separated by PFGE using a CHEF-DRII apparatus (Bio-Rad Laboratories) in a 1% (SpeI) or 0.8% (I-CeuI) agarose gel in 0.5X Tris-Borate-EDTA (TBE) buffer at 150 V and at 10°C. Pulse ramps were 5 to 35 s for 35 h followed by 2 to 10 s for 10 h (SpeI fragments) or 200 to 300 s for 24 h (I-CeuI fragments). The gels were stained with ethidium bromide and photographed under UV light. SpeI-digested DNA from strain O. intermedium LMG3301T was loaded on each gel in order to standardize the migration patterns. PFGE bands above 100 kbp were measured with the Mesurim software (http://pedagogie.ac-amiens.fr/svt/info/logiciels/Mesurim2/Telecharge.htm). The bands were scored as present (1) or absent (0) in a binary table, a tolerance of 2% in band position was applied. PFGE patterns were compared by calculation of the Dice correlation coefficient with the FAMD software (http://www.famd.me.uk/) and were clustered into a dendrogram by the unweighted pair group method with the arithmetic average clustering technique.
Gene amplification and sequencing
Genomic DNA was obtained using the MasterPure™ DNA purification kit (EpiCentre). Seven genes (dnaK, recA, rpoB, trpE, aroC, omp25 and gap) were amplified using primers and PCR conditions previously described for MLST scheme of O. anthropi [37]. PCR products and molecular weight marker (phage phiX DNA digested with HaeIII, New England Biolabs) were separated in 1.5% (w/v) agarose gels in 0.5X TBE buffer. Amplification products were sequenced in both directions using forward and reverse sequencing primers [37] on an ABI 3730xl automatic sequencer (Cogenics, France). The sequences were deposited to GenBank database with accession numbers KF825086 to KF825540 and KF866307 to KF866369. The primer ins1 was used in association with the universal primer 1492r for specific detection of rrs copies carrying a 46-bp atypical insertion previously described [9].
Phylogeny and decomposition analysis
Gene sequences were codon-aligned using ClustalX after translation with TRANSLATE (http://www.expasy.org). The size of the codon-aligned sequences used for further analyses is indicated in Table 3. Phylogenetic analyses were performed for each of the seven gene sequences and for the manually concatenated sequence. Evolutionary distance was analyzed using Phylip package v3.66 [59] by Neighbor-Joining after distance matrix construction using DNADIST (F84 as substitution model). Bootstrap values were calculated using SEQBOOT and CONSENSE after 1000 reiterations. Maximum-likelihood (ML) analysis was performed using phylogenetic analyses available at http://www.phylogeny.fr [60]. The general time-reversible (GTR) model plus gamma distribution and invariant sites was used as a substitution model. ML bootstrap support was computed after 100 reiterations. The sequences of O. anthropi ATCC49188T (PRJNA58921) and/or Rhizobium rhizogenes (PRJNA58269) were included in phylogenetic analyses in order to place an artificial tree root.
Along with the phylogenetic reconstruction, we performed a network reconstruction on concatenated data using the Neighbor-net algorithm available in Splitstree4 software [61].
Multi Locus Sequence Typing (MLST) and multilocus genetics
For each locus, each different allele was assigned to a different arbitrary number using a nonredundant database program available at http://linux.mlst.net/nrdb/nrdb.htm. The combination of allele numbers for each isolate defined the sequence type (ST). A Minimum Spanning (MS) tree was constructed using Prim's algorithm to determine the links among STs (http://www.pubmlst.org). Clonal complexes (CC) included STs that differed by 1 or 2 alleles. The singleton STs corresponded to STs differing from every other ST at 3 or more of the 7 loci.
The LIAN v3.5 program [62] was used to calculate the standardized IA (sIA) and to test the null hypothesis of linkage disequilibrium as well as to determine mean genetic diversity (H) and genetic diversity at each locus (h). To detect the presence of recombination events, we also performed the pairwise homoplasy index test, φw [63], implemented in Splitstree4 [61]. The number of synonymous (dS) and non-synonymous (dN) substitutions per site was determined on codon-aligned sequences using SNAP software [64].
