Abstract
Treatment of CNS malignancies typically involves radiotherapy to forestall tumor growth and recurrence following surgical resection. Despite the many benefits of cranial radiotherapy, survivors often suffer from a wide range of debilitating and progressive cognitive deficits. Thus, while patients afflicted with primary and secondary malignancies of the CNS now experience longer local regional control and progression free survival, there remains no clinical recourse for the unintended neurocognitive sequelae associated with their cancer treatments. Multiple mechanisms contribute to disrupted cognition following irradiation, including the depletion of radiosensitive populations of stem and progenitor cells in the hippocampus.
We have explored the potential of using intrahippocampal transplantation of human stem cells to ameliorate radiation-induced cognitive dysfunction. Past studies demonstrated the capability of cranially transplanted human embryonic (hESCs) and neural (hNSCs) stem cells to functionally restore cognition in rats 1 and 4-months post-head-only irradiation. The present study employed an FDA-approved fetal-derived human neural stem cell line capable of large scale-up under good manufacturing practice (GMP). Animals receiving cranial transplantation of these cells 1-month following irradiation showed improved hippocampal spatial memory and contextual fear conditioning performance compared to irradiated, sham surgery controls. Significant newly born (doublecortin positive) neurons and a smaller fraction of glial subtypes were observed within and nearby the transplantation core. Engrafted cells migrated and differentiated into neuronal and glial subtypes throughout the CA1 and CA3 subfields of the host hippocampus. These studies expand our prior findings to demonstrate that transplantation of fetal-derived human neural stem cells improves cognitive deficits in irradiated animals, as assessed by two separate cognitive tasks.
Keywords: Human fetal-derived neural stem cells, transplantation, radiation, cognition, hippocampus, novel place recognition, fear conditioning
INTRODUCTION
Cranial radiotherapy remains the most effective way to treat a range of CNS malignancies, from pediatric patients afflicted with medulloblastoma to adults stricken with glioblastoma multiforme. In the former case, radiotherapy can be curative (30), while in the latter case, irradiation improves progression free survival (28,55). These survival benefits however, come at the cost of debilitating cognitive decrements that adversely impact quality of life (15,22,40,41,51,56). Treatment-associated cognitive dysfunction is particularly problematic for pediatric patients in its severity, long-term nature and socioeconomic impact (38,44,53). At present, no long-term, satisfactory solutions exist for the treatment of cognitive impairments caused by both radio- and chemotherapy (25).
While improvements in radiotherapy treatment strategies have limited radiographically visible morphologic injury to the brain parenchyma, such as radionecrosis and edema, a progressive onset of cognitive dysfunction invariably occurs (1,2,36,46). The cognitive decrements are multifaceted and manifest as impaired hippocampal-dependent (and independent) learning and memory, and frequently include altered attention, concentration and executive functions, such as planning and multi-tasking (42) Interventions to combat the long-term neurocognitive sequelae resulting from such cytotoxic cancer therapies have yet to be developed (22).
Considerable evidence points to the depletion of neural stem and progenitor cells as one of the contributory mechanisms underlying radiation-induced cognitive dysfunction. Irradiation has a profound impact on the small but neurogenically-active population of stem cells in the subgranular zone (SGZ) of the hippocampal dentate gyrus (3,19,50,54,59). Many studies have now shown that ionizing radiation depletes radiosensitive populations of mulitpotent neural stem and progenitor cells in the hippocampus and elicits a persistent oxidative stress that fundamentally compromises the neurogenic niche, thereby leading to long-term impairments of neurogenesis (24,25). While the convergence of multiple mechanisms are certain to impact cognitive health, it is the temporal coincidence linking the loss of multipotent neural cells to the inhibition of neurogenesis and onset of hippocampal-dependent spatial learning and memory deficits that point to the importance of preserving neural stem cell pools (4,24,25).
The foregoing provides the backdrop for much of our work aimed at replacing those cells lost or damaged by irradiation. Our past work using an athymic nude rat model has demonstrated the efficacy of pluripotent human embryonic stem cells (hESCs) and multipotent human neural stem cells (hNSCs) in presvering cognitive function when transplanted intrahippocampally following cranial irradiation (3-5). Cells transplanted 2 days following irradiation were found to restore spatial memory performance on a novel place recognition task that relies on hippocampal function, and is sensitive to radiation-induced deficits (3-5). Beneficial effects of cognition were found to persist out to 4-months following a single round of transplantations (3-5). Further analysis revealed significant survival of engrafted cells that migrated throughout the septal-temporal axis of the hippocampus and differentiated along both glial and neuronal lineages (3-5).
This evidence suggests that stem cell-based strategies designed to preserve and/or replenish radiation-damaged and/or depleted stem cell pools in the brain may be capable of forestalling the development of cognitive impairments. In the present study, we have tested an FDA-approved, GMP human fetal-derived neural stem cell line to investigate its therapeutic potential in our established ATN rat model. Here we report that transplantation of fetal-derived hNSCs reverses irradiation-induced cognitive dysfunction using two cognitive tasks, and further show that engrafted cells exhibit robust survival and neuronal differentiation 1-month following cranial irradiation.
MATERIALS AND METHODS
Animals and irradiation procedure
All animal procedures described were approved by Institutional IACUC and comply with NIH guidelines. The immunocompromised athymic nude (ATN) rat model (strain 0N01, Cr:NIH-rnu) was used here, as in our previous studies (3-5). A total of 29 male ATN rats (2 month old, purchased from Frederick National Laboratory, NCI, MD, USA) were maintained in sterile housing conditions (20°C ± 1°C; 70% ± 10% humidity; 12h each light and dark cycle), and had free access to sterilized diet and water. Animals were divided into three groups: 0Gy (no irradiation) sham surgery controls (CON, n=12), 10Gy irradiated sham surgery (IRR, n=9) and 10Gy irradiated with engrafted neural stem cells (IRR+NSI, n=8). Rats were anesthetized, eyes and body were shielded and the head was exposed to cranial γ-irradiation (10Gy) using a 137Cs irradiator (J.L. Shepard, Mark I, CA, USA) at a dose rate of 2.07 Gy/min, as described previously (3).
