Abstract
The emergence of extended-release naltrexone (XR-NTX) raises the opportunity to explore the role of endorphin blockade on hedonic response during long-term alcohol dependence treatment. A hedonic survey was administered to 74 alcohol dependent patients treated for an average of 3.5 years with nearly continuous month-long intramuscular XR-NTX. The paper-and-pencil, one-time survey asked patients about the degree of pleasure they experience in the past 90 days with drinking alcohol, sex, exercise and other daily activities. The data revealed lower pleasure ratings for alcohol than for sex, exercise and 10 other common activities. Mean responses to drinking alcohol and gambling were significantly lower than to listening to music, sex, reading, being with friends, eating good food, eating spicy food and playing video/card games. This effect was independent of XR-NTX dose or duration. Although this exploratory study lacked baseline data, a comparison group or control for the impact of patient discontinuation, the data indicate the feasibility of examining long-term hedonic response in recovery. The differential hedonic ratings suggest that, in patients who persist with long-term continuous therapy, XR-NTX may selectively inhibit the pleasure associated with drinking alcohol, compared to a variety of other activities.
INTRODUCTION
Almost 4% of Americans age 18 or older are estimated to be dependent on alcohol.1 Although many individuals with an alcohol use disorder seek help from Alcoholics Anonymous or receive office-based or program-based psychosocial treatments, pharmacotherapy has increasingly become part of the treatment program. In fact, clinical guidelines from the National Institute on Alcohol Abuse and Alcoholism recommend that pharmacotherapy be considered for all alcohol dependent patients.2
One of the pharmacologic agents approved by the U.S. Food and Drug Administration (FDA) for the treatment of alcohol dependence is oral naltrexone, an opioid antagonist. Alcohol has been found to increase the release of the endogenous opioids in humans and animals.3,4 Blocking opioid receptors with naltrexone has been found to lead to less alcohol-induced pleasure, less euphoria and less craving for alcohol.5–8 In addition, administration of alcohol increases dopamine concentration in the nucleus accumbens.9,10 Animal studies and imaging studies in humans indicate that naltrexone reduces this alcohol-stimulated dopamine output in the nucleus accumbens.11–13 The nucleus accumbens has long been identified as the “reward center” of the brain,14 and is involved in the rewarding aspects of food15 and sex,16 in addition to multiple addictive drugs.
Based on its potential effects on the brain’s reward system, one concern with the use of naltrexone as a treatment for alcohol dependence is whether patients receiving naltrexone will experience less pleasure from a variety of experiences, not just from alcohol. Consistent with this concern, opioid antagonists (i.e., oral naltrexone, naloxone) have demonstrated an impact on the hedonic effects of physical exercise,17–19 gambling,20 eating,21 sex22 and shopping.23 However, when assessing the effect of naltrexone on the experience of pleasure among alcohol dependent individuals, studies need to examine the impact of current abstinence/non-abstinence on reports of pleasure because regular use of alcohol among those who are dependent is associated with dysphoria,24 and this may show up as low levels of reported pleasure.
In 2006 the FDA approved an extended-release formulation of naltrexone (XR-NTX) designed to be administered once every month by intramuscular injection. With XR-NTX, naltrexone is continuously released over the 1-month period with little within-day fluctuation following the injection.25–27 A multicenter placebo-controlled investigation demonstrated that, in alcohol dependent adults (n=82) who had at least 2 episodes per week of heavy drinking (≥ 5 drinks per day for men, ≥ 4 for women) in the 30 days prior to enrollment but were able to abstain at least 4 days prior to treatment initiation, XR-NTX plus psychosocial counseling produced a three-fold delay in time to first drink (41 vs. 12 days) and approximately a tripling in percent of patients who were abstinent (32% vs. 11%) after six months of treatment compared to placebo.28
Few treatment studies of alcohol dependence have directly examined the impact of a pharmacological agent on the experience of pleasure from everyday activities. Long-term treatment with an opioid antagonist provides a unique and never before possible opportunity to examine differential hedonic response in humans. The current investigation was therefore an exploratory study of whether or not long-term XR-NTX treatment impacts patients’ experience of pleasure from a variety of everyday activities in comparison to their experience of pleasure from alcohol.
