Abstract
OBJECTIVES
The efficacy of concomitant ablation techniques in patients with paroxysmal atrial fibrillation (AF) undergoing mitral valve surgery remains under debate. The aim of this prospective, randomized, single-centre study was to compare pulmonary vein isolation (PVI) only versus a left atrial maze (LAM) procedure in patients with paroxysmal AF during mitral valve surgery.
METHODS
Between February 2009 and June 2011, 52 patients with a mean age of 54.2 (standard deviation 7.2 years) underwent mitral valve surgery and concomitant bipolar radiofrequency ablation for paroxysmal AF. Patients were randomized into the PVI group (n = 27) and the LAM group (n = 25). After surgery, an implantable loop recorder for continuous electrocardiography (ECG) monitoring was implanted. Patients with an AF burden (AF%) of <0.5% were considered AF free (responders). The mean follow-up was 18.6 months (standard deviation 2.1 months), and the patient' data were evaluated every 3 months.
RESULTS
All patients were alive at discharge. No procedure-related complications occurred for either the ablation or the loop recorder implantation. Mean aortic clamping and ablation times were significantly longer in the LAM group than in the PVI group. The incidence of early AF paroxysm recurrence was significantly higher in the PVI group than in the LAM group (62.9 vs 24.0%, P < 0.001). At 20 months after surgery, 15 (55.6%) of the 27 patients in the PVI group and 22 (88.0%) of the 25 patients in the LAM group had no documented atrial arrhythmias and were considered responders (AF burden <0.5%). The mean AF burden during all follow-up periods was significantly lower in the LAM group (23.6 ± 8.7%) than in the PVI group (6.8 ± 2.2%) (P < 0.001).
CONCLUSIONS
According to continuous ECG monitoring data, freedom from AF was significantly higher after the concomitant LAM procedure than after PVI in patients with paroxysmal AF who underwent mitral valve surgery.
Keywords: Atrial fibrillation, Mitral valve surgery, Continuous electrocardiography monitoring, Radiofrequency ablation
INTRODUCTION
Atrial fibrillation (AF) is a common arrhythmia, and 40–60% of patients undergoing mitral valve operations have AF at the time of surgery [1, 2]. Surgical ablation of AF is the standard concomitant procedure during valve surgery, since it improves quality of life, reduces the risk of stroke and prolongs survival [3]. A number of studies have demonstrated the role of pulmonary veins as main triggers for lone paroxysmal AF, but the real underlying mechanism in patients with mitral valve lesions remains unknown [4–6]. It remains debatable whether pulmonary vein isolation (PVI) alone is sufficient for treating paroxysmal AF associated with mitral valve disease despite the description of positive results [7].
Recent evidence shows that focal activation in the left atrium close to the pulmonary veins plays an important role in patients with chronic AF and mitral valve disease. Experience with the surgical left atrial isolation procedure has also demonstrated the important role of the left atrium in the arrhythmogenesis of AF. Therefore, both cardiologists and surgeons are increasingly focusing on the left atrium with special interest in the pulmonary veins, and one could expect that left-sided procedures are highly effective [8, 9].
With regard to the rhythm assessment via electrocardiography (ECG) monitoring, office ECG and 24-h Holter monitoring are commonly used to assess cardiac rhythm after the ablation procedure, but they have limited abilities to detect AF paroxysmal recurrences [10, 11]. Continuous and precise rhythm monitoring may help clinicians uncover the true incidence and duration of AF and assess the efficacy of different AF ablation techniques during mitral valve surgery [12, 13].
The aim of this study was to compare different lesion patterns in patients undergoing mitral valve surgery and concomitant paroxysmal AF ablation based on continuous ECG monitoring.
MATERIALS AND METHODS
Patient population
Between February 2009 and June 2011, 52 consecutive patients with mitral valve lesions and paroxysmal AF were enrolled in the study (Fig. 1). In each patient, a primary mitral valve lesion was the main indication for surgery in accordance with the European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for the management of patients with valvular heart disease [14].