Transplantation surgery
The use of human stem cells in this study was approved by the Institutional Human Stem Cell Research Oversight Committee (hSCRO) under the material transfer agreement (MTA) with NeuralStem Inc. (MD, USA). The NSI566RSC cells (henceforth termed NSI) are human, fetal-derived neural stem cells developed by Neuralstem, Inc. (MD, USA). NSIs were prepared and isolated from the cervical-upper thoracic region of spinal cord obtained from a single 8-week human fetus after an elective abortion (31,35,64). The development and propagation of NSI cultures is described in detail previously (31,35,64). The NSI cell line displayed abundant expression of Sox2/Nestin and retained their capability to differentiate (neuronal and astro-glial phenotypes), as described previously (34,58,64).
A day prior to each surgery, cryopreserved vials derived from a well-characterized GMP cell bank were thawed, washed, prepared in a hibernation buffer and shipped overnight from NeuralStem to UCI. The following day, the cells were checked for viability and used directly for transplantation without further manipulation. On an average, the viability of NSI used in the present study was 80-85%.
The NSI cultures were transplanted in irradiated rats as described in the Figure 1A’s schematic (Fig. 1A). At two days post-irradiation, each rat received bilateral, intra-hippocampal transplantation of 70,000 live NSI (IRR+NSI) in 1 μl of cell suspension using a 33-gauge microsyringe at an injection rate of 0.25 μl/min. Both hippocampi received 4 distinct injections (total 2.8 × 105 live NSIs per hemisphere) using precise stereotaxic coordinates, as described previously (3). Sham-operated control (CON) and Irradiated (IRR) rats received sterile vehicle (hibernation buffer) at the same stereotaxic coordinates.
Figure 1.
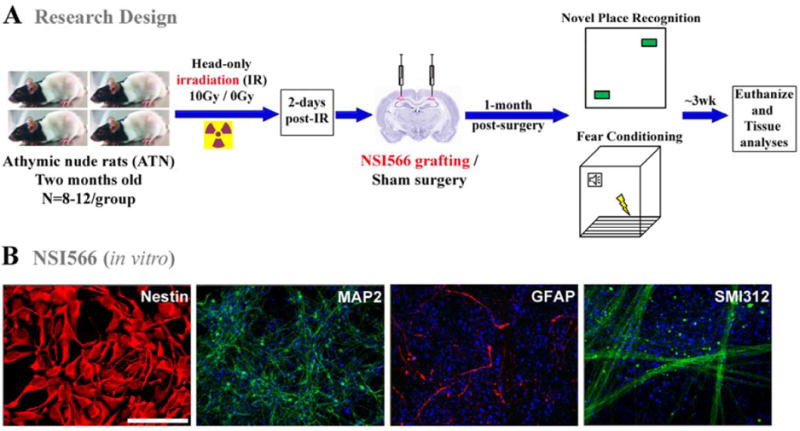
Schematics of research design. (A) Two month old athymic nude rats received 10Gy head-only γ-irradiation were transplanted two days later with human-fetal derived neural stem cells (NSI566). At 1-month post-transplantation surgery, animals were administered a novel place recognition and a fear conditioning tasks. Three weeks later, after cognitive testing, animals were euthanized for immunohistochemical analysis. Non-irradiated control and irradiated animals receiving sterile hibernation buffer served as sham surgery groups. (B) Immunocytochemical analysis of NSI566 stained with DAPI shows strong expression and co-localization of the multipotent marker (Nestin, red). In vitro differentiated NSIs express both neuronal (green, neurofilament proteins MAP2 and SMI312) and astrocytic (red, GFAP) markers. (Scale bars: Nestin, 10 μm; MAP2, SMI312 and GFAP, 50 μm).
Measurement of cognitive function
To evaluate the outcome of NSI-transplantation on cognitive function, at 1-month post-transplantation, rats from CON, IRR and IRR+NSI groups were tested on novel place recognition (NPR), contextual and cued fear conditioning (FC) tasks as described previously (3,4,19). NPR task assesses spatial recognition memory and relies on intact hippocampal function (3,4,19). FC task was administered in three phases over three days: a training phase, a context test and a cue test. Cued FC memory has been shown to rely on intact amygdala function, while contextual FC memory additionally engages the hippocampus (19). For all phases, rats were placed in a PhenoTyper 3000 (Noldus Information Technology, Leesburg, VA) comprised of a video camera for observation and live tracking during FC trials. The animals were tested on NPR followed by FC. For both tasks, video recording and automated tracking of the animals was carried out using a Noldus Ethovision XT system (version 7.0; Noldus Information Technology, Leesburg, VA). The detailed procedures for the NPR and FC tasks are as described previously (3,4,18,19).
Immunohistochemistry and confocal microscopy
After completion of cognitive testing, animals were deeply anesthetized with isoflurane, and euthanized by intracardiac perfusion with 4% paraformaldehyde (Acros Organics, NJ, USA). Tissues were processed in a sucrose gradient (10 to 30%) and 30 μm-thick sections cut coronally through the hippocampus using a cryostat (Leica Microsystems, Wetzlar, Germany) were then stored in phosphate buffered saline (PBS) with 0.02% sodium azide (Sigma-Aldrich, MO, USA).