Supporting Information
Maximum-Likelihood tree based on concatenated sequences of the seven housekeeping gene fragments of the MSLT scheme indicating the relative placement of 64 strains of O. intermedium and type strain of O. ciceri . The scale bar indicates the number of substitutions per nucleotide position. The numbers at the nodes are support values estimated with 100 bootstrap replicates. The position of the artificial root (black circle) corresponds to the branching node of the outgroup organism (O. anthropi ATCC49188T), included in the analysis but not shown on the tree. Clinical strains were noted in blue and environmental strains in green. Clonal complexes (CC) were also reported.
(TIFF)
PFGE of I- Ceu I-digested genomic DNA from O. intermedium and O. ciceri strains belonging to the CC68. (A) Lane 1, Saccharomyces cerevisiae ladder (Bio-Rad) as molecular size marker with most band sizes in kb; lane 2, ADV1 (ST68); lane 3, ADV107 (ST68); lane 4, ADV32 (ST68); lane 5, ADV9 (ST68); lane 6, Nim80 (ST68); lane 7, LMG5425 (ST68); lane 8, LMG5426 (ST68); lane 9, RT148-2 (ST68); lane 10, ADV143B (ST84). (B) Lane 1, Saccharomyces cerevisiae ladder (Bio-Rad) as molecular size marker with most band sizes in kb; lane 2, CCUG1838 (ST79); lane 3, CCUG44770 (ST80); lane 4, LMG379 (ST83); lane 5, O. ciceri DSM22292T (ST113); lane 6, LMG5443 (ST101).
(TIFF)
Strains or clones affiliated to O. intermedium / O. ciceri on the basis of 16S rRNA or recA genes sequence analysis in publications (see Materials and Methods ). Results were sorted by origin. The strains or clones not confirmed to belong to O. intermedium/O. ciceri were indicated in bold. NA, not applicable. a 46-bp atypical insertion in rrs described by Teyssier et al., 2003 [9].
(XLSX)
Deposited sequences in genetic databases not associated with a publication corresponding to strains or clones of O. intermedium / O. ciceri on the basis of 16S rRNA gene or 16S–23S rRNA intergenic spacer sequence analysis (see Materials and Methods ). Results were sorted by origin. The strains or clones not affiliated to O. intermedium/O. ciceri were indicated in bold. NA, not applicable. a 46-bp atypical insertion in rrs described by Teyssier et al., 2003 [9].
(XLSX)
Acknowledgments
We are particularly indebted to the microbiology lab team of the Montpellier academic hospital for providing clinical isolates. We also thank Corentine Alauzet, Christine Seconds and Anne Gouby for providing additional clinical isolates, Sylvie Pages for her help in isolating nematode-associated strains, Jean-Luc Jeannot for isolation of environmental strains and Hélène Marion for technical assistance.
