Abstract
Background
Limited access to HIV testing of children impedes early diagnosis and access to antiretroviral therapy. Our objective was to evaluate the feasibility and acceptability of routine pediatric HIV testing in an urban, fee-for-service, outpatient clinic in Durban, South Africa.
Methods
We assessed the number of patients (0–15 years) who underwent HIV testing upon physician referral during a baseline period. We then established a routine, voluntary HIV testing study for pediatric patients, regardless of symptoms. Parents/caretakers were offered free rapid fingerstick HIV testing of their child. For patients <18 months, the biological mother was offered HIV testing and HIV DNA polymerase chain reaction was used to confirm the infant’s status. The primary outcome was the HIV testing yield, defined as the average number of positive tests per month during the routine compared with the baseline period.
Results
Over a 5-month baseline testing period, 931 pediatric patients registered for outpatient care. Of the 124 (13%) patients who underwent testing on physician referral, 21 (17%, 95% confidence interval: 11–25%) were HIV infected. During a 13-month routine testing period, 2790 patients registered for care and 2106 (75%) were approached for participation. Of these, 1234 were eligible and 771 (62%) enrolled. Among those eligible, 637 (52%, 95% confidence interval: 49–54%) accepted testing of their child or themselves (biological mothers of infants <18 months). There was an increase in the average number of HIV tests during the routine compared with the baseline HIV testing periods (49 versus 25 tests/month, P = 0.001) but no difference in the HIV testing yield during the testing periods (3 versus 4 positive HIV tests/month, P = 0.06). However, during the routine testing period, HIV prevalence remains extraordinarily high with 39 (6%, 95% confidence interval: 4–8%) newly diagnosed HIV-infected children (median 7 years, 56% female).
Conclusions
Targeted and symptom-based testing referral identifies an equivalent number of HIV-infected children as routine HIV testing. Routine HIV testing identifies a high burden of HIV and is a feasible and moderately acceptable strategy in an outpatient clinic in a high prevalence area.
Keywords: routine HIV testing, pediatric, adolescent, South Africa, outpatient clinic
Of the approximately 2 million children in need of antiretroviral therapy (ART) globally, only 500,000 (23%) are on treatment.1 The large gap in treatment is concerning, given the rapid clinical progression of perinatally infected children not on ART and the large improvement in health and survival of those on treatment.2–4
A major barrier to increase access to HIV treatment in children is the limited testing opportunities after the postnatal period.5 In 2007, the World Health Organization recommended routine HIV testing, also known as provider-initiated testing, for children in inpatient and outpatient settings in epidemic areas.6 Although these guidelines endorse offering HIV testing irrespective of signs or symptoms, they offer little implementation guidance.6
Previous studies have examined the feasibility and acceptability of routine pediatric HIV testing in inpatient wards7–11 and newborn immunization clinics.12,13 Our objective was to assess the feasibility and acceptability of routine pediatric HIV testing in an urban outpatient clinic in an epidemic setting in Durban, South Africa.
MATERIALS AND METHODS
Study Setting
McCord Hospital is located in Durban, KwaZulu Natal, the province with the highest HIV prevalence in South Africa.14 McCord Hospital is an urban, state-aided facility where patients pay a subsidized fee for services. McCord has a general medical outpatient clinic and serves a predominantly Zulu-speaking population. The outpatient consultation fee is ZAR 280–300 (US $33–36). Patients <12 years receive care from dedicated physician staff, whereas those ≥12 years receive adult care.
McCord Hospital has a well-established HIV clinic that has served >10,000 adult and pediatric patients over the last 10 years.15,16 Patients pay an inclusive fee (ZAR 50–150 = US $6–18 per/visit) for HIV services.16–18 We received study approval from the McCord Hospital Ethics Committee (Durban, South Africa) and Boston Children’s Hospital (Protocol 10-06-0302).