Immunohistochemical studies employed both monoclonal and polyclonal antibodies. Primary antibodies were as follows: anti-Ku80 (human specific DNA telomere-binding nuclear protein, mouse, 1:100, Stem101, StemCell Inc., Cambridge, UK), anti-DCX (doublecortin, goat, Santa Cruz Biotechnology Inc., CA, USA), anti-NeuN (neuron specific nuclear antigen, rabbit, 1:250, Millipore, MA, USA), anti-GFAP (glial fibrillary acidic protein, rabbit, 1:500, Millipore, MA, USA), anti-S100 (S100β protein, rabbit, 1:200, Millipore, MA, USA), anti-MAP2 (neuronal filament protein, monoclonal AP20, mouse, 1:200, Sigma-Aldrich), anti-SMI312 (pan-axonal neurofilament, mouse, 1:200, EMD Millipore, MA, USA), anti-nestin (rabbit, 1:200, EMD Millipore). The secondary antibodies and detection reagents included biotinylated horse anti-goat IgG (1:200, Vector Labs, CA, USA), donkey anti-mouse and anti-rabbit conjugated with Alexa Fluor 488 or 594 (1:200, Invitrogen, CA, USA) and TOTO-3 iodide (infrared nuclear counterstain, Invitrogen, CA, USA).
To identify and track differentiated fate of transplanted human cells, representative sections were processed using dual immunoflourescence staining for human-specific nuclear antigen (Ku80) and various mature and immature neuronal (DCX, NeuN) and astro-glial (GFAP, S100) markers, as described (7,21). Serial sections taken through the middle of the hippocampus were selected for staining and stored in tris-buffered saline (TBS, 100 mM, pH 7.4, Sigma-Aldrich, MO, USA) overnight. Free floating sections were first rinsed in TBS followed by Tris-A (TBS with 0.1% Triton-X-100, Sigma-Aldrich, MO, USA), blocked with 10% normal donkey serum (NDS with Tris-A, Sigma-Aldrich, MO, USA) and incubated overnight in a mouse anti-Ku80 solution (1:100) prepared in 3% NDS and Tris-A. The next day, the sections were treated with donkey anti-mouse IgG conjugated with Alexa Fluor 594 (1:200) made with Tris-A and 3% NDS for 1h. The sections were light protected, washed with Tris-A, and blocked in serum and primary antibodies for neuronal or glial markers. Color development was facilitated by Alexa-Fluor conjugated secondary antibodies, as described above, and counter stained with TOTO-3 nuclear dye for visualization of hippocampal morphology. Immunostained sections were rinsed in TBS and mounted on clean Vectabond (Vector Labs, CA, USA) coated slides using SlowFade anti-fade mounting medium (Invitrogen, CA, USA). Ku80 positive cells were visualized under fluorescence as red and DCX, NeuN, GFAP or S100-positive cells were visualized as green. For quantification of differentiated phenotypes, dual-labeled sections from representative hippocampal sections were analyzed from distinct transplants derived from four animals in each group.
Laser scanning confocal analyses to identify phenotypic fate of graft-derived cells were performed using a Nikon Eclipse microscope (TE2000-U, EZ-C1 interface), as described previously (3,4). Z-stack analyses (1 μm intervals) and orthogonal image reconstruction were done using Nikon Elements AR software (v3.22, Nikon Instech Co. Ltd., Japan).
Statistical analyses
Statistical analyses were carried out using PASW Statistics 18 (SPSS, IBM Corporation, Somers, NY). All analyses were 2-tailed, and a value of p ≤ 0.05 was considered statistically significant. In all cases, normal distribution of the data (Kolmogorov-Smirnov test), and homogeneity of variance (Levene’s test of equality of error variances) were confirmed. When a statistically significant overall group effect was found, multiple comparisons were made using Fisher’s protected least significant different (FPLSD) post hoc tests to compare the individual groups.
For the NPR taks, exploration ratio, or the proportion of total time spent exploring the novel spatial location (tnovel/tnovel + tfamiliar), was used as the main dependent measure. The behavior of the animals during minute 1 of the 5-minute and 24-hour test phases was analyzed [for details, see (3,19)]. The number of animals included for NPR task analyses were: CON (n=12), IRR (n=9) and IRR+NSI (n=8).
For the FC task, percentage of time spent freezing was used as the main dependent measure. Freezing was assessed during the final minute of the baseline (i.e. before tone-shock pairings were administered) and post-training (i.e. after tone-shock pairings) phases. For the context test, freezing was assessed over the entire 5-minute trial. For the pre-cue test, freezing was assessed during the first minute, in which no tone was sounded, and for the cue test, freezing was assessed across the three minute interval that the tone was sounded and for the final minute of the trial in which no tone was sounded. Repeated measures ANOVA were used to assess group (between subjects factor) and phase (within subjects factor) effects on freezing behavior [for details, see (19)]. A separate cohort of animals for each group (CON, IRR and IRR+NSI), n=8 animals were analyzed for the FC task analyses.
RESULTS
NSI express stem cells and neural markers in vitro
Prior to transplantation, NSI cells were tested in vitro for the expression of multipotent and neural markers. Robust expression of the neural stem cell marker nestin (red) validated the undifferentiated state of NSIs (Fig. 1B). Additional in vitro differentiation analysis demonstrated the capability of NSIs to generate cell types positive for the neuronal neurofilament proteins MAP2 and SMI312 (green) and astrocytic GFAP (red) following growth factor deprivation for 7 days (Fig. 1B).
