Abstract
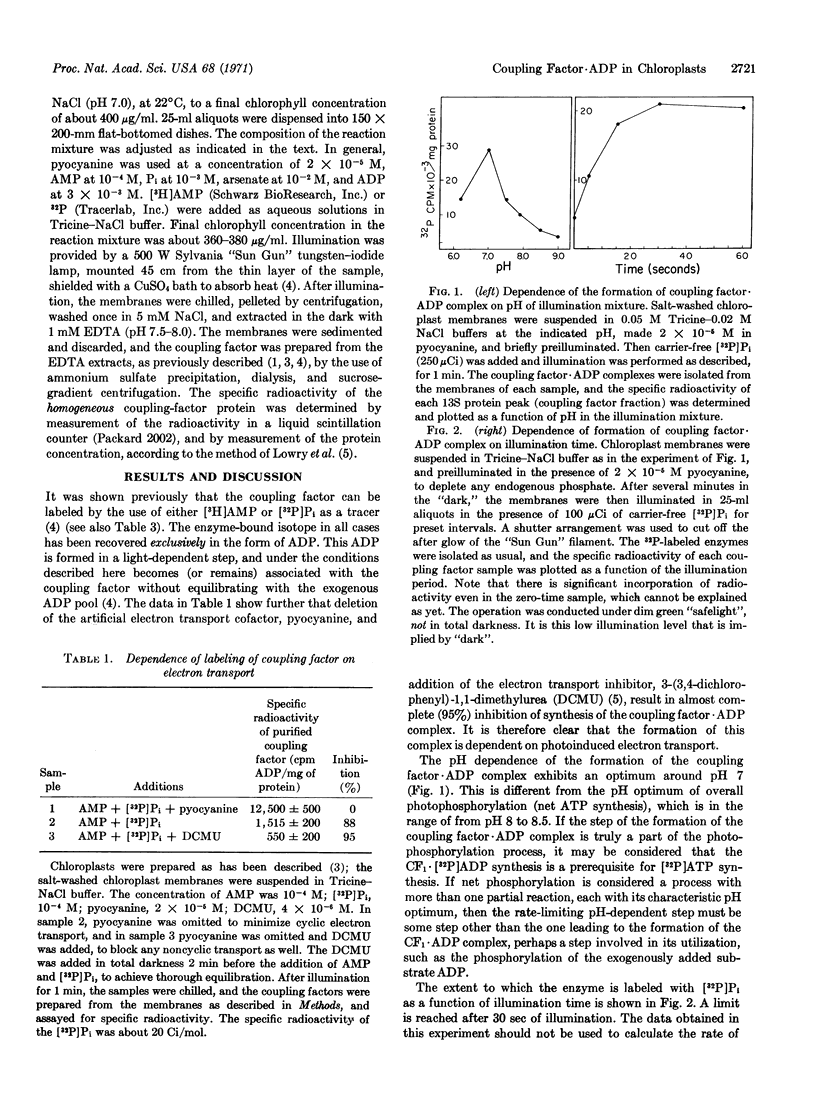
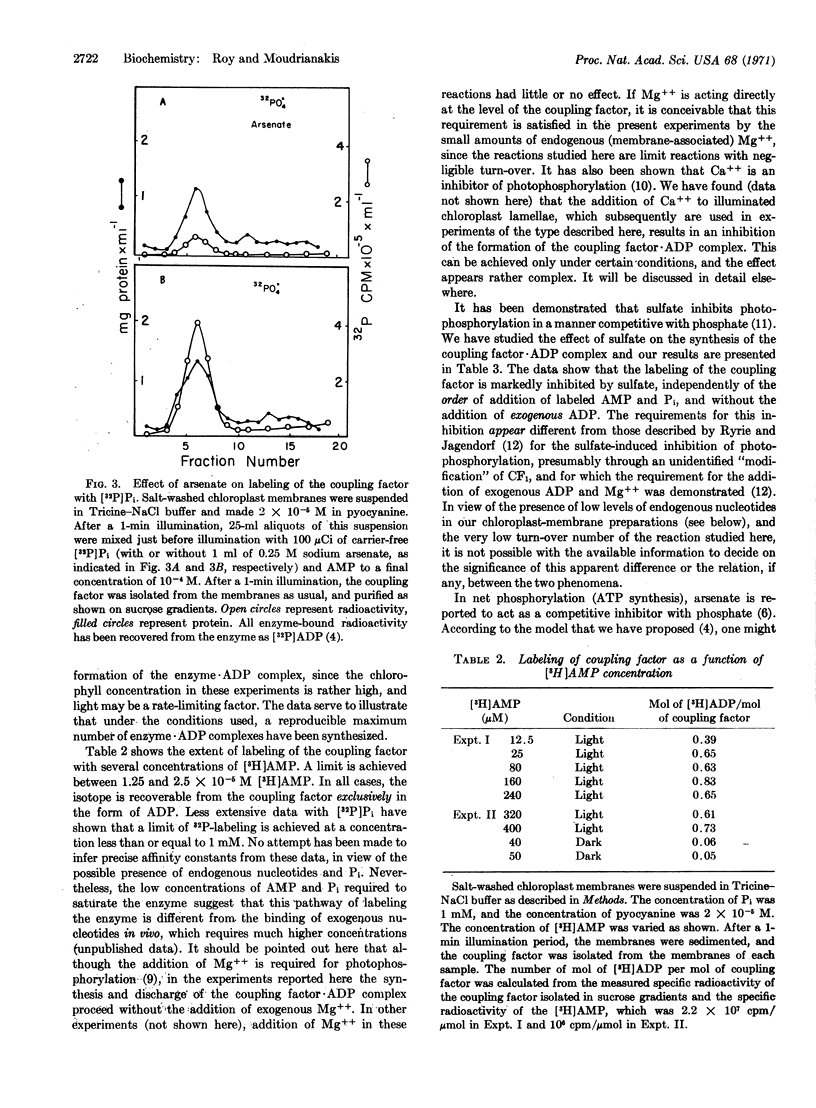
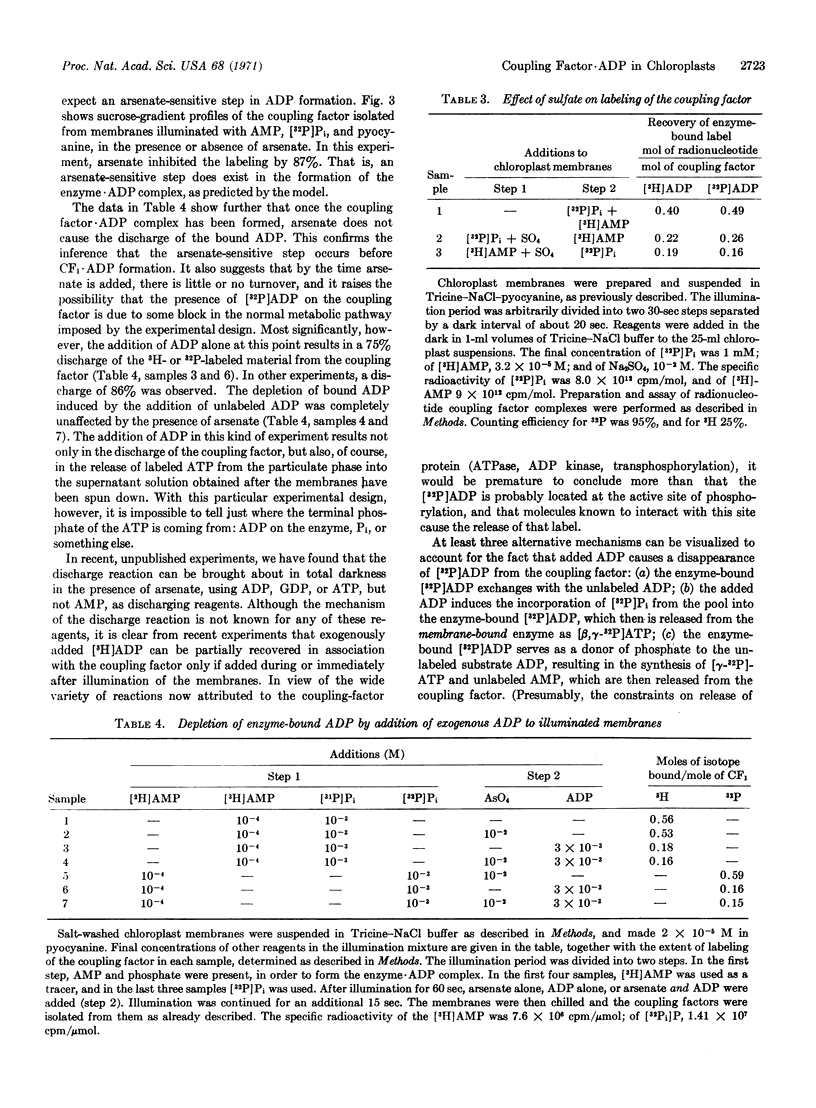
The formation of a coupling factor·ADP complex is shown to be dependent on photoinduced electron transport, AMP, and Pi, and sensitive to arsenate and sulfate. The stability of the complex is unaffected by subsequent addition of arsenate, but is quite markedly sensitive to the addition of ADP. The data are discussed in relation to possible models of photophosphorylation, and in particular, to one in which coupling factor-bound, photosynthetically generated, ADP serves as a phosphoryl donor to substrate ADP.
Keywords: light-dependent electron transport, arsenate, sulfate, photophosphorylation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T., EVANS M. Photosynthetic phosphorylation in a partially purified system. Biochim Biophys Acta. 1957 Nov;26(2):262–269. doi: 10.1016/0006-3002(57)90004-5. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Function of the "quantasome" in photosynthesis: structure and properties of membrane-bound particle active in the dark reactions of photophosphorylation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1261–1268. doi: 10.1073/pnas.58.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGENDORF A. T., MARGULIES M. Inhibition of spinach chloroplast photosynthetic reactions by p-chlorophenyll, 1-dimethylurea. Arch Biochem Biophys. 1960 Oct;90:184–195. doi: 10.1016/0003-9861(60)90566-x. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Moudrianakis E. N. Fractionation and comparative studies of enzymes in aqueous extracts of spinach chloroplasts. Arch Biochem Biophys. 1969 Feb;129(2):655–671. doi: 10.1016/0003-9861(69)90226-4. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H., Moudrianakis E. N. Interactions between ADP and the coupling factor of photophosphorylation. Proc Natl Acad Sci U S A. 1971 Feb;68(2):464–468. doi: 10.1073/pnas.68.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J., Jagendorf A. T. Inhibition of photophosphorylation in spinach chloroplasts by inorganic sulfate. J Biol Chem. 1971 Feb 10;246(3):582–588. [PubMed] [Google Scholar]
- SCHULZ A. R., BOYER P. D. The fate of oxygens of inorganic phosphate in photophosphorylation. Arch Biochem Biophys. 1961 May;93:335–337. doi: 10.1016/0003-9861(61)90275-2. [DOI] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]