METHODS
Subjects
Subjects had participated in one of two randomized clinical trials evaluating the efficacy and safety of XR-NTX as a treatment for alcohol dependence. For study 1,29 patients were recruited at 24 U.S. public hospitals, private and Veterans Administration clinics, and tertiary care medical centers. Study 230 was also conducted at 24 similar sites in the U.S. Following 6-month (study 1) or 1-year (study 2) initial randomized trials, patients were eligible to participate in open-label extension phases lasting 3–5 years. At the completion of the extension phases of the clinical trials, subjects who continued on XR-NTX were invited to participate in the current questionnaire study.
Adult outpatients (ages 18 and older) meeting criteria for a DSM-IV diagnosis of alcohol dependence were eligible to participate in the original randomized trials. Study 2 also included some opioid-dependent patients in the original trial. Only those who were alcohol dependent were approached for participation in the current study. Alcohol dependent patients also needed to have a minimum of 2 episodes of heavy drinking (≥ 5 standard drinks/day for men; ≥ 4 for women) per week in the 30 days before screening. Key exclusion criteria included untreated major depression with suicidal ideation, psychosis, bipolar disorder and dependence within the past year on benzodiazepines, opiates (with the exception of the subgroup in Study 2 not included here) or cocaine (use of other illicit drugs was not an exclusion), evidence of liver failure, and any pending legal proceedings that had the potential for incarceration during the study period.
Patients provided written, informed consent for the original randomized trials, extension phases and the current questionnaire study. All protocols were approved by Institutional Review Boards or ethics committee.
Treatment
XR-NTX was given every 4 weeks as an intramuscular gluteal injection, alternating sides with each visit. In Study 1, patients were randomly assigned to six months of treatment with XR-NTX 380 mg (VIVITROL®; Alkermes, Waltham, MA), XR-NTX 190 mg or placebo. In Study 2, patients were originally randomized to one year of treatment with XR-NTX 380 mg or oral naltrexone 50 mg/day. In the extension phase of Study 1, patients on XR-NTX continued on their same dosage, while patients receiving placebo during the initial phase were switched to either XR-NTX 380 mg or XR-NTX 190 mg. In Study 2, all patients received XR-NTX 380 mg in the extension phase. Extension phases lasted from 3 to 5 years after the completion of the initial 6 month (Study 1) or 1-year (Study 2) trials.
Patients were not required to be abstinent at the time of initial injection and were not withdrawn if they continued to use alcohol. In addition to receiving study medication, patients were also provided with psychosocial support with the Biopsychosocial, Report, Empathy, Needs, Direct advice and Assessment (BRENDA) model31 at study visits during the initial randomized trials. Although not required, patients were encouraged to continue attending BRENDA sessions during the extension phases.
Hedonic Response Questionnaire
For the current study, a 13-item self-report hedonic response questionnaire was created (Appendix 1) because no other questionnaire assessing pleasure from a range of activities was available. This questionnaire was designed to inquire about a range of types of activities that might produce positive feelings. Items for the questionnaire were derived from a literature review focusing on pleasurable activities that might potentially be affected by opioid antagonists. This review identified studies of the hedonic effects of opioid antagonists on physical exercise,17–19 gambling,20 eating,21 sex22 and shopping.23 In addition to these domains from the literature, other common pleasant activities (listening to music; being with friends; reading; watching sports; playing cards or board games or video games) were selected to represent a range of potentially enjoyable everyday activities.
For each item, the respondent rated how pleasurable each activity was for him/her in the past 90 days on a 1 (“not at all”) to 5 (“very much so”) scale. A response of “not applicable” was also available if the respondent did not engage in the activity in the past 90 days. The paper-and-pencil survey was administered once to patients.
Statistical Analyses
Initial analyses focused on the mean hedonic response to each of the rated activities for patients who reported that they were continuing to drink alcohol during the past 90 days compared to those who were not drinking alcohol during the past 90 days. These comparisons were conducted using independent t-tests.