Transplanted human NSIs improves cognition
Novel place recognition task
One month post-transplantation, animals were habituated and tested on the NPR task (Fig. 2A-C). Analysis of total time spent exploring both objects during the initial familiarization phase revealed an overall group difference (Fig. 2A, P<0.0001). IRR+NSI animals explored more than CON and IRR groups (P’s<0.0001). Following a 5min retention interval, IRR animals spent a significantly lower proportion of time exploring the novel place compared to CON and IRR+NSI groups (Fig. 2B, P’s<0.0001). On the other hand, after the 5min retention interval, IRR+NSI animals did not differ from CON animals. Moreover, one-sample t-tests comparing the exploration ratios of individual groups to chance (0.5, dashed line, Fig. 2B) revealed that CON (P<0.001) and IRR+NSI (P<0.0001) animals spent significantly more time exploring the novel place than expected by chance (dashed line, Fig. 2B), while IRR animals explored the novel spatial location less than expected by chance. Following an additional 24hr retention interval (Fig. 2C), the overall group difference was not significant (P=0.09). One-sample t-tests showed that none of the groups explored the novel spatial location significantly more or less than expected by chance following the 24hr delay. There was however, a trend for the IRR animals to explore less (Fig. 2C).
Figure 2.
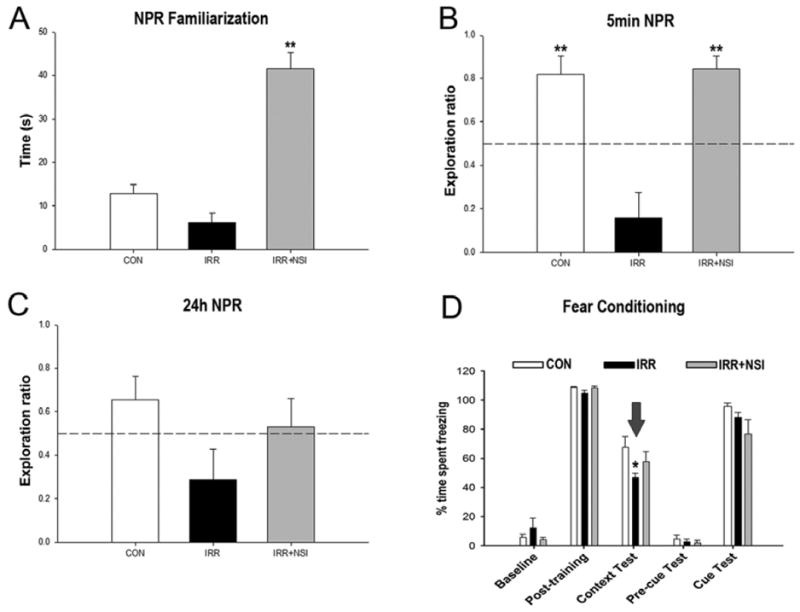
Human fetal-derived neural stem cell transplantation improved radiation-induced cognitive impairments at 1-month post-transplantation. For the novel place recognition task (NPR), animals were first familiarized with two identical objects in specific spatial locations in an open field arena, and total time spent exploring both identical objects was assessed. Following a 5-min retention interval, animal were placed in the same arena with one object moved to a novel spatial location. (A) Analysis of total time spent exploring both objects during the initial familiarization phase of NPR task revealed NSI-transplanted (IRR+NSI) animals explore more than controls- (CON) and irradiated-sham (IRR) groups (P’s<0.001, post hoc). Exploration ratios were calculated as, timenovel/timenovel+timefamiliar, for the first minute of 5min (B) and 24h (C) test sessions in NPR task. (B) In 5min test phase, IRR animals spent a significantly lower proportion of time exploring the novel place (P’s<0.001 vs. CON, and vs. IRR+NSI, post hoc), while CON and IRR+NSI animals did not differ. IRR animals did not spent more time exploring the novel place than expected by chance (dashed line at 50%). (C) 24h after the initial familiarization phase, animals were presented with the same two objects, with one moved to a new spatial location. None of the groups spent more time exploring the novel place than expected by chance. For the context and cued fear-conditioning task (D), baseline freezing levels were established using a series of 5 tone-shock pairings (post-training bars, D), and all groups showed increased post-training freezing behavior. 24h hour later, a context test was administered, and the IRR group spent significantly less time freezing compared to CON, while CON and IRR+NSI did not differ (P=0.014, post hoc comparisons, indicated by arrow). 48h after the initial training phase, the context was changed, which resulted in a substantial reduction in freezing behavior in all groups (pre-cue bars, D). Further, freezing levels were restored in all groups following the tone sound (cue test bars, D), indicating intact amygdala function in all groups Data are presented as Means + 1 SEM. P values were derived from FPLSD post hoc comparisons. *, P=0.014 indicates significant difference versus CON and IRR+NSI, and **, P=0.001 indicates significant difference versus IRR animals.
Fear-conditioning task
The three phases of the FC task (training, cue and context tests) were administered over 3 days. A significant overall group × phase interaction effect (P=0.03) was found by repeated measures ANOVA for the percentage time spent freezing during the FC task (Fig. 2D). Subsequent individual one-way ANOVAs conducted for each phase of the task revealed a significant group effect (P=0.046) for the context test phase of the FC task (context text bars, Fig. 2D). Post hoc FPLSD tests showed that IRR animals spent significantly decreased percentages of time freezing compared to the CON group (P=0.014), while CON and IRR+NSI groups did not differ. No significant group difference was observed in freezing behavior across baseline, post-training, pre-cue test and cue test phases (Fig. 2D), indicating a selective deficit on the hippocampal-dependent contextual memory phase of the task. Furthermore, since all groups demonstrated significant increases in freezing behavior after the tone-shock pairings (post-training phase), irradiation did not impair motor or sensory function. The fact that cued memory was intact demonstrates that the acquisition of the tone-shock pairing was not impaired, and that the deficit was specific to the memory of the context in which the pairing was learned.