Funding Statement
The funder is the French Ministry of Higher Education and Research. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Finlay BB, Falkow S (1997) Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 61: 136–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgiades K, Raoult D (2011) Defining Pathogenic Bacterial Species in the Genomic Era. Front Microbiol 1: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith JM, Smith NH, O'Rourke M, Spratt BG (1993) How clonal are bacteria? Proc Natl Acad Sci U S A 90: 4384–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joyce EA, Chan K, Salama NR, Falkow S (2002) Redefining bacterial populations: a post-genomic reformation. Nat Rev Genet 3: 462–473. [DOI] [PubMed] [Google Scholar]
- 5. Suen G, Goldman BS, Welch RD (2007) Predicting prokaryotic ecological niches using genome sequence analysis. PLoS ONE 2: e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rezzonico F, Smits THM, Montesinos E, Frey JE, Duffy B (2009) Genotypic comparison of Pantoea agglomerans plant and clinical strains. BMC Microbiol 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graindorge A, Menard A, Monnez C, Cournoyer B (2012) Insertion sequence evolutionary patterns highlight convergent genetic inactivations and recent genomic island acquisitions among epidemic Burkholderia cenocepacia . J Med Microbiol 61: 394–409. [DOI] [PubMed] [Google Scholar]
- 8. Kaiser S, Biehler K, Jonas D (2009) A Stenotrophomonas maltophilia Multilocus Sequence Typing Scheme for Inferring Population Structure. J Bacteriol 191: 2934–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teyssier C, Marchandin H, Simeon De Buochberg M, Ramuz M, Jumas-Bilak E (2003) Atypical 16S rRNA Gene Copies in Ochrobactrum intermedium Strains Reveal a Large Genomic Rearrangement by Recombination between rrn Copies. J Bacteriol 185: 2901–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teyssier C, Marchandin H, Masnou A, Jeannot J-L, de Buochberg M, et al. (2005) Pulsed-field gel electrophoresis to study the diversity of whole-genome organization in the genus Ochrobactrum . Electrophoresis 26: 2898–2907. [DOI] [PubMed] [Google Scholar]
- 11. Pandey S, Ghosh PK, Ghosh S, De TK, Maiti TK (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J Microbiol 51: 11–17. [DOI] [PubMed] [Google Scholar]
- 12. Wackerow-Kouzova N (2007) Ochrobactrum intermedium ANKI, a nitrogen-fixing bacterium able to decolorize azobenzene. Applied Biochemistry and Microbiology 43: 403–406. [PubMed] [Google Scholar]
- 13. Waranusantigul P, Lee H, Kruatrachue M, Pokethitiyook P, Auesukaree C (2011) Isolation and characterization of lead-tolerant Ochrobactrum intermedium and its role in enhancing lead accumulation by Eucalyptus camaldulensis . Chemosphere 85: 584–590. [DOI] [PubMed] [Google Scholar]
- 14. Kavita B, Keharia H (2012) Reduction of hexavalent chromium by Ochrobactrum intermedium BCR400 isolated from a chromium-contaminated soil. 3 Biotech 2: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boukhatem ZF, Domergue O, Bekki A, Merabet C, Sekkour S, et al. (2012) Symbiotic characterization and diversity of rhizobia associated with native and introduced acacias in arid and semi-arid regions in Algeria. FEMS Microbiol Ecol 80: 534–547. [DOI] [PubMed] [Google Scholar]
- 16. Teyssier C, Marchandin H, Jean-Pierre H, Diego I, Darbas H, et al. (2005) Molecular and phenotypic features for identification of the opportunistic pathogens Ochrobactrum spp. J Med Microbiol 54: 945–953. [DOI] [PubMed] [Google Scholar]
- 17. Dean AS, Crump L, Greter H, Schelling E, Zinsstag J (2012) Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis 6: e1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wattam AR, Williams KP, Snyder EE, Almeida NF Jr, Shukla M, et al. (2009) Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J Bacteriol 191: 3569–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwashita S, Callahan TP, Haydu J, Wood TK (2004) Mesophilic aerobic degradation of a metal lubricant by a biological consortium. Appl Microbiol Biotechnol 65: 620–626. [DOI] [PubMed] [Google Scholar]