Figure 1:
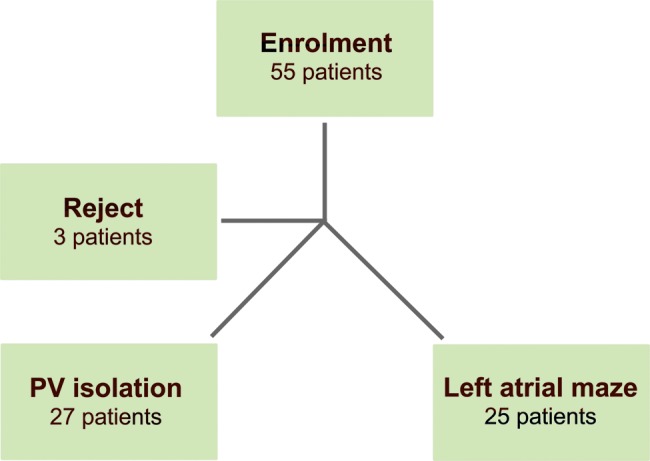
Patient enrolment flow chart.
The study was planned as a prospective, open and randomized trial with two arms. This study was approved by the local ethics committee and conducted in compliance with the protocol and in accordance with standard operating procedures. All patients signed an informed consent form prior to participating in the study.
The baseline characteristics of the patients are given in Table 1. Patients in both groups were compared by age, gender, New York Heart Association (NYHA) class and specific risk markers for AF recurrence (AF paroxysm duration and left atrium size).
Table 1:
Patient baseline dates
| PV isolation (n = 27) | LAM (n = 25) | P-value | |
|---|---|---|---|
| Age (years) | 56.5 ± 6.9 | 58.2 ± 7.9 | 0.123 |
| Gender (female) | 16 (59.3%) | 14 (56.0%) | 0.243 |
| Mean NYHA functional class | |||
| II | 11 (40.7%) | 7 (28.0%) | 0.059 |
| III | 16 (59.3%) | 18 (72.0%) | 0.053 |
| AF paroxysms duration (months) | 18.4 ± 7.9 | 25.1 ± 11.3 | 0.091 |
| History of stroke | 2 (7.4%) | 1 (3.7%) | 0.063 |
| LVEF (%) | 59.2 ± 4.9 | 54.4 ± 6.7 | 0.219 |
| Mean LA size (mm) | 63.6 ± 1.9 | 66.1 ± 1.5 | 0.367 |
| Mitral valve lesion | |||
| Rheumatic | 15 (55.6%) | 13 (52.0%) | 0.362 |
| Degenerative | 10 (37.0%) | 11 (44.0%) | 0.197 |
| Endocarditis | 2 (7.4%) | 1 (4.0%) | 0.063 |
Continuous data as mean ± standard deviation and categorical data as number (percentage).
LVEF: left ventricular ejection fraction; LA: left atrium; LAM: left atrial maze; PV: pulmonary vein.
Enrolment protocol
All patients had symptomatic paroxysmal AF detected using standard ECG or Holter. Paroxysmal AF was defined as the occurrence in the previous 6 months of one or more episodes lasting <7 days, all of which terminated spontaneously.
Inclusion criteria:
History of paroxysmal AF.
Scheduled for mitral valve surgery.
Exclusion criteria:
Repeated cardiac surgical procedure.
Class IV NYHA heart failure symptoms.
Left atrial size ≥80 mm.
Documented atrial flutter.
Aortic valve surgery requirement.
Emergent cardiac surgery requirement.
Left ventricle ejection fraction <35%.
Unwillingness to participate in the study.
Patients were randomly assigned to receive PVI only (PVI group; n = 27) or undergo a complete left atrial maze (LAM) procedure (LAM group; n = 25). Eligible patients were included in one of the groups immediately before the ablation procedure according to a computer-generated randomization list.