Survival and Location of NSI in irradiated rat hippocampus
At ~ 2-months post-transplantation, following cognitive testing, 8 animals in each group were euthanized for immunohistochemical analysis to determine the pattern of integration of transplanted NSIs (Fig. 3). Examination of Ku80 (human-specific nuclear antigen, green) immunofluorescence stained sections from IRR+NSI group demonstrated the presence of grafts along the entire septo-temporal axis of the hippocampus (Fig. 3). Visualization with Ku80 marker (green) revealed that transplanted cells remained in clusters at the transplant site, and very few Ku80+ cells were observed migrating away from the transplant core in the irradiated host hippocampus (Fig. 3C-D). As shown in our previous studies (3-5), we aimed placement of transplants above the CA1 region, without disturbing the hippocampal milieu. With the exception of 2 animals (wherein transplant was found above DG), transplant-derived cells were located above the CA1 subfield with projections into corpus callosum (CC), and they formed a transplant core (Tc, Fig. 3A-C). As shown in Fig. 3, the location of transplant did not distort the host hippocampal cytoarchitecture (pink, TOTO-3 nuclear counter stain, Fig. 3).
Figure 3.

Survival and location of transplanted NSIs. At ~ 2 months post-transplantation, NSI are located near the injection site (dual labeled cells displayed as white, Nt, needle track; Tc, transplant core, A-E, 5 to 60× magnification) and CA1 and corpus callosum (CC) areas. Transplanted NSI (green) did not show extensive migration patterns in the host hippocampus (dentate gyrus, DG; dentate hilus DH, CA3 subfields). Transplanted NSIs were detected by human specific nuclear antigen (Ku80, green) and counterstained with nuclear dye (TOTO-3, pink). Insert (E) represents orthogonal reconstruction of confocal Z-stacks. (Scale bars: A-B, 100 μm; C, 50 μm; D, 20 μm; E, 10 μm and E-insert, 5 μm).
Differentiation of transplanted NSI
To determine the phenotypic fate of transplant-derived cells in the irradiated animals following NSI transplantation, dual immunofluorescence and confocal Z-stack analyses were carried out in representative sections of IRR+NSI group. Examination of dual immunofluorescence-stained Ku80+ and various neuronal and astrocytic markers demonstrated the presence of both phenotypes in the irradiated host hippocampus (Fig. 4). Transplant-derived cells expressed immature (DCX+, Fig. 4A) and mature (NeuN+, Fig. 4B) neuronal markers. Moreover, assessment of astrocytic differentiation using Ku80 revealed the presence of double-labeled immature (GFAP+, Fig. 4C) and mature (S100+, Fig. 4D) phenotypes. Orthogonal reconstructions of confocal Z-stacks are shown for each marker (Fig. 4a-d). Qualitative examination of all animals indicated that the majority of transplanted cells differentiated into neurons that were still in the developmental stage (DCX+, Fig. 4A) and located primarily in the transplant core, whereas a minority of transplant-derived cells expressed astrocytic markers (Fig. 4C-D).
Figure 4.
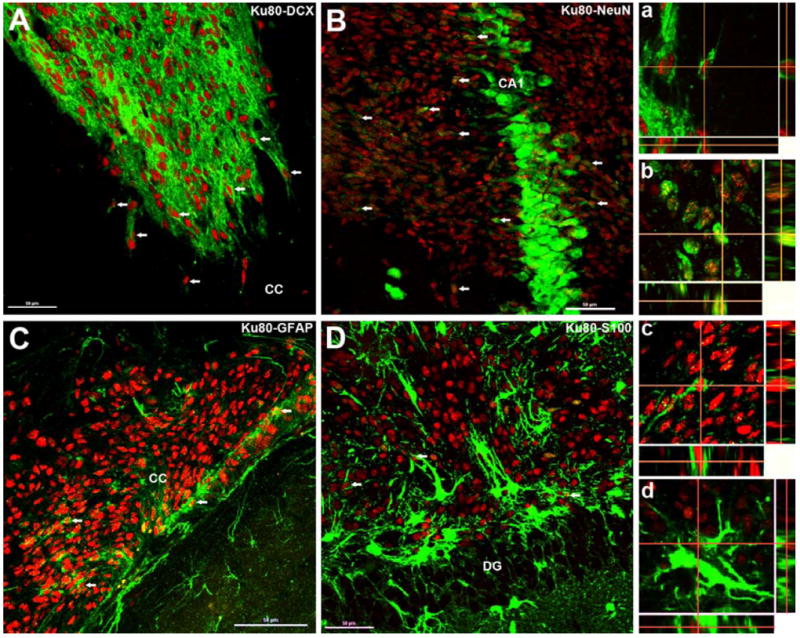
Differentiation of transplanted NSIs in the irradiated hippocampus. At ~ 2 months post-transplantation, Ku80 positive (human specific nuclear antigen, red) NSIs differentiated into immature (doublecortin, DCX, A and a, green) and mature neurons (neuron specific nuclear antigen, NeuN, B and b, green) as visualized by dual labeling of neuron-specific markers with Ku80 (red). A similar pattern of differentiation was observed for immature (glial fibrillary acidic protein, GFAP, C and c, green) and mature (S100 protein, D and d, green) astrocytes. Arrows indicate representative dual-labeled NSI transplant-derived cells (A-D). Confocal Z-stack orthogonal reconstructions of dual-labeled cells are shown for each neuronal (NeuN, a; DCX, b) and astrocytic (GFAP, c; S100, d) phenotypes. DG, dentate gyrus; CC, corpus callosum. (Scale bars: A-D, 50 μm and a-d, 10 μm).
Quantification of differentiated phenotypes revealed that the majority of engrafted cells expressed the immature neuronal marker DCX (24.5 +/- 2.2%, Fig. 5) with a smaller percentage going on the express the mature neuronal marker NeuN (3.65 +/- 1.0%, Fig. 5). Engrafted cells developing astrocytic fates were much smaller as the percentage of GFAP positive (2.21 +/- 0.2%, Fig. 5) and S100β positive (2.29 +/- 0.09%, Fig. 5) cells were lower in comparison. Thus, engrafted NSIs were found to preferentially commit to neuronal rather than astrocytic fates.