HIV Testing Procedures
As per the South African National Guidelines, the testing procedure included pretest and posttest counseling, consent and 2 concurrent free rapid blood HIV 1/2 tests for children ≥18 months, the biological mothers of those <18 months and for infants <18 months presenting without their biological mothers.19 Because consent is needed for pediatric HIV testing, opt-out testing is not possible. Those infants <18 months whose test was positive or whose biological mother had positive testing were deemed HIV exposed and referred for HIV DNA polymerase chain reaction (PCR). The DNA PCR was processed at the National Health Laboratory free of charge or for expedited results for a fee (ZAR 390 = US $47). Mothers of infants undergoing DNA PCR were asked to return in 2–4 weeks to collect results. Newly diagnosed HIV-infected patients were referred for a free CD4 cell count and to the HIV clinic.
HIV Testing During the Baseline Period
The outpatient clinic has had an adult routine HIV testing program since 2008.20 Per hospital guidelines, patients ≥12 years who are HIV status unknown/negative are offered routine HIV testing by a counselor; patients can also self-refer for testing.20 Per South African law, patients ≥12 years can consent for HIV testing without guardian approval.17 Before our study, patients <12 years were referred for testing if the physician suspected HIV or on caretaker request. Patients <5 years were also referred for testing using the World Health Organization–integrated management of childhood illness guidelines.21,22
From May to September 2010, we prospectively collected outpatient registrations and HIV testing data for patients 0–15 years. We collected the number of registrations when an HIV counselor was available (Monday–Friday, 7:00 am–4:00 pm), number of tests, and the reason for testing referral. We recorded the number of positive HIV tests and the number/results of those obtaining a CD4 count.
HIV Testing During the Routine Testing Period
From October 2010 to November 2011, we offered routine, voluntary HIV testing to patients 0–15 years presenting to the outpatient clinic within a study context as this was not the current standard of care. Study activities were performed by a dedicated HIV counselor and included consent/assent, HIV testing and caretaker questionnaire. Eligibility criteria included written biological parent or primary caretaker consent for all participants, consent from those 7 to 11 years, and consent from those ≥12 years. Patients who were known HIV infected or presented without their biological parent/primary caretaker were ineligible. Patients who were critically ill were ineligible and were tested outside the study if clinically indicated.
All patients and their caretakers were referred to the pediatric HIV counselor after triage and offered study participation. We recorded the reason that caretakers were not eligible or declined participation. After the pediatric HIV counselor obtained consent, he performed the HIV test and administered the caretaker questionnaire. The pediatric HIV counselor contacted newly diagnosed HIV-infected patients who had not had an initial HIV clinic visit 1 month after diagnosis and then every 2 weeks for a minimum of 6 months thereafter.
Data collected during the routine testing period included the number of outpatient registrations (Monday–Friday, 7:00 am–4:15 pm), tests performed, positive HIV tests, and the number and CD4 count results. For those newly diagnosed HIV infected, we recorded the number who had an initial HIV clinic visit and initiated ART over a minimum 6-month follow-up period.
Caretaker Questionnaire
Caretakers were offered a verbal questionnaire, irrespective of consenting for HIV testing. The questionnaire was administered before receiving HIV test results and included demographics and caretaker HIV testing history and status.13,23–25 Caretakers were asked: “How comfortable did you feel about your child being offered an HIV test at this clinic today” and also about the potential advantages/disadvantages of routine HIV testing of children in the outpatient setting.
Statistical Analysis
The primary outcome of the study was the HIV testing yield, defined as the average number of positive tests per month during the routine compared with the baseline period. We compared the average number of HIV tests per month during the 2 periods. We also measured the HIV prevalence among those tested in each study period. Caretaker acceptability was measured as the proportion of eligible patients who underwent testing. Acceptability was also assessed from the response to the question: “how comfortable did you feel about your child being offered an HIV test at this clinic today.”
We compared categorical data from the 2 testing periods using the χ2 test and the Student’s t test for continuous variables. Median CD4 counts were compared using the Wilcoxon rank sum test. All analyses were performed using R software (R version 2.11.1).26
RESULTS
Baseline HIV Testing Period
During the 5-month baseline HIV testing period, 931 pediatric patients 0–15 years registered in the outpatient clinic. One hundred twenty-four (13%) underwent HIV testing and 21 (17%, 95% confidence interval [CI]: 11–25%) were HIV infected. On average, there were 25 pediatric HIV tests per month and 4 new pediatric HIV diagnoses per month (Table 1). The common reasons for HIV testing referral were HIV exposure (n = 12), pneumonia (n = 12) and weight loss/malnutrition (n = 6).