Surgical procedure
After median sternotomy, standard cardiopulmonary bypass with bicaval cannulation was instituted and moderate hypothermia (33–34°C) was achieved. Cold crystalloid (Custadiol; Dr Kohler Pharma, Alsbach-Hahnlein, Germany) cardioplegic arrest was initiated with antegrade root flow in all patients.
The ablation procedure was performed using a dry bipolar radiofrequency (RF) ablation clamp only (AtriCure, Inc., Cincinnati, OH, USA) in all patients. The technique used in the PVI group consisted of ablations around the right and left PV orifices (Fig. 2A), usually before aortic cross-clamping. The encircling PV applications were performed five times epicardially. The scheme of RF ablation in the LAM group was performed as previously described (Fig. 2B) [13]. In all cases, anatomic exclusion of the left atrial appendage was performed using an external double-layer 4–0 polypropylene suture. Valve surgery techniques were standard and similar between groups.
Figure 2:
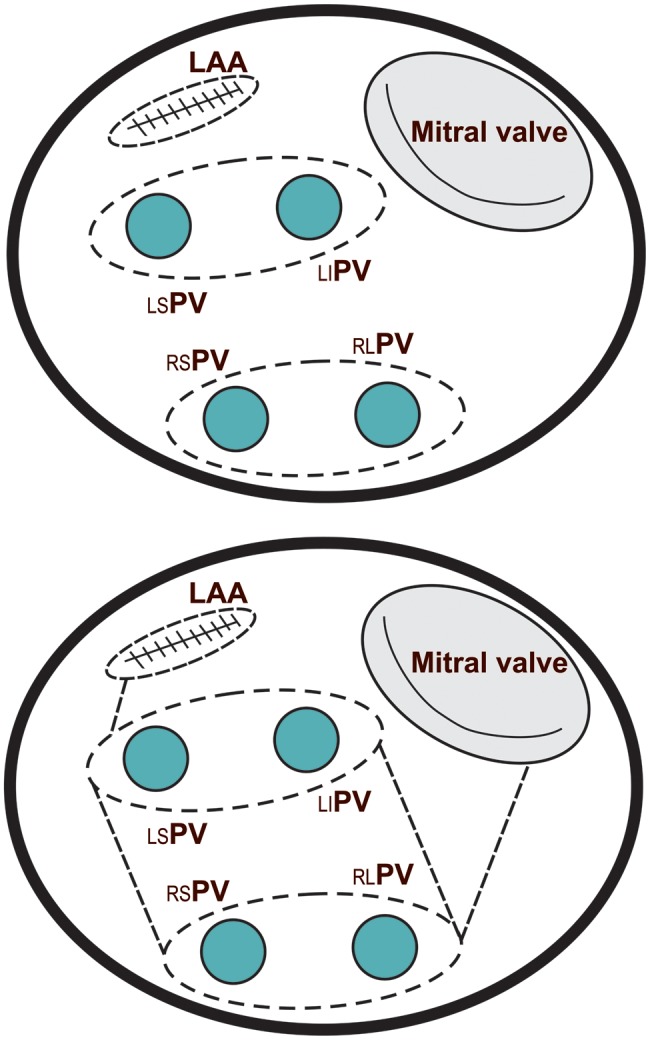
Ablation lines scheme: (A) lesion set in the pulmonary vein isolation (PVI) group and (B) lesion set in the left atrial maze (LAM) group.
Cardiac rhythm assessment
Continuous subcutaneous monitoring was performed using the REVEAL XT implantable loop recorder (Medtronic, Inc., Minneapolis, MN, USA). The definition of procedural success was considered an AF burden of <0.5% (based on implantable loop recorder data) at each follow-up examination. The same criterion to define AF-free (responders) has been used in earlier studies published by our group [13]. Patients with an AF burden of >0.5% were classified as non-responders. AF was visually adjudicated by the investigators through analysis of each stored ECG.
