Abstract
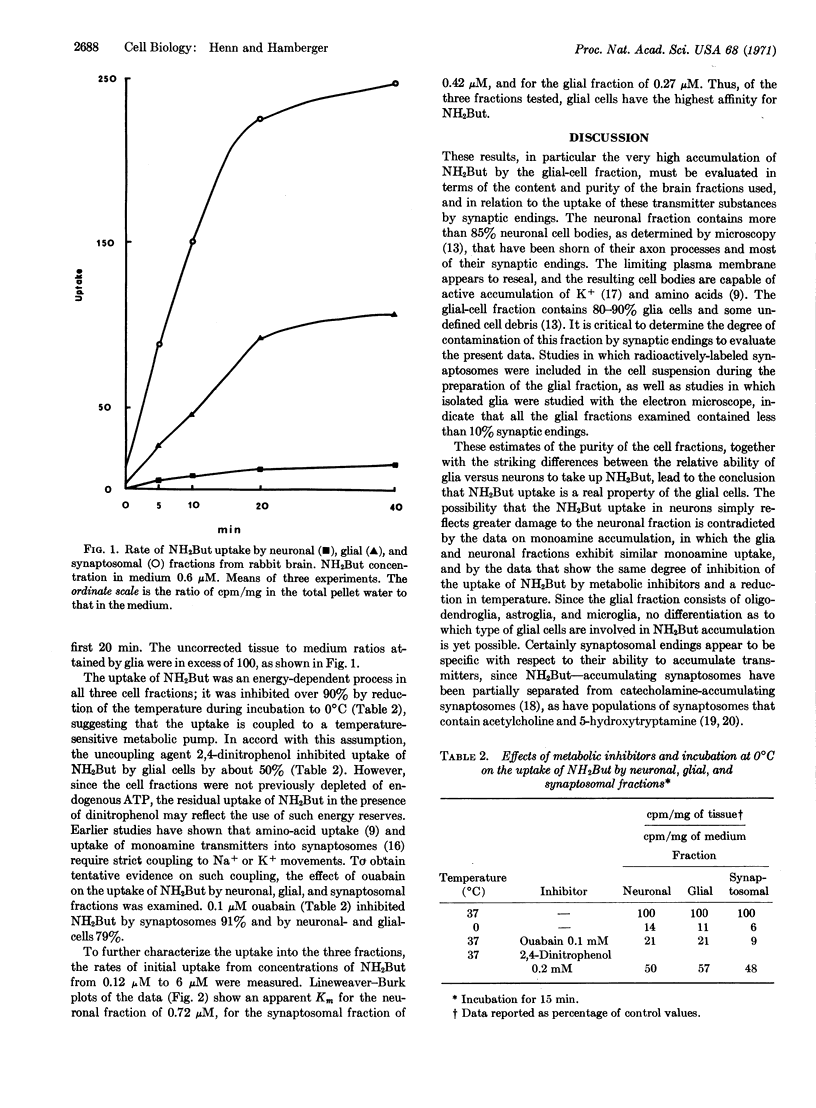
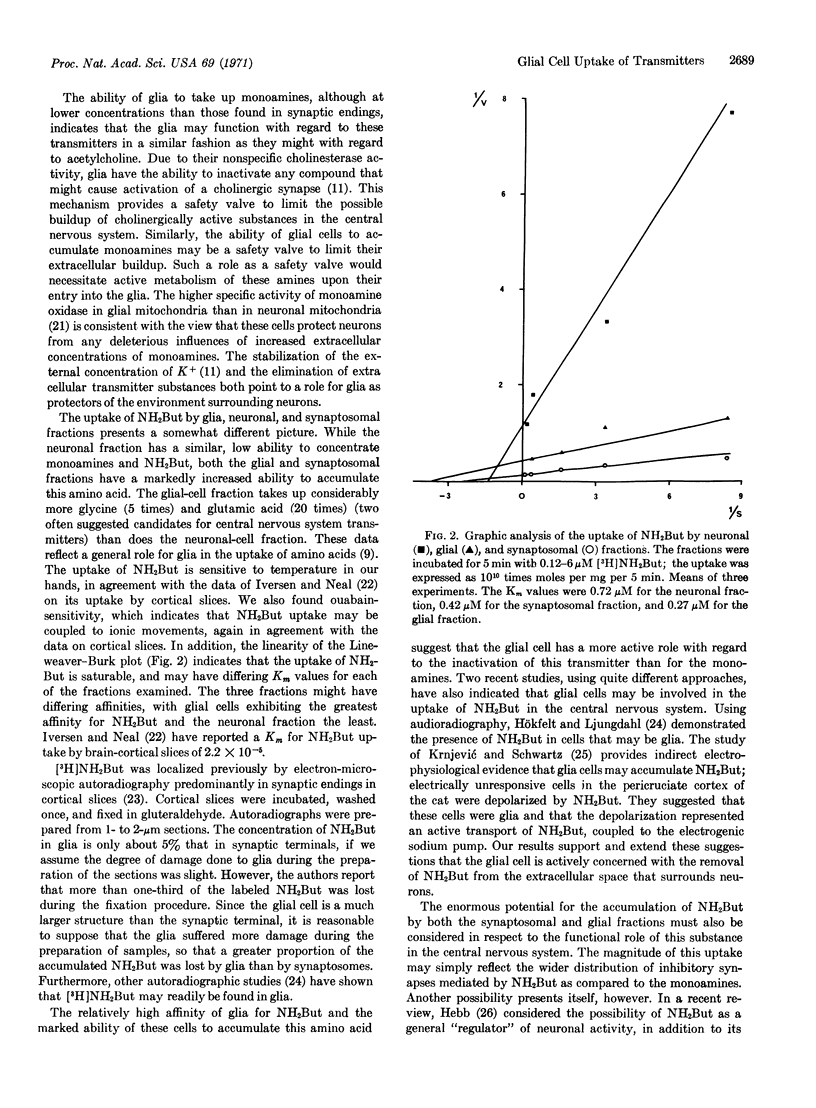
Rabbit-brain fractions enriched in neuronal cell bodies and in glial cells accumulated norepinephrine, serotonin, dopamine, and γ-aminobutyric acid, substances believed to serve as neurotransmitters in the central nervous system. Both neurons and glia were able to concentrate the monoamine transmitters about 4-fold from a medium containing 0.1-1 μM concentrations. However, the glial-cell fraction concentrated aminobutyrate over a 100-fold from the medium, in contrast to the neuronal fraction, which concentrated this amino acid only 4-fold. The uptake of aminobutyrate by glial cells was 30-50% of that of synaptosome preparations. Its uptake in all fractions was temperature sensitive, sensitive to metabolic inhibitors, and exhibited Km values of 0.72 μM for the neuronal fraction, 0.42 μM for the synaptosomal fraction, and 0.27 μM for the glial-cell fraction. These results are interpreted as evidence that the glial cell is involved in limiting the extracellular build-up of substances that might trigger synaptic transmission by removing any transmitters that may diffuse out of the synaptic cleft during the transmission of impulses. The possible function of the enormous ability of glia and synaptosomes to accumulate aminobutyrate is discussed in light of the actions and distribution of this substance in the central nervous system.
Keywords: rabbit brain, γ-aminobutyric acid, serotonin, dopamine, norepinephrine
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomstrand C., Hamberger A. Amino acid incorporation in vitro into protein of neuronal and glial cell-enriched fractions. J Neurochem. 1970 Aug;17(8):1187–1195. doi: 10.1111/j.1471-4159.1970.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Blomstrand C., Hamberger A. Protein turnover in cell-enriched fractions from rabbit brain. J Neurochem. 1969 Sep;16(9):1401–1407. doi: 10.1111/j.1471-4159.1969.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Iversen L. L. Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature. 1971 Feb 26;229(5287):628–630. doi: 10.1038/229628a0. [DOI] [PubMed] [Google Scholar]