TABLE 1.
Number of Registrations, HIV Tests, Positive HIV Tests and Characteristics of Newly Diagnosed HIV-infected Children in the Baseline Compared With the Routine Pediatric HIV Testing Period
| Baseline Testing (May–Sept 2010) |
Routine Testing (Oct 2010–Nov 2011)* |
P | |
|---|---|---|---|
| Total number of registrations | 931 | 2790 | NA |
| Average number of registrations per month | 186 | 215 | 0.08 |
| Total number of HIV tests | 124 | 637 | NA |
| Average number of HIV tests per month | 25 | 49 | 0.001 |
| Total number of positive HIV tests | 21 | 39 | NA |
| Average number of positive HIV tests per month | 4 | 3 | 0.06 |
| Newly diagnosed HIV-infected children | N = 21 | N = 39 | |
| Female | 14 (67%) | 22 (56%) | 0.6 |
| Median age (yr, interquartile range) | 9 (3–11) | 7 (4–11) | 0.8 |
| Age <5 yr | 6 (29%) | 12 (31%) | 1.0 |
| Had CD4 count | 17/21 (est.) | 32/39 (82%) | 1.0 |
| Median CD4 percentage/count (cells/mm3) | 17/209 | 13/288 | 0.4 |
| Had first HIV clinic visit | NA | 31/39 (79%) | NA |
| On ART if eligible | NA | 26/30 (87%) | NA |
| Death | NA | 3/39 (8%) | NA |
| Loss to follow-up | NA | 4/39 (10%) | NA |
| Repeat rapid testing negative at other clinic | NA | 1/39 (3%) | NA |
The study enrolled for 13 months.
NA indicates not applicable.
Newly Diagnosed HIV-Infected
Among the 21 who were HIV infected, 14/21 (67%) were female and their median age was 9 years (interquartile range 3–11 years; Table 1). Of these, 17 (81%) had a CD4 count and median CD4 was 17%, 209 cells/mm3 (interquartile range 67–757 cells/mm3).
Routine HIV Testing Period
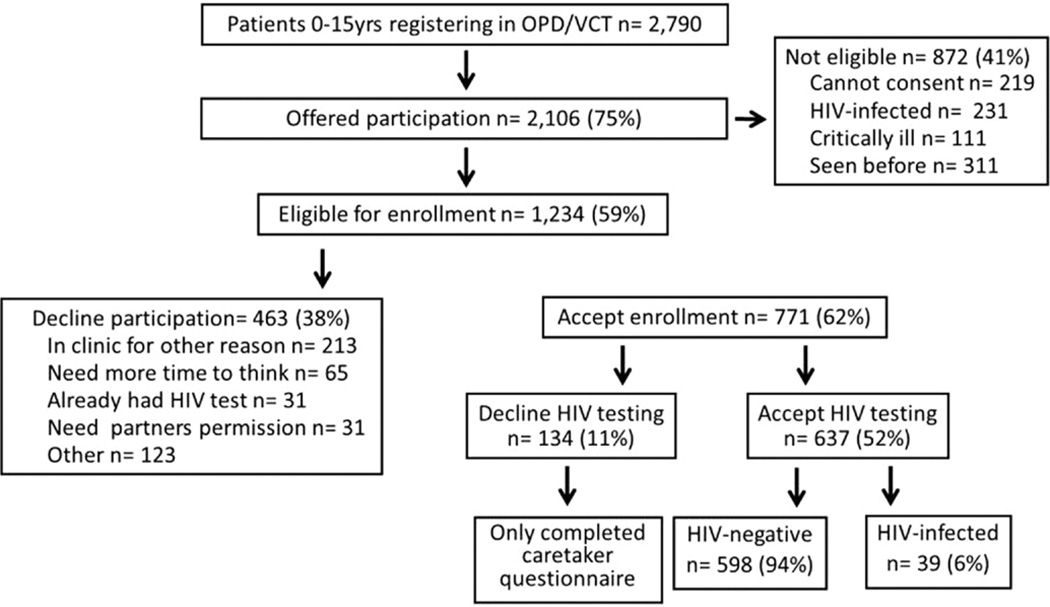
During the 13-month routine testing period, 2790 pediatric patients 0–15 years registered in the outpatient clinic. We offered participation to 2106 (75%) patients, and of these, 1234 (59%) were eligible (Fig. 1) and 219/872 (25%) of the ineligible patients presented with a caretaker that could not consent (Fig. 1). Seven hundred sevenety-one (62%) eligible children and caretakers participated; the median age of all participating children was 3 years and 47% were female. Of the 463 (38%) who declined study participation, 213 “were there for another reason,” 65 “needed more time to think,” 31 had “already been HIV tested” and 31 “needed their partner’s permission” (Fig. 1). In all, 637/1234 (52%, 95% CI: 49–54%) accepted routine HIV testing for their child or for themselves if they were the biological mother of an infant <18 months (Fig. 1).