Postoperative management and follow-up
In cases of stable sinus rhythm, amiodarone administration was started with an intravenous bolus of 300 mg after cardiopulmonary bypass, followed by an infusion rate of 900 mg/day for 3 days after surgery; thereafter, 200 mg/day was administered orally. Three (5.8%) patients with contraindications to amiodarone were given sotalol instead. Early AF recurrence during hospital stay, after antiarrhythmic saturation, and exclusion of intracardiac thrombosis by transoesophageal echocardiography were treated with electrical cardioversion. After hospital discharge, such medications were continued for the blanking period and all antiarrhythmics were discontinued after 3 months in all cases. Oral anticoagulants were discontinued after valve repair or valve tissue replacement in responders as documented by implantable loop recorder 3 months after the procedure; in addition, low-dose aspirin (100 mg/day) was started. Patients with mechanical valves were kept on lifelong anticoagulants.
Patient follow-up examinations were performed at our outpatient clinic or at the referral cardiologist's office. The primary endpoint of this study was freedom from AF at follow-up (AF burden <0.5%).
Statistical analysis
Results are expressed as mean values ± standard deviation or as numbers and related percentages as appropriate. Unpaired t-tests were used to compare continuous data, while χ2 or Fisher's exact test was used to compare categorical data. Freedom from AF recurrence was estimated by the Kaplan–Meier method, the curves from which were compared with those of the log-rank test. All cases in which recurrence occurred between 3 and 18 months were considered failures. All reported P-values were based on two-sided tests, and P < 0.05 was considered significant. All statistical calculations were performed using the SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA).
RESULTS
Intraoperative data
The patients' intraoperative data are described in Table 2. The mean aortic clamping and ablation times were significantly longer in the LAM group than in the PVI group (Table 3). Exit block of the pulmonary veins (electrophysiological disconnection of the PV from the left atrium during pacing) was assessed after ablation prior to cross-clamping. A consolidated conduction block was successfully achieved in all patients. At the end of the surgical procedure, the loop recorder was implanted in the left parasternal area, requiring an implant time of 6.1 ± 2.8 min.
Table 2:
Procedure characteristics
| PV isolation (n = 27) | LAM (n = 25) | P-value | |
|---|---|---|---|
| Mitral valve repair | 14 (51.9%) | 11 (44.0%) | 0.072 |
| Mitral valve replacement | 13 (48.1%) | 14 (56.%) | 0.096 |
| Mechanical valve | 11 (40.7%) | 10 (40.0%) | 0.721 |
| Tissue valve | 2 (7.4%) | 4 (16.0%) | 0.033 |
| Left atrium thrombectomy | 2 (7.4%) | 0 | 0.045 |
| Tricuspid valve repair | 10 (37.0%) | 8 (32.0%) | 0.143 |
| CABG | 3 (11.1%) | 2 (8.0%) | 0.081 |
LAM: left atrial maze; PV: pulmonary vein.
Table 3:
Operative data
| PV isolation (n = 27) | LAM (n = 25) | P-value | |
|---|---|---|---|
| Aortic clamping time (min) | 63.2 ± 12.3 | 78.7 ± 16.5 | 0.023 |
| Bypass time (min) | 79.7 ± 10.1 | 99.2 ± 13.4 | 0.032 |
| Mean ablation time (min) | 7.1 ± 2.3 | 23.8 ± 4.1 | 0.001 |
LAM: left atrial maze; PV: pulmonary vein.
Early outcomes
There were no early deaths in either group. No procedure-related complications occurred with regard to either ablation or the monitoring device. The mean intensive care unit stay was 2.1 ± 1.1 days for the PVI group and 2.3 ± 1.4 days for the LAM group (P = 0.621), while the median hospitalization was 14.2 ± 5.4 days for the PVI group and 15.1 ± 6.2 days for the LAM group (P = 0.394).