Figure 5.
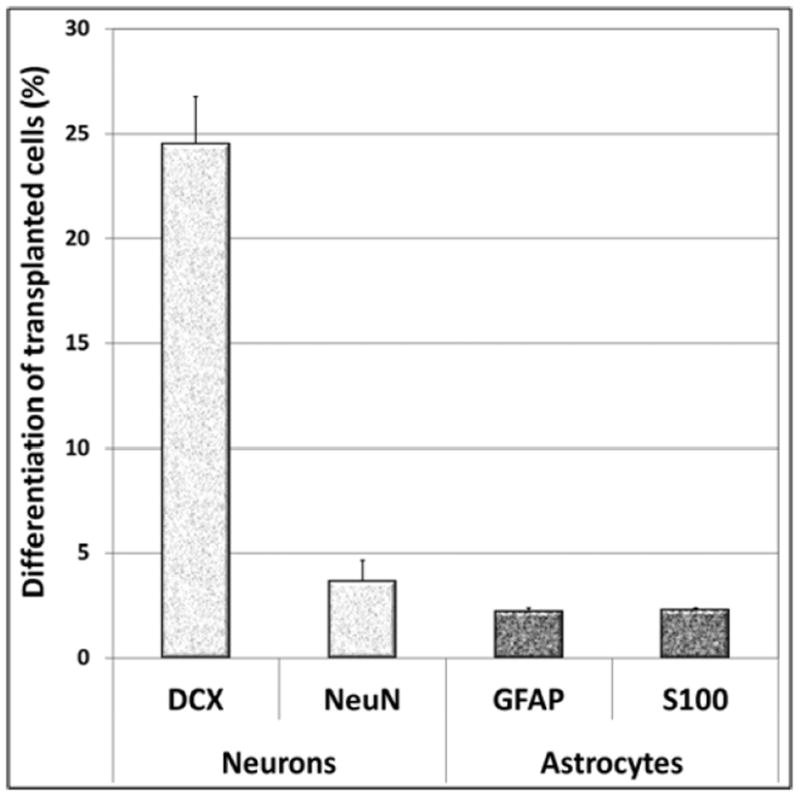
Engrafted NSIs differentiated into neuronal and astrocytic phenotypes 2-months after irradiation and transplantation. The majority of NSI graft-derived cells (Ku80+) differentiated into immature neurons (doublecortin, DCX). Transplanted NSI also differentiated into mature (S100β+) and immature (GFAP+) astrocytes, though minimal oligodendrocytic differentiation was observed at this time-point (not shown). The data is represented as the Mean ± S.E.M. of 4 independent observations for each marker.
DISCUSSION
The progressive and debilitating cognitive decrements associated with radiotherapeutic management of brain cancer have a significant and adverse impact on quality of life. While advancements in cancer treatment have improved the survival rate and lifespan of cancer survivors, many still suffer from neurocognitive sequelae with no satisfactory, long-term clinical recourse for their condition. Our past and present findings showing that cranially-engrafted stem cells can either reverse or prevent the development of radiation-induced cognitive impairments suggests that stem cell replacement strategies might one day provide a much needed intervention for forestalling this serious side effect.
Our past data analyzed at 1 and 4 months post-transplantation indicated that hNSCs provided slightly better cognitive benefits than hESCs (26). There was also no evidence of teratoma formation at these post-transplantation times. Despite these positive results, translating such findings to the clinic invariably leads to important decisions regarding the best choice of stem cells, with safety and efficacy of chief concern. With this in mind, we decided to initiate the present studies using an FDA approved, GMP human neural stem cell from Neuralstem, Inc., referred to as NSI566RSC (i.e. NSI). The behavior of these cells and their differentiation into motor neurons in the rodent spinal cord has been characterized over the years (31,35,64). NSI cells have been successfully engrafted into the lumbar spinal cord of SOD1 transgenic rodent models of motor neuron disease (60,61,63). Engrafted cells underwent extensive neuronal differentiation, formed new synaptic contacts, released neurotrophic factors and showed an advanced degree of structural integration into the motor circuitry, effects that likely contributed to the delayed onset and progression of disease in these models. More recent studies have found that engraftment of NSI cells in rats subjected to complete spinal cord resection stimulated functional recovery (37). Data show that grafted neurons were capable of overcoming the inhibitory microenvironment of the injured spinal cord, where they underwent extensive axonal growth and synapse formation to form new neuronal relays capable of restoring electrophysiological activity and behavior (37). These promising findings led the FDA to approve the first phase 1 safety trial of direct intraspinal transplantation of NSI cells into patients with amyotrophic lateral sclerosis (ALS). All 12 patients enrolled in this ongoing trial that received stem cell injections have tolerated the surgical procedures and transplants in the absence of any accelerated disease progression (12,26,49). Thus, current data demonstrate safety and may well lead to efficacy studies aimed at protecting motor neuron pools in ALS patients.