FIGURE 1.
Routine pediatric HIV testing study participation flow chart.
On average, there were 49 pediatric HIV tests per month and 3 new pediatric HIV diagnoses per month (Table 1). Most participants had 2 concurrent rapid blood HIV tests, except 26 who had a DNA PCR. Compared with baseline, there was a significant increase in the number of HIV tests during the routine testing period (49 versus 25 tests/month, P = 0.001).
During the routine testing period, there were 39 new HIV diagnoses with an HIV prevalence among those tested of 39/637 (6%, 95% CI: 4–8%). There was no difference in the HIV testing yield during the routine compared with baseline periods (3 versus 4 positive HIV tests/month, P = 0.06; Table 1).
Newly Diagnosed HIV-Infected
Among the newly diagnosed HIV-infected participants, 22/39 (56%) were female and the median age was 7 years (interquartile range 4–11 years; Table 1). Their median age was lower than the baseline period (7 versus 9 years, P = 0.8). A total of 32/39 (82%) had a CD4 count, and the median results were 13%, 288 cells/mm3 (interquartile range 181–523 cells/mm3; Table 1).
After a minimum of 6 months of follow-up, 31/39 (79%) subjects were in HIV care and nearly all ART-eligible subjects were receiving treatment (26/30, 87%). Among the remaining children, 3/39 (8%) died, 4/39 (10%) were lost to follow-up and 1/39 (3%) reportedly retested HIV negative at another clinic (Table 1).
Among the newly diagnosed HIV-infected children, 26/39 (67%) caretakers completed a questionnaire. Based on the responses, 7 (27%) of the HIV-infected children had a history of pneumonia and 4 (15%) tuberculosis. Only 7 (27%) of the newly diagnosed HIV-infected children had a prior HIV test and 19/26 (73%) presented with their biological mother. Of these mothers, 4/19 (21%) reported their own HIV status as negative; the median age of these 4 children was 7 years (interquartile range 5–12 years).
Caretaker Acceptability
Among the 608 (79%) caretakers who completed the questionnaire, 586/608 (96%) reported a prior HIV test and 188/586 (32%) reported being HIV infected (Table 2). The cohort characteristics were the same irrespective of testing uptake, except that caretakers who accepted testing were more likely to receive a government welfare grant than those who declined (40% versus 17%, P < 0.01, data not shown). Among the biological mothers, 516/608 (85%) completed the questionnaire, 164/516 (32%) reported being HIV infected and of these, only 105/164 (64%) of their children had a prior HIV test (data not shown).
TABLE 2.