Re-exploration due to bleeding was required in 1 patient in each group during the early postoperative period (3.7% in the PVI group and 4.0% in the LAM group by Fisher's exact test; P = 0.812), but none was related to the ablation procedure.
Ischaemic stroke occurred in 1 (3.7%) patient in the PVI group (P = 0.063; Fisher's exact test) immediately after the operation and was validated by a computed tomography brain scan. This patient experienced a recurrence of a cerebral ischaemic event and a neurological deficit prior to surgery. There were no deep sternal infections in either groups, but a superficial sternal wound infection was found in 2 (8.0%) patients in the LAM group and no patients in the PVI group (P = 0.042; Fisher's exact test).
The incidence of early AF paroxysmal recurrence was significantly higher in the PVI group than in the LAM group (62.9 vs 24.0%; P < 0.001), which necessitated electrical cardioversion in 29.6 and 8.0% (P < 0.001) of patients, respectively. Pacemaker implantation before discharge due to sinus node dysfunction was required by 1 patient in the LAM group (P = 0.089). All of the other patients were discharged in stable sinus rhythm.
Follow-up
The mean follow-up for all 52 patients was 18.6 months (standard deviation 2.1 months). Follow-up examinations were scheduled every 3 months. One (3.7%) patient in the PVI group died suddenly 13 months after the operation. The clinical data suggested that the most probable cause of death was a cerebrovascular event, but our request for a post-mortem examination was refused.
During the follow-up examination, 15 (55.6%) of the 27 patients in the PVI group and 22 (88.0%) of 25 patients in the LAM group had no documented atrial arrhythmias and were considered responders (AF burden < 0.5%) (log-rank test; P = 0.031; Fig. 3). At the end of the follow-up period, the calculated mean AF burden was significantly lower in the LAM group than in the PVI group (23.6 ± 8.7 and 6.8 ± 2.2%, respectively; P < 0.001).
Figure 3:
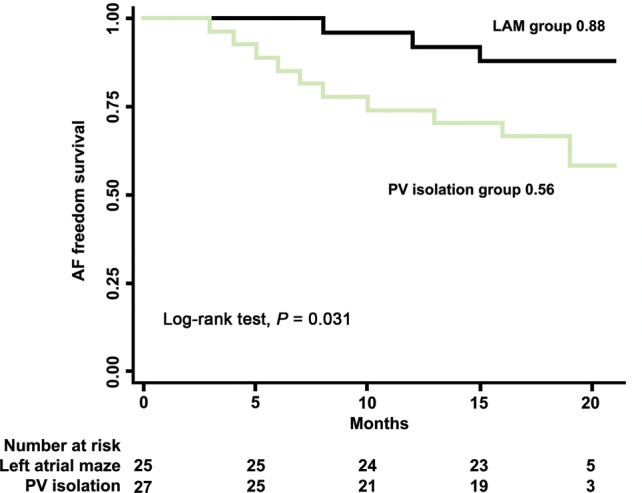
Time to first atrial fibrillation (AF) paroxysm (AF burden >0.5%) after surgery pulmonary vein isolation (PVI) (black line) and the LAM group (green line).
Two (3.9%) patients had atrial flutter. In 1 case in the PVI group, left atrial flutter (mitral valve annulus-related) was found 7 months after the surgery. In this case, the patient underwent catheter ablation, and RF lesions from left inferior PV to the mitral annulus were required to terminate the atrial flutter. In the other patient in the LAM group, an electrophysiological study indicated a counter-clockwise typical flutter. A linear block was created on the right atrial isthmus, and sinus rhythm was restored. No further episodes of atrial flutter were observed in either patient.