Using the sample of patients who did report drinking during the past 90 days, paired t-tests were used to compare the mean hedonic response to drinking alcohol to the mean hedonic response for each of the non-alcohol activities. Effect sizes were calculated using Cohen’s d (mean difference divided by the standard deviation of the difference scores). To examine whether any differences between the degree of pleasure from drinking alcohol and the degree of pleasure from each of the other activities varied as a function of dosage of XR-NTX (380 mg vs. 190 mg) or duration of naltrexone treatment, repeated measures analyses of variance were conducted on an exploratory basis (due to low statistical power) with measure (drinking alcohol vs. another activity) as the within subject factor and dosage, or duration (in approximate years), as the between group factor. A significant interaction effect in these models would indicate that the difference in degree of pleasure between drinking alcohol and another activity varied depending on dosage or duration.
RESULTS
Baseline Characteristics
Of the 187 patients who initially agreed to long-term XR-NTX treatment (participated in one of the two extension phase studies), 75 were available and willing to participate in the current study after having received XR-NTX for several years, with one case failing to complete the hedonic survey, thereby leaving a sample of 74 patients available for analysis. Demographic characteristics of the sample and mean severity of drinking are shown in Table 1. Baseline characteristics of these patients were similar to those of the sample of patients who originally began the extension phase of the two parent studies.
Table 1.
Demographic Characteristics of Sample
| Entered Extension Phase Study 1 |
Respondents from Study 1 |
Entered Extension Phase Study 2 |
Respondents from Study 2 |
Aggregate Sample |
|
|---|---|---|---|---|---|
| N=108 | N=44 | N=79 | N=31 | N=75 | |
| Sex, n (%) | |||||
| Male | 69 (64%) | 26 (59%) | 51 (65%) | 17 (55%) | 43 (57%) |
| Female | 39 (36%) | 18 (41%) | 28 (35%) | 14 (45%) | 32 (43%) |
| Age, mean (SD) | 46.9 (9.5) | 46.3 (8.3) | 44.7 (8.8) | 44.3 (9.2) | 47.0 (8.1) |
| Race, n (%) | |||||
| White | 90 (83%) | 37 (84%) | 66 (83%) | 27 (87%) | 64 (85%) |
| Black | 7 (6%) | 3 (7%) | 9 (11%) | 3 (10%) | 6 (8%) |
| Latino | 7 (6%) | 2 (5%) | 3 (4%) | 1 (3%) | 3 (4%) |
| Other | 4 (4%) | 2 (4%) | 1 (1%) | 0 | 2 (2%) |
| Drinking At Intake | |||||
| % Heavy drinking days in past 30 days, mean (SD) | 60.2 (26) | 60.2 (26) | 51.9 (35) | 52.0 (34) | 56.6 (30) |
Note. One case was missing all survey response data and is not included in results but is retained for demographic purposes.
Treatment Received
At the time of administration of the survey for the current study, 53 (71%) patients were receiving 380 mg of XR-NTX and 21 (29%) were receiving 190 mg of XR-NTX. Subjects received XR-NTX for an average of 41.5 (SD = 8.7l) months. Adherence with monthly injections of XR-NTX over these extended time periods among these subjects averaged 99.8% for Study 1 and 99.7% for Study 2.
Pleasure from Drinking Alcohol
At this long-term assessment point, patients who had consumed any alcohol during the past 90 days showed relatively low ratings of pleasure derived from drinking (mean = 2.57 on 1 to 5 scale) with only 19% rating “drinking alcohol” as “very much” or “quite a bit” pleasurable. A total of 21 (28%) of the 74 patients who completed the survey reported no drinking of alcohol in the past 90 days.