Caretaker Characteristics and Perceptions of Routine Pediatric HIV Testing
| Total N = 608 (%) |
|
|---|---|
| Female | 537 (88) |
| Relationship to child | |
| Mother | 516 (85) |
| Father | 68 (11) |
| Grandparent | 11 (2) |
| Step parent | 4 (0.7) |
| Aunt | 4 (0.7) |
| Ethnicity | |
| Black-South African | 500 (83) |
| Asian | 40 (7) |
| Black-Other | 31 (5) |
| Colored (mixed race) | 26 (4) |
| White | 9 (1.5) |
| Education | |
| High school or higher | 460 (77) |
| Receives government welfare grant | 211 (36) |
| Previous HIV testing | 586 (96) |
| HIV infected | 188 (32) |
| How comfortable did you feel with your child being offered an HIV test at this clinic today? | 3 (0.5) |
| Not comfortable | 10 (1.6) |
| Somewhat not comfortable | 84 (14) |
| Neutral | 85 (14) |
| Comfortable | 407 (67) |
| Very comfortable | 19 (3) |
| Did not answer question | |
| Possible advantages of routine pediatric HIV testing | |
| Confirms child’s status | 602 (99) |
| Allows for ART | 605 (99) |
| Allows for opportunistic infection prophylaxis | 605 (99) |
| Helps determine whether safe to breast-feed baby | 588 (97) |
| Gives peace of mind | 589 (97) |
| Useful to share with family | 585 (96) |
| Possible disadvantages of routine pediatric HIV testing | |
| Partner may leave them | 129 (21) |
| Other people may find out status | 89 (15) |
| Not ready to find out child’s status | 44 (7) |
| No support in taking care of child | 23 (4) |
| Find out child’s status | 18 (3) |
| Find out own status | 20 (3) |
Caretaker’s perspectives on pediatric routine HIV testing were favorable when assessed before HIV testing: 407 (67%) were very comfortable, 85 (14%) were comfortable and 84 (14%) were neutral with routine pediatric HIV testing (Table 2). Potential disadvantages included the following: 129 (21%) expressed that their partner might leave them based on the child’s status and 89 (15%) commented that other people may find out the child’s status (Table 2). There was no difference in these responses by testing uptake (data not shown).
Family-centered HIV Testing
Although we did not offer HIV testing to accompanying family members, an additional 146 (19%) family members requested a test. Among the 96 (66%) mothers, 44 (30%) fathers and 6 (4%) siblings who underwent testing, there were a total of 9 (11%) new diagnoses with 7 new diagnoses in mothers and 2 in fathers.
DISCUSSION
Routine HIV testing in the outpatient clinic resulted in nearly 2 times the average number of pediatric tests per month compared with testing by physician and self-referral (49 versus 25 tests/month, P = 0.001). We did not detect a difference in the mean number of new pediatric HIV diagnoses per month between the routine and the baseline testing periods in this public-private, fee-for-service clinic. However, the HIV prevalence among children presenting for acute medical services during the routine testing period was extraordinarily high at 6% (95% CI: 4–8%). During routine testing, 52% (95% CI: 49–54%) of eligible caretakers accepted HIV testing of their child.
Previous studies have documented successful implementation of routine pediatric HIV testing programs in inpatient wards,7–11 immunization clinics12,13 and home-based testing for preschool children.27 Routine pediatric HIV testing has also been shown to be feasible in primary healthcare clinics among South African infants28 and Zimbabwean adolescents 10–18 years.29 We demonstrate successful implementation and a large increase in the number of HIV tests per month with a routine pediatric testing program in an outpatient clinic where children receive acute medical services.
The feasibility of this routine pediatric HIV testing program was influenced by having a dedicated HIV counselor, staff support and testing supplies. Our routine HIV testing office was located within the pediatric outpatient clinic and adjacent to the triage area, which likely facilitated testing. Our newly diagnosed HIV-infected participants had follow-up and access to a pediatric HIV clinic.
Previous studies have reported an HIV prevalence of 7.5% among children 2–60 months in primary healthcare clinics in Kwa-Zulu Natal.30 Our study also found a high HIV prevalence of 6% among children 0–15 years presenting to an outpatient clinic for acute medical services in the same province. Unlike previous routine HIV testing studies in the pediatric inpatient9,10 and adult outpatient clinic setting,20 we were unable to show an increase in the number of new HIV diagnoses per month. The similar number of new HIV diagnoses between the 2 testing periods is likely due to a number of factors. Our study population is not representative of the population of children who receive care in the government sector where care is free. Because we offered participation to a high proportion of children and some sought care more than once during the study period, the pool of eligible participants and thus, HIV-infected children likely decreased over time. In addition, children were only offered testing during “office hours” (8 hours/d, on week days). Critically ill patients, who are more likely to have a high HIV prevalence,8,31 had an opportunity to test out of the study.