DISCUSSION
This study is the first prospective randomized study using continuous monitoring to evaluate the efficacy of two different ablation strategies, PVI and LAM, for patients with paroxysmal AF undergoing mitral valve surgery. The main finding is that the LAM procedure is significantly more effective than PVI alone for concomitant paroxysmal AF ablation during mitral valve surgery. The incidence of early AF paroxysms recurrence was significantly higher in the PVI group than in the LAM group (62.9 vs 24.0%; P < 0.001). Also the mean AF burden during the follow-up period was significantly lower in the LAM group than in the PVI group (23.6 ± 8.7 and 6.8 ± 2.2%, respectively; P < 0.001). This study's findings can be seen as the first confirmation that minimizing the lesion sets as alternative modifications to the LAM procedure can reduce surgical trauma while retaining its effectiveness.
It is now generally accepted that the underlying mechanism of paroxysmal AF originates in the myocardial junction of the pulmonary veins [4]. However, this theory was proved in patients with paroxysmal lone AF without structural heart disease.
Further most of the studies regarding concomitant ablation during surgery involved patients with paroxysmal as well as persistent (or long-standing persistent) AF in a heterogeneous group (mitral valve and non-mitral valve or coronary patients). The only study of dedicated surgery for paroxysmal AF in the setting of mitral valve disease is the work of Gillinov et al. [7]. They reported that lesions set of the ablation procedure had no statistically significant impact on AF prevalence; however, to detect paroxysmal recurrences, a special statistical technique was used that included discrete ECG only. To the best of our knowledge, any intermittent method of monitoring that is commonly used to estimate cardiac rhythm after the ablation procedure has limited capability especially in patients with paroxysmal AF. Our study used continuous rhythm monitoring as a more accurate and reliable diagnostic tool to detect AF recurrences [11, 12, 15, 16]. Gillinov et al. [17] estimated the effectiveness of the different lesion sets in patients with permanent AF and found that PVI alone or lesion sets without mitral lines were less effective than the LAM procedure. These data are thus confirmatory with regard to the leading role of the pulmonary veins in paroxysmal AF genesis.
Significant reduction in the recurrence of AF in patients undergoing LAM supports the hypothesis that a mitral valve lesion, associated with electrophysiological changes in the entire left atrium triggered paroxysmal AF at the initial stage, which can be addressed more fully with the specific procedure. Moreover, our data demonstrated that the additional connecting ablation lines rather than mere PV isolating islands, added some extra cross-clamp and cardiopulmonary bypass time but did not affect postoperative morbidity and mortality. The effect of complete electrical isolation of the posterior left atrium in the maze procedure has been clearly shown by Voeller et al. In most cases concomitant ablation was performed during mitral valve surgery and >60% patients had paroxysmal AF. This fact is indirect evidence that the pulmonary veins are not the only AF triggers, even in paroxysmal patients and an important role is played by isolating the posterior left [18].
In our study, we performed complete left atrial procedure in the LAM group using dry bipolar RF ablation clamp only, including the left isthmus line. Surgical technique and the efficacy of electrophysiology in performing mitral line with bipolar clamp alone has been described by Benussi et al. [19]. However, the work of Castellá et al. [20] noted the anatomical impossibility of forming the line with a bipolar clamp. In our study, we strictly followed Benussi's technique. First we ablated only the thicker part of the atrial wall, clamping the mitral annulus, with a fissure between the thicker part of the left atrial wall and the jaws of the bipolar forceps. When transmurallity was reached at this position, the clamp was slightly pulled back, tightly clutching the thin atrial wall. Performing ablation to the mitral annulus with bipolar RF entails circumferential ablation of the coronary sinus. Such ablation may increase AF elimination, but the main aim of the left isthmus line is prophylaxis left atrial flutter around the ‘box’ and mitral annulus [21]. There were no statistical differences in atrial flutter between the two groups in our work, but we have one left atrial flutter (around mitral annulus) in the PVI group.
Resuming concomitant LAM may be beneficial in patients with paroxysmal AF during mitral valve surgery and seems to be a suitable procedure.