Pleasure from Everyday Activities
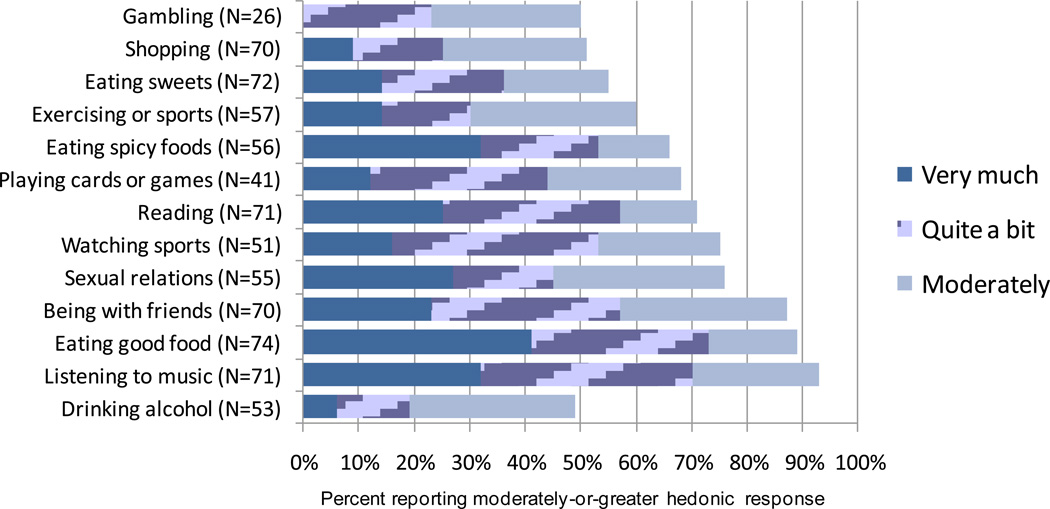
For all patients who completed the questionnaire, most of the non-drinking everyday activities, including eating good food, sex, and exercise, were rated on average as moderately pleasurable or better, with the exception of gambling and shopping (Figure 1). The proportion of patients rating a respective activity as “very much,” “quite a bit” or “moderately” pleasurable was high for listening to music (93%), eating good food (89%), being with friends (87%) and exercising or participating in sports (60%).
Figure 1.
Hedonic Response to Everyday Activities: Percent of Patients Reporting Moderately-or-Greater Hedonic Response during Treatment with XR-NTX.
There were several significant mean differences on ratings of pleasure from everyday activities between the patients who reported drinking during the past 90 days compared to those who reported not drinking during the past 90 days (Table 2). In particular, non-drinking patients reported more pleasure from eating sweets (p = .035), reading (p = .0013), and shopping (p = .0092), compared to those who were drinking alcohol in the past 90 days.
Table 2.
Mean Hedonic Response to Pleasurable Activities Following 3-5 Years of XR-NTX Treatment
| Activity | Mean ± SD (N) Patients Who Reported No Drinking |
Mean ± SD (N) Patients Who Reported Drinking |
Effect Size vs. Alcohol |
P-Value vs. Alcohol |
|---|---|---|---|---|
| Eating good food | 4.14 ± 0.96 (21) | 3.94 ± 1.12 (53) | 1.08 | <0.001 |
| Listening to Music | 4.00 ± 1.03 (20) | 3.92 ± 0.93 (51) | 0.99 | <0.001 |
| Being with friends | 3.85 ± 1.18 (20) | 3.54 ± 1.01 (50) | 0.71 | <0.001 |
| Reading | 4.26 ± 0.87 (19) | 3.23 ± 1.23 (52) | 0.44 | 0.003 |
| Eating spicy food | 4.00 ± 1.07 (15) | 3.24 ± 1.43 (41) | 0.48 | 0.004 |
| Sexual relations | 3.92 ± 1.16 (12) | 3.30 ± 1.23 (43) | 0.54 | 0.001 |
| Watching Sports | 3.71 ± 1.07 (14) | 3.11 ± 1.35 (37) | 0.32 | 0.06 |
| Playing cards or board games or video games | 3.56 ± 1.13 (9) | 3.00 ± 1.24 (32) | 0.56 | 0.003 |
| Eating sweets | 3.43 ± 1.50 (21) | 2.75 ± 1.09 (51) | 0.16 | 0.26 |
| Exercising | 2.93 ± 1.33 (15) | 2.90 ± 1.21 (42) | 0.25 | 0.12 |
| Shopping | 3.26 ± 1.41 (19) | 2.45 ± 1.01 (51) | −0.04 | 0.79 |
| Gambling | 2.75 ± 0.96 (4) | 2.41 ± 1.18 (22) | −0.37 | 0.096 |
| Drinking alcohol | --- | 2.57 ± 1.10 (53) | --- | --- |
Notes. N represents number of patients reporting the activity in the prior 90 days. Effect sizes are calculated as the mean of the difference scores (each item mean minus alcohol mean) divided by the standard deviation of the difference scores for patients who reported drinking during the past 90 days. P-values from paired t-tests comparing the mean of each activity to the mean hedonic response from drinking alcohol for those who reported drinking during the past 90 days.