The acceptability of all eligible caretakers was moderate (52%, 95% CI: 49–54%). In our analysis, we conservatively assumed that all the 463 caretakers who declined study participation would have refused HIV testing of their child. Among surveyed caretakers, the acceptability of routine pediatric HIV testing was high. Caretakers reported a high level of comfort with routine pediatric HIV testing and acknowledged potential advantages of this testing strategy. A number of previous studies have also reported the acceptability of routine pediatric HIV testing in different settings.7–13,27,29 Some of the caretakers we surveyed endorsed that routine pediatric HIV testing have some disadvantages including other people finding out their child’s status. Caretakers might be encouraged to accept HIV testing of their child if the confidentiality of the testing process is highlighted during pretest counseling and testing is conducted in a private space.
Some caretakers reported that routine pediatric HIV testing was also disadvantageous because they feared that their partner may leave them depending on their child’s status. This highlights the importance of exploring family-centered testing opportunities.32 Although the utility of partner-based testing has been explored in the prevention of mother to child transmission (PMTCT) programs,33 there are limited studies on the efficacy of family-centered HIV testing in the outpatient setting.34 In our study, an additional 146 (19%) of participating caretakers requested an HIV test of which 44 (30%) were fathers who are typically a difficult-to-access population in testing programs.35,36 These findings underscore the role of routine pediatric testing as a venue for family-centered testing.
As awareness of pediatric HIV increases and ART during pregnancy is more available, the context for routine pediatric HIV testing is changing. Although PMTCT programs and the World Health Organization–integrated management of childhood illness program are used to identify HIV-infected children in South Africa,5,21,22,37 there are limitations to these programs including loss to follow-up38–40 and the mother correctly knowing her HIV status.21,22 In our study, the median age of newly diagnosed HIV-infected children was 7 years in the routine and 9 years in the baseline testing periods. As PMTCT programs have improved over time, it is possible that routine testing would identify older children who were vertically infected and have not been diagnosed. In addition, 36% of HIV-infected mothers who completed the caretaker questionnaire had not had a prior HIV test for their child. Furthermore, 21% of biological mothers of newly diagnosed HIV-infected children reported an HIV-negative status, which could reflect that the mothers seroconverted after their last HIV test, did not know their status or did not want to disclose. Taken together, the large number of HIV-infected mothers who had not had their child HIV tested and the misreporting of maternal HIV status highlight the shortcomings of existing testing programs and the role of routine pediatric HIV testing programs in identifying children who are missed by these efforts.
This study has several additional limitations. Our study population is not representative of the large volume government primary healthcare clinics. Therefore, we cannot make any definitive conclusions about the efficacy of routine pediatric HIV testing in other outpatient clinics in epidemic settings. Although opt-out HIV testing may have changed our testing acceptability, this testing scheme was not possible because consent is needed for pediatric HIV testing as per National Testing Guidelines.19 The largest limitation of our study is that we have minimal information on the 463 caretakers who declined study participation. Understanding the viewpoint of this group would allow a more comprehensive assessment of the range of opinions of routine testing for children.
Routine pediatric HIV testing in an outpatient clinic increased testing, identified a large burden of HIV infection and was feasible and moderately acceptable. Although we were unable to show an increase in the HIV testing yield between the routine and the baseline testing periods, future studies are needed in a more representative pediatric population to determine whether routine testing is more beneficial and cost-effective than targeted, symptom-based testing referral.
ACKNOWLEDGMENTS
The authors thank the children, families and HIV counselors, social workers and psychologists at McCord Hospital. In addition, they thank Tamaryn Crankshaw, Emily Walsh and Senica Chetty.