Study limitation
The main limitation of our study is its small number of patients. The cause of the very small number of patients was the main inclusion criterion—mitral valve lesion associated with paroxysmal AF proved by standard ECG or Holter. Another limitation factor was the patient's desire to be implanted with a subcutaneous loop recorder. On the other hand, we ensured homogeneous management of the populations in both groups and a standardized approach to each procedure. It is also important to mention that the study was prospective and randomized but was conducted in a single centre, implying the difficulty of generalizing its results. As such, further larger-scale clinical trials are needed on this issue.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr C. Muneretto (Brescia, Italy): Despite the fact that the number of patients is low, your paper analyses some very interesting considerations.
The first question is how do you explain the better results in the maze group, since this is a type of paroxysmal atrial fibrillation in which we know that usually the origin of the problem is in the pulmonary vein? This is a typical focal disease and so, by definition, this disease should be treated by a simple pulmonary vein isolation. What is your explanation for the failure in such patients? Was it the failure of your isolation of the pulmonary vein? Was it the failure of your diagnosis because those patients were not in paroxysmal atrial fibrillation, or what?
Secondly, we have used the continuous loop recorder for the last five years, and we know that most of the events are artefacts. So if you look at the automatic records, you will have a lot of mistakes because most of those events are not AF. Indeed, they are a very different type of artefact, mainly including some type of focal atrial arrhythmia. Could you comment on that?
Dr Bogachev-Prokophiev: Regarding the first question about explaining the low effectiveness of pulmonary vein isolation, as we know from the previous work of Hessinger, the problem is in the pulmonary vein. But we think that half of our patients were rheumatic patients with problems not only in the pulmonary vein, but also damage of the left atrial myocardium by the rheumatic process. Maybe this fact explains the better results in the left atrial maze group. As for the second question, we have a lot of experience of patients with the implantable loop recorder, different patients, coronary patients and patients after valve procedures, about 1,000 patients who had catheter ablation or thoracoscopic ablation. We did not encounter any differences in signal problems.
Dr P. Punjabi (London, UK): I have a very specific question, hopefully with a very specific answer. In terms of your atrial transport function, although not surprisingly you did not find any difference, what was the exact method of evaluating that? And why was it that there was no difference, if you can very quickly summarize?
Dr Bogachev-Prokophiev: We used the echo method. Also, we performed it in some patients to compare with the MRI technique when we performed valvuloplasty without any foreign material in the heart. And we have a lot of experience of assessing the transport function of the left atrium after the Maze procedure. As for methods, we used the velocity integral of peak A and peak E to measure the atrial activity.
As for differences in the early postoperative period, we explained these by the damage by bipolar ablation and oedema of the myocardium, and after some months (the last follow-up period was 17 months), the left atrial function was normal in both groups. The left atrial appendage accounts for about 20% of atrial activity, and was excluded in both groups.