- Fahn S., Côté L. J. Regional distribution of gamma-aminobutyric acid (GABA) in brain of the rhesus monkey. J Neurochem. 1968 Mar;15(3):209–213. doi: 10.1111/j.1471-4159.1968.tb06198.x. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Hamberger A., Blomstrand C., Lehninger A. L. Comparative studies on mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J Cell Biol. 1970 May;45(2):221–234. doi: 10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb C. CNS at the cellular level: identity of transmitter agents. Annu Rev Physiol. 1970;32:165–192. doi: 10.1146/annurev.ph.32.030170.001121. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Cellular localization of labeled gamma-aminobutyric acid (3H-GABA) in rat cerebellar cortex: an autoradiographic study. Brain Res. 1970 Sep 16;22(3):391–396. doi: 10.1016/0006-8993(70)90480-4. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Snyder S. H. Synaptosomes: different populations storing catecholamines and gamma-aminobutyric acid in homogenates of rat brain. Nature. 1968 Nov 23;220(5169):796–798. doi: 10.1038/220796a0. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical identification of acetylcholinesterase in cholinergic, adrenergic and sensory neurons. J Pharmacol Exp Ther. 1955 Jun;114(2):167–184. [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. Some properties of unresponsive cells in the cerebral cortex. Exp Brain Res. 1967;3(4):306–319. doi: 10.1007/BF00237557. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- MICHAELSON I. A., WHITTAKER V. P. The subcellular localization of 5-hydroxytryptamine in guinea pig brain. Biochem Pharmacol. 1963 Feb;12:203–211. doi: 10.1016/0006-2952(63)90185-0. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kuriyama K. Biochemical-physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968 Apr;8(1):1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Rose S. P. Preparation of enriched fractions from cerebral cortex containing isolated, metabolically active neuronal cells. Nature. 1965 May 8;206(984):621–622. doi: 10.1038/206621a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Coyle J. T. Regional differences in H3-norepinephrine and H3-dopamine uptake into rat brain homogenates. J Pharmacol Exp Ther. 1969 Jan;165(1):78–86. [PubMed] [Google Scholar]
- White T. D., Keen P. The role of internal and external Na+ and K+ on the uptake of [3H] noradrenaline by synaptosomes prepared from rat brain. Biochim Biophys Acta. 1970;196(2):285–295. doi: 10.1016/0005-2736(70)90016-7. [DOI] [PubMed] [Google Scholar]
- Wood J. D. A possible role for gamma-aminobutyric acid in the homeostatic control of brain metabolism under conditions of hypoxia. Exp Brain Res. 1967;4(1):81–84. doi: 10.1007/BF00235219. [DOI] [PubMed] [Google Scholar]