L.R.-A., D.P. and I.V.B. conceived and designed the study; L.R.-A. and S.S. collected and assembled the data; L.R.-A., F.N. and E.L. analyzed the results; L.R.-A., F.N., J.G., E.L., R.P.W. and I.V.B. interpreted the data; L.R.-A. and I.V.B drafted the article; and L.R.-A., F.N., D.P., J.G., E.L., R.P.W. and I.V.B. critically reviewed the article for intellectual content.
Supported by the Boston Children’s Hospital Aerosmith Endowment Grant (L.R.-A.), the Harvard Global Health Institute (L.R.-A.), National Institute of Child Health and Human Development T32 HD 055148-02 (L.R.-A.), National Institute of Allergy and Infectious Diseases (NIAID) T32 AI 007433 (L.R.-A.), NIAID P30 AI060354 (Harvard University Center for AIDS Research) (L.R.-A.), National Institute of Mental Health R01 MH090326 (I.V.B.), R01 MH073445 (R.P.W.), Harvard University Program on AIDS and the Claflin Distinguished Scholar Award of the Massachusetts General Hospital (I.V.B.).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.World Health Organization. Pediatric HIV data and statistics. [Accessed June 8, 2012]; Available at: http://www.who.int/hiv/topics/paediatric/data/en/index1.html.
- 2.Meyers T, Moultrie H, Naidoo K, et al. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis. 2007;196(suppl 3):S474–S481. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, et al. Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 5.Kellerman S, Essajee S. HIV testing for children in resource-limited settings: what are we waiting for? PLoS Med. 2010;7:e1000285. doi: 10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidance on provider-Initiated HIV testing and counseling in health facilities: 2007. [Accessed August 1, 2012]; Available at: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf.
- 7.Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanyenze RK, Nawavvu C, Ouma J, et al. Provider-initiated HIV testing for paediatric inpatients and their caretakers is feasible and acceptable. Trop Med Int Health. 2010;15:113–119. doi: 10.1111/j.1365-3156.2009.02417.x. [DOI] [PubMed] [Google Scholar]
- 9.McCollum ED, Preidis GA, Golitko CL, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–e81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel R, Kamthunzi P, Mwansambo C, et al. Effect of provider-initiated testing and counselling and integration of ART services on access to HIV diagnosis and treatment for children in Lilongwe, Malawi: a pre- post comparison. BMC Pediatr. 2009;9:80. doi: 10.1186/1471-2431-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCollum ED, Preidis GA, Kabue MM, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS One. 2010;5:e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollins N, Little K, Mzolo S, et al. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–1347. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 13.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 14.South African National Department of Health. The 2010 National antenatal sentinel HIV and syphilis prevalence survey in South Africa. [Accessed May 1, 2012]; Available at: http://www.doh.gov.za/docs/reports/2011/hiv_aids_survey.pdf.
- 15.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa? not everyone who should. AIDS. 2010;24(suppl 1):S37–S44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Avila L, Regan S, Giddy J, et al. Depressive symptoms and their impact on health-seeking behaviors in newly-diagnosed HIV-infected patients in Durban, South Africa. AIDS Behav. 2012;16:2226–2235. doi: 10.1007/s10461-012-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Department of Health Republic of South Africa. Guidelines for the management of HIV in children. [Accessed April 16, 2011]; Available at: http://www.health-e.org.za/documents/35cc37337b5448b6d06f48440fb424cc.pdf. [Google Scholar]
- 20.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46:181–186. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwood C, Vermaak K, Rollins N, et al. Paediatric HIV management at primary care level: an evaluation of the integrated management of childhood illness (IMCI) guidelines for HIV. BMC Pediatr. 2009;9:59. doi: 10.1186/1471-2431-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Integrated management of childhood illness for high HIV settings. [Accessed August 1, 2012]; Available at: http://whqlibdoc.who.int/publications/2008/9789241597388_eng.pdf. [PubMed]