REFERENCES
- 1.Kawaguchi A, Kosakai Y, Sasako Y, Eishi K, Nakano K, Kawashima Y. Risks and benefits of combined maze procedure for atrial fibrillation associated with organic heart disease. J Am Coll Cardiol. 1996;28:985–90. doi: 10.1016/s0735-1097(96)00275-6. [DOI] [PubMed] [Google Scholar]
- 2.Handa N, Schaff H, Morris J, Anderson B, Kopecky S, Enriquez-Sarano M. Outcome of valve repair and the Cox maze procedure for mitral regurgitation and associated atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:626–35. doi: 10.1016/S0022-5223(99)70007-3. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck K, Cappato R, Brugada J, Camm A, Chen S, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Marrouche NF, Dresing T, Cole C, Bash D, Saad E, Balaban K, et al. Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation: impact of different catheter technologies. J Am Coll Cardiol. 2002;40:464–74. doi: 10.1016/s0735-1097(02)01972-1. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42:185–97. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 7.Gillinov AM, Bakaeen F, McCarthy PM, Blackstone EH, Rajeswaran J, Pettersson G, et al. Surgery for paroxysmal atrial fibrillation in the setting of mitral valve disease: a role for pulmonary vein isolation? Ann Thorac Surg. 2006;81:19–28. doi: 10.1016/j.athoracsur.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 8.Nitta T, Ishii Y, Miyagi Y, Ohmori H, Sakamoto S, Tanaka S. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or re-entrant activation as the mechanism in atrial fibrillation. J Thorac Cardiovasc Surg. 2004;127:770–8. doi: 10.1016/j.jtcvs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80:373–80. [PubMed] [Google Scholar]
- 10.Stulak J, Sundt T, III, Dearani J, Daly R, Orsulak T, Schaff H. Ten-year experience with the Cox-Maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg. 2007;83:1319–24. doi: 10.1016/j.athoracsur.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Ad N, Henry L, Hunt S, Barnett S, Stone L. The Cox maze III procedure success rate: comparison by electrocardiogram, 24-hour Holter monitoring and long-term monitoring. Ann Thorac Surg. 2009;88:101–5. doi: 10.1016/j.athoracsur.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Hanke T, Charitos EI, Stierle U, Karluss A, Kraatz E, Graf B, et al. Twenty-four-hour Holter monitor follow-up does not provide accurate heart rhythm status after surgical atrial fibrillation ablation therapy up to 12 months experience with a novel permanently implantable heart rhythm monitor device. Circulation. 2009;120(Suppl.):S177–84. doi: 10.1161/CIRCULATIONAHA.108.838474. [DOI] [PubMed] [Google Scholar]
- 13.Bogachev-Prokophiev A, Zheleznev S, Romanov A, Pokushalov E, Pivkin A, Corbucci G, et al. Ablation for atrial fibrillation during mitral valve surgery: 1-year results through continuous subcutaneous monitoring. Interact CardioVasc Thorac Surg. 2012;15:37–41. doi: 10.1093/icvts/ivs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vahanian A, Alfieri O, Andreotti F, Antunes M, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). The joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42:S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 15.Pokushalov E, Romanov A, Cherniavsky A, Corbucci G, Pak I, Kareva Y, et al. Ablation of paroxysmal atrial fibrillation during coronary artery bypass grafting: 12 months follow-up through implantable loop recorder. Eur J Cardiothorac Surg. 2011;40:405–11. doi: 10.1016/j.ejcts.2010.11.083. [DOI] [PubMed] [Google Scholar]
- 16.Camm AJ, Corbucci G, Padeletti L. Usefulness of continuous electrocardiographic monitoring for atrial fibrillation. Am J Cardiol. 2012;110:270–6. doi: 10.1016/j.amjcard.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Gillinov AM, Bhavani S, Blackstone EH, Rajeswaran J, Svensson LG, Navia JL, et al. Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Ann Thorac Surg. 2006;82:502–13. doi: 10.1016/j.athoracsur.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Voeller RK, Bailey MS, Zierer A, Lall SC, Sakamoto S, Aubuchon K, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–7. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 19.Benussi S, Nascimbene S, Galanti A, Fumero A, Dorigo E, Zerbi V, et al. Complete left atrial ablation with bipolar radiofrequency. Eur J Cardiothorac Surg. 2008;33:590–5. doi: 10.1016/j.ejcts.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Castellá M, García-Valentín A, Pereda D, Colli A, Martinez A, Martinez D, et al. Anatomic aspects of the atrioventricular junction influencing radiofrequency Cox maze IV procedures. J Thorac Cardiovasc Surg. 2008;136:419–23. doi: 10.1016/j.jtcvs.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Cox JL. Atrial fibrillation II: rationale for surgical treatment. J Thorac Cardiovasc Surg. 2003;126:1693–9. doi: 10.1016/j.jtcvs.2003.06.003. [DOI] [PubMed] [Google Scholar]