- 20. Scholz HC, Al Dahouk S, Tomaso H, Neubauer H, Witte A, et al. (2008) Genetic diversity and phylogenetic relationships of bacteria belonging to the Ochrobactrum-Brucella group by recA and 16S rRNA gene-based comparative sequence analysis. Syst Appl Microbiol 31: 1–16. [DOI] [PubMed] [Google Scholar]
- 21. Boucher D, Laffaire JB, Jaziri F, David C, Biderre-Petit C, et al. (2011) Bacterial community composition of biological degreasing systems and health risk assessment for workers. Microb Ecol 62: 868–881. [DOI] [PubMed] [Google Scholar]
- 22. Perkins SD, Angenent LT (2010) Potential pathogenic bacteria in metalworking fluids and aerosols from a machining facility. FEMS Microbiol Ecol 74: 643–654. [DOI] [PubMed] [Google Scholar]
- 23. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, et al. (2009) Topographical and Temporal Diversity of the Human Skin Microbiome. Science 324: 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teyssier C, Jumas-Bilak E, Marchandin H, Jean-Pierre H, Jeannot JL, et al. (2003) Identification d'espèce et épidémiologie moléculaire des bactéries du genre Ochrobactrum . Pathologie Biologie 51: 5–12. [DOI] [PubMed] [Google Scholar]
- 25. Babic I, Fischer-Le Saux M, Giraud E, Boemare N (2000) Occurrence of natural dixenic associations between the symbiont Photorhabdus luminescens and bacteria related to Ochrobactrum spp. in tropical entomopathogenic Heterorhabditis spp. (Nematoda, Rhabditida). Microbiology 146: 709–718. [DOI] [PubMed] [Google Scholar]
- 26. Kulkarni G, Dhotre D, Dharne M, Shetty S, Chowdhury S, et al. (2013) Draft genome of Ochrobactrum intermedium strain M86 isolated from non-ulcer dyspeptic individual from India. Gut Pathog 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins CS, Murtough SM, Williamson E, Hiom SJ, Payne DJ, et al. (2001) Resistance to antibiotics and biocides among non-fermenting Gram-negative bacteria. Clin Microbiol Infect 7: 308–315. [DOI] [PubMed] [Google Scholar]
- 28. Tian Y-S, Xiong A-S, Xu J, Zhao W, Gao F, et al. (2010) Isolation from Ochrobactrum anthropi of a novel class II 5-enopyruvylshikimate-3-phosphate synthase with high tolerance to glyphosate. Appl Environ Microbiol 76: 6001–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandey S, Saha P, Barai PK, Maiti TK (2010) Characterization of a Cd(2+)-resistant strain of Ochrobactrum sp. isolated from slag disposal site of an iron and steel factory. Curr Microbiol 61: 106–111. [DOI] [PubMed] [Google Scholar]
- 30. Human Microbiome Project Consortium (2012) A framework for human microbiome research. Nature 486: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thoma B, Straube E, Scholz HC, Al Dahouk S, Zöller L, et al. (2009) Identification and antimicrobial susceptibilities of Ochrobactrum spp. International Journal of Medical Microbiology 299: 209–220. [DOI] [PubMed] [Google Scholar]
- 32. Nishino K, Yamaguchi A (2008) Role of xenobiotic transporters in bacterial drug resistance and virulence. IUBMB Life 60: 569–574. [DOI] [PubMed] [Google Scholar]
- 33. Matyar F, Kaya A, Dinçer S (2008) Antibacterial agents and heavy metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Sci Total Environ 407: 279–285. [DOI] [PubMed] [Google Scholar]
- 34. van Mansfeld R, Jongerden I, Bootsma M, Buiting A, Bonten M, et al. (2010) The Population Genetics of Pseudomonas aeruginosa Isolates from Different Patient Populations Exhibits High-Level Host Specificity. PLoS ONE 5: e13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bidet P, Mahjoub-Messai F, Blanco J, Blanco J, Dehem M, et al. (2007) Combined multilocus sequence typing and O serogrouping distinguishes Escherichia coli subtypes associated with infant urosepsis and/or meningitis. J Infect Dis 196: 297–303. [DOI] [PubMed] [Google Scholar]