Compared to ratings of pleasure from drinking alcohol, mean pleasure ratings were significantly higher for most other activities: listening to music, sex, reading, being with friends, eating good food, eating spicy food, and playing video/card games (all p-values < 0.005) (Table 2). Among patients who gambled in the previous 90 days (n = 22), hedonic response to this activity was not significantly different (p = 0.096) from hedonic response to drinking alcohol. Effect sizes comparing everyday activities to drinking alcohol were moderate to large for eating good food, listening to music, being with friends, playing games, sex, eating spicy food and reading (Table 2).
No significant interaction effects emerged in the exploratory repeated measures analyses for dosage or duration effects on the difference between pleasure from alcohol and pleasure from any of the other activities.
DISCUSSION
The availability of data from two long-term open-label extension studies of continuous treatment with XR-NTX provided a unique opportunity to evaluate the hedonic effect of 3–5 years of continuous blockade of the μ-opioid receptor in an alcohol dependent patient sample. In this exploratory study, continuous long-term treatment with XR-NTX was found to primarily inhibit the hedonic response associated with drinking alcohol, while sparing the experience of pleasure associated with other activities such as listening to music, being with friends, sex, eating good food, and reading. Of 13 activities studied, gambling, drinking alcohol and shopping received the lowest mean ratings of hedonic experience among patients treated with XR-NTX for 3–5 years. Thus, the data indicate that clinical use of XR-NTX by alcohol dependent patients is compatible with pleasurable response to a variety of everyday activities while selectively inhibiting the pleasure associated with drinking alcohol.
Previous literature has suggested that opioid antagonists may impact hedonic response to activities such as exercise,17–19 eating21 and sex.22 Eating, exercise and sex were not found to have reduced pleasure (in comparison to drinking alcohol) in the current study. There are several potential explanations for these differences between the current study and previous studies with oral naltrexone. For one, the methodologies of the studies are considerably different. Studies of the impact of oral naltrexone or naloxone on exercise, eating and sex were typically short-term studies that assessed acute subject reactions 1–2 hours after administration (sometimes in large doses) of the opioid antagonist.19,22,32 In contrast, the current study evaluated pleasure in various activities occurring in the prior 90 days in patients who had received 3–5 years of continuous XR-NTX treatment. These reports are also consistent with the clinical experience of the first author who has prescribed oral naltrexone for opioid addicts since 1974 and to alcoholics since 1983. Patients who have been on naltrexone for an extended period of time have rarely complained of lack of pleasure in daily life while receiving the medication.
Differences in dosage and drug metabolism may also explain the effects found here compared to those in the literature. The dosage of XR-NTX is not directly comparable to oral naltrexone because of the differences in mode of administration. Due to the continuous release of naltrexone, the approved monthly dose (380 mg) of XR-NTX is substantially less than the dosage (1500 mg) of oral naltrexone that would be administered over the course of a month (50 mg/day for 30 days). However, XR-NTX has reduced first-pass metabolism compared to oral naltrexone.25,33 Oral naltrexone is primarily metabolized to 6β-naltrexol, with resultant plasma concentrations of 6β-naltrexol more than 20X higher than naltrexone.26,34 While the effects of oral naltrexone on the brain are likely due to both 6β-naltrexol and naltrexone, XR-NTX injections result in mostly naltrexone entering the brain. In addition, plasma levels fluctuate dramatically over a 24-hour period with oral naltrexone.35 In contrast, with XR-NTX, plasma levels are fairly continuous within each day, with negligible accumulation on repeat dosing.26 The area-under-the-curve steady state blood concentration levels of naltrexone using XR-NTX are actually about 4 times that of oral naltrexone over a 28 day period.26 However, the maximum level of plasma 6β-naltrexol achieved with oral naltrexone (approximately 1 hour after dosing) is about 5 times higher than that occurring XR-NTX.26 The previous studies of the immediate impact of opioid antagonists on hedonic response to various activities may have been influenced by measuring hedonic response at or near maximum levels of 6β-naltrexol.