These findings were instrumental in our decision to promote the translation of our findings to the clinic and analyze the capability of the FDA-approved NSI cells to ameliorate radiation-induced cognitive dysfunction. Engraftment of NSIs was found to elicit significant improvements in cognition 1 month post-transplantation in rats receiving head-only irradiation, as assessed by two well characterized and widely used cognitive task (Fig. 2). In contrast to irradiated animals that underwent sham transplantation surgery, the performance of NSI-engrafted animals was indistinguishable from unirradiated controls on the 5 min NPR task (Fig. 2B), with both controls and transplanted animals showing significant preference for exploring the novel place. Differences in exploration ratios between cohorts re-tested 24h later on the NPR task were not found to be significant, as animals only showed a trend for exploring the novel location at this time (Fig. 2C). While we and other independent laboratories have shown that the novel place recognition task is sensitive to radiation-induced deficits as well as cognitive improvements following transplantation surgery, group differences in locomotor activity and/or anxiety levels could confound this behavioral paradigm that relies on spontaneous exploration. However, group differences in anxiety levels assessed using an elevated plus maze were not observed (data not shown). Locomotor activity (distance traveled and velocity of movement) assessed during the initial familiarization phase of the NPR task revealed that irradiated animals, regardless of surgery, showed reduced locomotion and velocity compared to non-irradiated controls (data not shown). Thus, this prompted additional behavioral testing using a contextual fear-conditioning (FC) task that does not rely on spontaneous exploration but is known to engage the hippocampus.
Using the FC task, a specific impairment was detected (Fig. 2D). Animals subjected to cranial irradiation spent significantly less time engaged in freezing behavior than controls during the context phase of the task. This finding suggests that irradiation disrupted 24-hour memory for the shock-context association, which has been shown to rely on intact hippocampal function (47). Interestingly, animals engrafted with NSI cells demonstrated intact freezing behavior, and were statistically indistinguishable from controls in their contextual fear memory. This finding suggests that stem cell transplants ameliorated a radiation-induced deficit in hippocampal function. The amount of post-training freezing observed was comparable between all experimental cohorts, indicating that irradiation, sham surgery and/or transplantation of NSI cells did not affect initial acquisition of the conditioned freezing response. Similarly, the experimental conditions imposed on animal cohorts did not affect freezing behavior during the cue test phase, indicating intact acquisition and memory for the conditioned tone stimulus, which has been shown to rely on intact amygdala function (47). The specific deficits in contextual fear memory observed in the present study suggest that irradiation selectively disrupts hippocampal function, which is consistent with the NPR deficits and disruptions in hippocampal neurogenesis that we observed in the present and many past studies (47,59). Similarly, the capability of engrafted NSI cells to reverse or prevent radiation-induced cognitive dysfunction corroborates our past data, and points to the potential utility of these cells in another injury model of the CNS.
To date, the only routine application for stem cell therapy involves hematopoietic stem cell transplantation for the treatment of leukemia and lymphomas (29), which reconstitutes the immune system after whole-body irradiation (29) but can also facilitate the repair of brain injury in rodent models (45). Stem cell-based approaches have been used successfully in animal models to ameliorate neurodegenerative conditions such as Alzheimer’s disease (10,62), as well as other disorders such as epilepsy (6,11,39) and traumatic brain injury (48). Importantly, we have now demonstrated that intrahippocampal transplantation of hNSCs can rescue cognition following exposure to cranial irradiation using three sources of stem cell (3,4). Our present findings demonstrate the utility of a GMP-derived stem cell line that has been granted FDA approval. Engrafted NSI cells survived and differentiated throughout the septo-temporal axis of the hippocampus in sufficient yield to significantly ameliorate cognitive dysfunction at 1-month follow-up in animals receiving 1Gy head-only irradiation.
In past studies, we have used a well-characterized immunohistochemical technique that capitalizes on the rapid and predictable expression of activity-related cytoskeletal protein (Arc) to address some of the possible mechanisms by which transplanted stem cells improve cognitive function. Arc is an activity-induced gene that correlates both temporally and spatially with the stimulus that induced its transcription (13,32,52). Our findings that transplanted cellss express Arc suggest that engrafted cell can functionally integrate into neuronal circuits to ameliorate cognitive decline. This however, is unlikely to account for all the potentially beneficial effects of transplanted stem cells, and trophic support derived from engrafted cells is likely important (10). In support of this idea is the observation that the transplant core has an extremely high yield of doublecortin positive cells. These enriched areas of engrafted cells may promote the secretion of beneficial factors throughout the hippocampus
In the present study, the majority of grafted cells developed neuronal phenotypes as validated by dual immunoflourescence staining of human specific nuclear antigen (Ku80) with DCX or NeuN (Fig. 4). This result corroborates previous observations in which rodent models of ischemic paraplegia and SOD1 G93A were transplanted with the same NSI cells (20,63). The microtubule-associated protein, doublecortin, is exclusively expressed (with some exception) by immature neurons from embryonic day 10 to ~2-3 weeks post-mitosis (14,27,43). At the transplant core, there was marked differentiation of grafted cells into DCX positive cells (Fig. 4), suggesting that the majority of NSI graft-derived cells developed neuronal phenotypes by 3 to 4 weeks post-grafting. Careful examination of the morphologic phenotypes of engrafted cells positive for either immature or mature neuronal or astrocytic markers did not reveal any overt aberrant features. Furthermore, there were no apparent disruptions to hippocampal architecture caused by the presence of the engrafted cells. Quantification of differentiated phenotypes (Fig. 5) confirmed that engrafted NSIs preferentially committed to neuronal rather than astrocytic cell fates. While a further time course study is needed to quantify the fate of individual cells, current results indicate robust survival of grafted cells ~ 2 months following transplantation in the irradiated CNS. Further, while evidence suggests that NSI graft-derived cells undergo extensive proliferative within the transplant core, this does not appear to translate to teratoma formation over the duration of our study.