- 36. Hoffmaster AR, Novak RT, Marston CK, Gee JE, Helsel L, et al. (2008) Genetic diversity of clinical isolates of Bacillus cereus using multilocus sequence typing. BMC Microbiol 8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romano S, Aujoulat F, Jumas-Bilak E, Masnou A, Jeannot JL, et al. (2009) Multilocus sequence typing supports the hypothesis that Ochrobactrum anthropi displays a human-associated subpopulation. BMC microbiology 9: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aujoulat F, Jumas-Bilak E, Masnou A, Salle F, Faure D, et al. (2011) Multilocus Sequence-Based Analysis Delineates a Clonal Population of Agrobacterium (Rhizobium) radiobacter (Agrobacterium tumefaciens) of Human Origin. J Bacteriol 193: 2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pirnay J-P, Bilocq F, Pot B, Cornelis P, Zizi M, et al. (2009) Pseudomonas aeruginosa Population Structure Revisited. PLoS ONE 4: e7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spilker T, Vandamme P, LiPuma JJ (2012) A Multilocus Sequence Typing Scheme Implies Population Structure and Reveals Several Putative Novel Achromobacter Species. Journal of Clinical Microbiology 50: 3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bevivino A, Costa B, Cantale C, Cesarini S, Chiarini L, et al. (2011) Genetic relationships among Italian and Mexican maize-rhizosphere Burkholderia cepacia complex (BCC) populations belonging to Burkholderia cenocepacia IIIB and BCC6 group. BMC Microbiology 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arvand M, Viezens J (2007) Evaluation of pulsed-field gel electrophoresis and multi-locus sequence typing for the analysis of clonal relatedness among Bartonella henselae isolates. Int J Med Microbiol 297: 255–262. [DOI] [PubMed] [Google Scholar]
- 43. Michaux-Charachon S, Bourg G, Jumas-Bilak E, Guigue-Talet P, Allardet-Servent A, et al. (1997) Genome structure and phylogeny in the genus Brucella . Journal of Bacteriology 179: 3244–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bourg G, O'Callaghan D, Boschiroli ML (2007) The genomic structure of Brucella strains isolated from marine mammals gives clues to evolutionary history within the genus. Vet Microbiol 125: 375–380. [DOI] [PubMed] [Google Scholar]
- 45. Sällström B, Andersson SGE (2005) Genome reduction in the alpha-Proteobacteria. Curr Opin Microbiol 8: 579–585. [DOI] [PubMed] [Google Scholar]
- 46. Aujoulat F, Roger F, Bourdier A, Lotthé A, Lamy B, et al. (2012) From Environment to Man: Genome Evolution and Adaptation of Human Opportunistic Bacterial Pathogens. Genes 3: 191–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Imran A, Hafeez FY, Frühling A, Schumann P, Malik KA, et al. (2010) Ochrobactrum ciceri sp. nov., isolated from nodules of Cicer arietinum . Int J Syst Evol Microbiol 60: 1548–1553. [DOI] [PubMed] [Google Scholar]
- 48. Woo S-G, Ten LN, Park J, Lee M (2011) Ochrobactrum daejeonense sp. nov., a nitrate-reducing bacterium isolated from sludge of a leachate treatment plant. Int J Syst Evol Microbiol 61: 2690–2696. [DOI] [PubMed] [Google Scholar]
- 49. Huber B, Scholz HC, Kämpfer P, Falsen E, Langer S, et al. (2010) Ochrobactrum pituitosum sp. nov., isolated from an industrial environment. Int J Syst Evol Microbiol 60: 321–326. [DOI] [PubMed] [Google Scholar]
- 50. Fischer D, Lorenz N, Heuser W, Kämpfer P, Scholz HC, et al. (2012) Abscesses associated with a Brucella inopinata-like bacterium in a big-eyed tree frog (Leptopelis vermiculatus). J Zoo Wildl Med 43: 625–628. [DOI] [PubMed] [Google Scholar]
- 51. Eisenberg T, Hamann H-P, Kaim U, Schlez K, Seeger H, et al. (2012) Isolation of potentially novel Brucella spp. from frogs. Appl Environ Microbiol 78: 3753–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caro-Hernández P, Fernández-Lago L, de Miguel M-J, Martín-Martín AI, Cloeckaert A, et al. (2007) Role of the Omp25/Omp31 family in outer membrane properties and virulence of Brucella ovis . Infect Immun 75: 4050–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barquero-Calvo E, Conde-Alvarez R, Chacón-Díaz C, Quesada-Lobo L, Martirosyan A, et al. (2009) The differential interaction of Brucella and Ochrobactrum with innate immunity reveals traits related to the evolution of stealthy pathogens. PLoS ONE 4: e5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zalasiewicz J, Williams M, Steffen W, Crutzen P (2010) The New World of the Anthropocene. Environ Sci Technol 44: 2228–2231 doi:10.1021/es903118j [DOI] [PubMed] [Google Scholar]