- 23.Birbeck G, Chomba E, Atadzhanov M, et al. The social and economic impact of epilepsy in Zambia: a cross-sectional study. Lancet Neurol. 2007;6:39–44. doi: 10.1016/S1474-4422(06)70629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltzer K, Mngqundaniso N, Petros G. HIV/AIDS/STI/TB knowledge, beliefs and practices of traditional healers in KwaZulu-Natal, South Africa. AIDS Care. 2006;18:608–613. doi: 10.1080/09540120500294206. [DOI] [PubMed] [Google Scholar]
- 25.Family Health International 360. Behavioral Surveillance Surveys: Guidelines for repeated behavioral surveys in populations at risk of HIV. [Accessed August 1, 2012]; Available at: http://www.fhi360.org/en/HIVAIDS/pub/guide/bssguidelines.htm. [Google Scholar]
- 26.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Accessed August 1, 2012]. Available at: http://www.R-project.org. [Google Scholar]
- 27.Chhagan MK, Kauchali S, Arpadi SM, et al. Failure to test children of HIV-infected mothers in South Africa: implications for HIV testing strategies for preschool children. Trop Med Int Health. 2011;16:1490–1494. doi: 10.1111/j.1365-3156.2011.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin M, Mathema H, Stinson K, et al. Acceptability, feasibility and impact of routine screening to detect undiagnosed HIV infection in 17 – 24-month-old children in the western sub-district of Cape Town. S Afr Med J. 2012;102:245–248. [PubMed] [Google Scholar]
- 29.Ferrand RA, Trigg C, Bandason T, et al. Perception of risk of vertically acquired HIV infection and acceptability of provider-initiated testing and counseling among adolescents in Zimbabwe. Am J Public Health. 2011;101:2325–2332. doi: 10.2105/AJPH.2011.300250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwood C, Butler LM, Vermaak K, et al. Disease profile of children under 5 years attending primary health care clinics in a high HIV prevalence setting in South Africa. Trop Med Int Health. 2011;16:42–52. doi: 10.1111/j.1365-3156.2010.02672.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghani AS, Morrow BM, Hardie DR, et al. An investigation into the prevalence and outcome of patients admitted to a pediatric intensive care unit with viral respiratory tract infections in Cape Town, South Africa. Pediatr Crit Care Med. 2012;13:e275–e281. doi: 10.1097/PCC.0b013e3182417848. [DOI] [PubMed] [Google Scholar]
- 32.Rochat TJ, Bland R, Coovadia H, et al. Towards a family-centered approach to HIV treatment and care for HIV-exposed children, their mothers and their families in poorly resourced settings. Future Virol. 2011;6:687–696. doi: 10.2217/fvl.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betancourt TS, Abrams EJ, McBain R, et al. Family-centred approaches to the prevention of mother to child transmission of HIV. J Int AIDS Soc. 2010;13(suppl 2):S2. doi: 10.1186/1758-2652-13-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis Kulzer J, Penner JA, Marima R, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 2012;15:8. doi: 10.1186/1758-2652-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nglazi MD, van Schaik N, Kranzer K, et al. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59:e28–e34. doi: 10.1097/QAI.0b013e31824445f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snow RC, Madalane M, Poulsen M. Are men testing? Sex differentials in HIV testing in Mpumalanga Province, South Africa. AIDS Care. 2010;22:1060–1065. doi: 10.1080/09540120903193641. [DOI] [PubMed] [Google Scholar]
- 37.Qazi SA, Muhe LM. Integrating HIV management for children into the Integrated Management of Childhood Illness guidelines. Trans R Soc Trop Med Hyg. 2006;100:10–13. doi: 10.1016/j.trstmh.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Sherman GG, Jones SA, Coovadia AH, et al. PMTCT from research to reality–results from a routine service. S Afr Med J. 2004;94:289–292. [PubMed] [Google Scholar]
- 39.Paintsil E, Andiman WA. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr. 2009;21:94–101. doi: 10.1097/MOP.0b013e32831ec353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horwood C, Haskins L, Vermaak K, et al. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women’s health services. Trop Med Int Health. 2010;15:992–999. doi: 10.1111/j.1365-3156.2010.02576.x. [DOI] [PubMed] [Google Scholar]