A secondary finding of the current study was that patients who were continuing to drink alcohol reported less pleasures from certain activities (eating sweets, reading, shopping) compared to those who were not drinking alcohol in the previous 90 days. Although alcohol is known to produce a euphoric state, potentially mediated through the release of endogenous opioids,36 alcohol dependent individuals have been found to be have dysphoria upon drinking alcohol.24 This dysphoria may be responsible for reduced pleasure from some everyday activities. However, it may also be important to consider that this effect of drinking on hedonic ratings was found in the context of patients receiving XR-NTX. When individuals drink alcohol in the presence of naltrexone, they report increased subjective fatigue and nausea37, i.e., adverse consequences of alcohol consumption. In the current study, these interactive effects between naltrexone and alcohol may have contributed to reduced pleasure from certain activities among those who were drinking in the past 90 days compared to those who were not drinking. Despite this potential interaction between naltrexone and drinking, pleasure from most everyday activities remained significantly higher (with levels in the moderate to highly pleasurable range) than the pleasure reported from drinking alcohol.
Several limitations of this exploratory study are important to note. First, the absence of a baseline hedonic effect measurement precludes us from knowing to what extent patient ratings changed after administration of XR-NTX. Differences between pleasure from alcohol and pleasure from other activities may have pre-dated treatment with XR-NTX. Second, without a placebo control arm in the extension studies, it is unknown whether the effects that were obtained are directly attributable to the medication. Third, these data are from a self-selected sample of patients who remained on XR-NTX for an extended time period and as such may not be representative of the larger population of alcohol dependent individuals. Patients who have remained on XR-NTX for a long period of time may be those individuals who can tolerate it better and therefore may be less likely to also have a reduction in experienced pleasure from daily activities. Finally, the measure used has not been validated in previous studies. However, the data presented here showing differential patterns of reduction in hedonic response across a variety of activities provides some initial validity for the instrument.
The above limitations notwithstanding, the nearly continuous persistence (>99%) on XR-NTX for such a long duration makes this set of findings an important contribution to our understanding of the μ-opioid system’s role in hedonic response and its differentiation. All prior published research in this area consist of short-term studies with relatively focused categories of reward producing behavior. In contrast, this exploratory study demonstrates that, with the emergence of XR-NTX, research on the endorphin system’s role in hedonic response can move out of the short-term human laboratory paradigm and into naturalistic, longitudinal settings. Furthermore, these data characterize the hedonic impact of a clinical treatment for alcohol dependence as relatively selective for alcohol dependence. Future prospective randomized, placebo-controlled studies with baseline assessment of hedonic responses to a variety of activities are needed to confirm the preliminary findings from the current study.
Acknowledgments
Declaration of Interest
Funding for the study was provided by Alkermes, Inc., Cambridge, MA (manufacturer of XR-NTX). Alkermes provided funding for the preparation of the manuscript and participated in the analysis of the data, the writing of the manuscript, and the decision to submit the paper for publication.
Dr. O’Brien in the past 5 years has served as a paid consultant to Alkermes, Cephalon, Merck, US World Meds, Abbott and Purdue Pharma; and is supported in part by the National Institutes of Health Center Grant DA P60-001586-22 and AA RO1-17164-01. Dr. Pettinati receives research support from Alkermes and Eli Lilly. Drs. Gastfriend and Forman are full-time employees of Alkermes, the company that markets XR-NTX. Dr. Schweizer has received income as a consultant from Alkermes, Pfizer, Eli Lilly, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Neurocrine Biosciences, and Memory Pharmaceuticals.
Appendix 1
Hedonic Response Survey
In the past 90 days, how pleasure were the following activities?
| Not applicable |
Not at all |
A little bit |
Moderately | Quite a bit |
Very much so |
|
|---|---|---|---|---|---|---|
| a) Listening to music | ○ | ○ | ○ | ○ | ○ | ○ |
| b) Eating good food | ○ | ○ | ○ | ○ | ○ | ○ |
| c) Playing cards, board games or video games | ○ | ○ | ○ | ○ | ○ | ○ |
| d) Watching sports | ○ | ○ | ○ | ○ | ○ | ○ |
| e) Exercising or participating in sports | ○ | ○ | ○ | ○ | ○ | ○ |
| f) Sexual relations | ○ | ○ | ○ | ○ | ○ | ○ |
| g) Reading | ○ | ○ | ○ | ○ | ○ | ○ |
| h) Gaming | ○ | ○ | ○ | ○ | ○ | ○ |
| i) Eating with friends | ○ | ○ | ○ | ○ | ○ | ○ |
| j) Eating spicy food | ○ | ○ | ○ | ○ | ○ | ○ |
| k) Drinking alcoholic beverages | ○ | ○ | ○ | ○ | ○ | ○ |
| l) Eating sweets (candy, cake, ice cream) | ○ | ○ | ○ | ○ | ○ | ○ |
| m) Shopping | ○ | ○ | ○ | ○ | ○ | ○ |
REFERENCES
- 1.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.National Institute on Alcohol Abuse and Alcoholism. [Accessed June 5, 2008];Helping Patients Who Drink Too Much: A Clinician’s Guide. 2005 ed. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/ clinicians_.htm.