- 55. Mira A, Pushker R, Rodríguez-Valera F (2006) The Neolithic revolution of bacterial genomes. Trends Microbiol 14: 200–206. [DOI] [PubMed] [Google Scholar]
- 56. Teyssier C, Marchandin H, Jean-Pierre H, Masnou A, Dusart G, et al. (2007) Ochrobactrum pseudintermedium sp. nov., a novel member of the family Brucellaceae, isolated from human clinical samples. Int J Syst Evol Microbiol 57: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 57. Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, et al. (2008) Database indexing for production MegaBLAST searches. Bioinformatics 24: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, et al. (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Felsenstein J (1984) Distance Methods for Inferring Phylogenies: A Justification. Evolution 38: 16–24. [DOI] [PubMed] [Google Scholar]
- 60. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 62. Haubold B, Hudson RR (2000) LIAN 3.0: detecting linkage disequilibrium in multilocus data. Linkage Analysis. Bioinformatics 16: 847–848. [DOI] [PubMed] [Google Scholar]
- 63. Bruen TC, Philippe H, Bryant D (2006) A simple and robust statistical test for detecting the presence of recombination. Genetics 172: 2665–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korber B (2000) HIV Signature and Sequence Variation Analysis. Computational Analysis of HIV Molecular Sequences. Dordrecht, Netherlands: Kluwer Academic Publishers. pp. 55–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum-Likelihood tree based on concatenated sequences of the seven housekeeping gene fragments of the MSLT scheme indicating the relative placement of 64 strains of O. intermedium and type strain of O. ciceri . The scale bar indicates the number of substitutions per nucleotide position. The numbers at the nodes are support values estimated with 100 bootstrap replicates. The position of the artificial root (black circle) corresponds to the branching node of the outgroup organism (O. anthropi ATCC49188T), included in the analysis but not shown on the tree. Clinical strains were noted in blue and environmental strains in green. Clonal complexes (CC) were also reported.
(TIFF)
PFGE of I- Ceu I-digested genomic DNA from O. intermedium and O. ciceri strains belonging to the CC68. (A) Lane 1, Saccharomyces cerevisiae ladder (Bio-Rad) as molecular size marker with most band sizes in kb; lane 2, ADV1 (ST68); lane 3, ADV107 (ST68); lane 4, ADV32 (ST68); lane 5, ADV9 (ST68); lane 6, Nim80 (ST68); lane 7, LMG5425 (ST68); lane 8, LMG5426 (ST68); lane 9, RT148-2 (ST68); lane 10, ADV143B (ST84). (B) Lane 1, Saccharomyces cerevisiae ladder (Bio-Rad) as molecular size marker with most band sizes in kb; lane 2, CCUG1838 (ST79); lane 3, CCUG44770 (ST80); lane 4, LMG379 (ST83); lane 5, O. ciceri DSM22292T (ST113); lane 6, LMG5443 (ST101).
(TIFF)
Strains or clones affiliated to O. intermedium / O. ciceri on the basis of 16S rRNA or recA genes sequence analysis in publications (see Materials and Methods ). Results were sorted by origin. The strains or clones not confirmed to belong to O. intermedium/O. ciceri were indicated in bold. NA, not applicable. a 46-bp atypical insertion in rrs described by Teyssier et al., 2003 [9].
(XLSX)
Deposited sequences in genetic databases not associated with a publication corresponding to strains or clones of O. intermedium / O. ciceri on the basis of 16S rRNA gene or 16S–23S rRNA intergenic spacer sequence analysis (see Materials and Methods ). Results were sorted by origin. The strains or clones not affiliated to O. intermedium/O. ciceri were indicated in bold. NA, not applicable. a 46-bp atypical insertion in rrs described by Teyssier et al., 2003 [9].
(XLSX)