- 3.Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- 4.Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King A, Volpicelli J, Frazer A, O’Brien C. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley SS, Jaffe AJ, Rode S, Rounsaville BJ. Experience of a ’slip” among alcoholics treated with naltrexone and placebo. Am J Psychiatry. 1996;153:281–283. doi: 10.1176/ajp.153.2.281. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli JR, Watson MT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- 9.Mocsary Z, Bradberry C. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fisher 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middaugh LD, Szumlinski KK, Patten YV, Marlowe ALB, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 13.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue–induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 16.Tsai HW, Shui HA, Liu HS, Tai MY, Tsai YF. Monoamine levels in the nucleus accumbens correlate with male sexual behavior in middle-aged rats. Pharmacol Biochem Behav. 2006;83:265–270. doi: 10.1016/j.pbb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Daniel M, Martin AD, Carter J. Opiate receptor blockade by naltrexone and mood state after acute physical activity. Br J Sports Med. 1982;26:111–115. doi: 10.1136/bjsm.26.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janal MN, Colt EWD, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1994;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 19.Järvekülg A, Viru A. Opioid receptor blockade eliminates mood effects of aerobic gymnastics. Int J Sports Med. 2002;23:155–157. doi: 10.1055/s-2002-23168. [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- 21.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MR, Checkley SA, Seckl JR, Lightman SL. Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab. 1990;71:1056–1058. doi: 10.1210/jcem-71-4-1056. [DOI] [PubMed] [Google Scholar]
- 23.Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry. 1998;59:159–164. [PubMed] [Google Scholar]
- 24.Freed EX. Alcohol and mood: an updated review. Int J Addict. 1978;13:173–200. doi: 10.3109/10826087809039273. [DOI] [PubMed] [Google Scholar]
- 25.Bartus RT, Emerich DF, Hotz J, et al. Vivitrex, an injectable, extended-release formulation of naltrexone, provides pharmacokinetic and pharmacodynamic evidence of efficacy for 1 month in rats. Neuropsychopharmacology. 2003;28:1973–1982. doi: 10.1038/sj.npp.1300274. [DOI] [PubMed] [Google Scholar]
- 26.Dunbar JD, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single and multiple dose pharmacokinetics of long-acting naltrexone. Alcohol Clin Exp Res. 2006;30:480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BA, Ait-Daoud N, Aubin HJ, et al. A pilot evaluation of the safety and tolerability of repeat dose administration of long-acting injectable naltrexone (Vivitrex) in patients with alcohol dependence. Alcohol Clin Exp Res. 2004;28:1356–1361. doi: 10.1097/01.alc.0000139823.30096.52. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley S, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone (XR-NTX) in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharm. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- 29.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 30.Alkermes, Data on File [Google Scholar]
- 31.Volpicelli JR, Pettinati HM, McLellan AT, O’Brien CP. Combining Medication and Psychosocial Treatments for Addictions: The BRENDA Approach. New York: The Guilford Press; 2001. [Google Scholar]
- 32.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and references for sweet high-fat foods: evidence for opioid involvement. Physiol Behav. 1992;51:371–379. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- 33.Wall ME, Brine DR, Perez-Reyes M. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab Dispos. 1981;9:369–375. [PubMed] [Google Scholar]
- 34.Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- 35.Verebey K. The clinical pharmacology of naltrexone: pharmacology and pharmacodynamics. NIDA Res Monogr. 1980;28:147–158. [PubMed] [Google Scholar]
- 36.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- 37.McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]