Collectively our data point to the importance of preserving the pool of multipotent cells in the brain. Similar concepts have been considered by the radiotherapy community, as improved treatments for brain cancer now routinely implement more sophisticated technologies and treatment planning designed to maximize dose to the tumor bed while minimizing radiation injury to the normal tissues of the brain (8). Stereotactic radiosurgical approaches (e.g. Intensity-Modulated Radiation Therapy, IMRT and Image-Guided Radiation Therapy, IGRT) provide the capability to design hippocampal sparing treatment plans in efforts to reduce adverse neurocognitive sequelae (8). The clinical benefit of protecting neurogenesis during the radiotherapeutic management of pediatric medulloblatoma has recently been estimated (9). When a variety of treatment plans designed to minimize dose to the hippocampus were evaluated, the estimated risk for developing memory impairment was reduced by 33-47% (9). Confounding this potential beneficial strategy however is a recent study retrospectively analyzing patients that were treated for malignant glioma (23). Patients receiving larger doses to the stem cell niches did better, suggesting that stem cell niches in the brain harbor glioma stem cells (23). Therefore, while sparing the hippocampus may lead to improved cognitive outcomes at the risk of compromising progression free survival, targeting the hippocampus may improve progression-free survival at the expense of debilitating cognitive decrements. A plausible but unconfirmed resolution to this dilemma may involve an intentional boost of irradiation to the neurogenic regions of the brain. This may enhance kill of migrant and resident cancer stem cells in efforts to prolong progression free survival, which could be followed by cranial stem cell transplantation to restore cognition.
While stem cell transplantation may one day provide relief to the many cancer survivors trying to manage their neurocognitive sequelae, it is premature at this point to presume efficacy in the absence of any human data. With this in mind, the present efforts to initiate preclinical testing of an FDA-approved, GMP-derived NSI stem cell line move these finding closer to the clinical arena. We are cognizant that additional mechanisms involving disruptions to the blood brain barrier and/or enhanced permeability of the specialized vascular niche in which stem cells reside (57), altered cerebral blood flow (16,33) and perturbations to neuronal anatomy (17,65) may contribute to the onset and progression of radiation-induced dementia. Nonetheless, lack of suitable and efficacious treatment options for the devastating side effects of cranial radiotherapy underscore the urgency for developing satisfactory interventions for this long-term mental health problem. Our efforts to thwart cognitive dysfunction by cell replacement therapy employing a GMP-derived cell line may provide the experimental backdrop for a potential solution.
Acknowledgments
This work was supported by the National Institutes of Health NINDS Grant R01 NS074388 (CLL) and by Neuralstem Inc. We thank Katherine Tran, Nicole Chmielewski and Lara Riparip for excellent technical assistance.
Footnotes
Authors declare no conflicts of interest.
References
- 1.Abayomi OK. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol. 2002;41(4):346–351. doi: 10.1080/028418602760169389. [DOI] [PubMed] [Google Scholar]
- 2.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 3.Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, Limoli CL. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106(45):19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71(14):4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya MM, Christie LA, Lan ML, Limoli CL. Comparing the functional consequences of human stem cell transplantation in the irradiated rat brain. Cell Transplant. 2013;22(1):55–64. doi: 10.3727/096368912X640565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84(4):363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson AJ, Cummings BJ, Cotman CW. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer’s disease: association with pathology. Exp Neurol. 1994;125(2):286–295. doi: 10.1006/exnr.1994.1031. [DOI] [PubMed] [Google Scholar]
- 8.Barani IJ, Cuttino LW, Benedict SH, Todor D, Bump EA, Wu Y, Chung TD, Broaddus WC, Lin PS. Neural stem cell-preserving external-beam radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):978–985. doi: 10.1016/j.ijrobp.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Blomstrand M, Brodin NP, Munck Af Rosenschold P, Vogelius IR, Sanchez Merino G, Kiil-Berthlesen A, Blomgren K, Lannering B, Bentzen SM, Bjork-Eriksson T. Estimated clinical benefit of protecting neurogenesis in the developing brain during radiation therapy for pediatric medulloblastoma. Neuro Oncol. 2012;14(7):882–889. doi: 10.1093/neuonc/nos120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6(2):278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulis NM, Federici T, Glass JD, Lunn JS, Sakowski SA, Feldman EL. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8(3):172–176. doi: 10.1038/nrneurol.2011.191. [DOI] [PubMed] [Google Scholar]
- 13.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28(46):11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 15.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7(6):517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Tsien CI, Sundgren PC, Nagesh V, Normolle D, Buchtel H, Junck L, Lawrence TS. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for prediction of radiation-induced neurocognitive dysfunction. Clin Cancer Res. 2009;15(5):1747–1754. doi: 10.1158/1078-0432.CCR-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 2012;7(7):e40844. doi: 10.1371/journal.pone.0040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christie LA, Acharya MM, Limoli CL. Quantifying cognitive decrements caused by cranial radiotherapy. Journal of visualized experiments : J Vis Exp. 2011;56:3108. doi: 10.3791/3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 20.Cizkova D, Kakinohana O, Kucharova K, Marsala S, Johe K, Hazel T, Hefferan MP, Marsala M. Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience. 2007;147(2):546–560. doi: 10.1016/j.neuroscience.2007.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH, Beute GN, Sluimer JD, Slotman BJ, Reijneveld JC, Heimans JJ. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 23.Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, Pajonk F. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18(1):115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19(2):122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30(6):1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 27.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 28.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, Campone MM, Twelves CC, Raymond E, Hegi ME, Lacombe D, van den Bent MJ. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Greenberger JS, Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol. 2009;19(2):133–139. doi: 10.1016/j.semradonc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, Sainte-Rose C, Dellatolas G, Raquin MA, Jambaque I, Kalifa C. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45(1):137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tiss Eng Regen Med. 2010;4(3):181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 33.Hahn CA, Zhou SM, Raynor R, Tisch A, Light K, Shafman T, Wong T, Kirkpatrick J, Turkington T, Hollis D, Marks LB. Dose-dependent effects of radiation therapy on cerebral blood flow, metabolism, and neurocognitive dysfunction. Int J Radiat Oncol Biol Phys. 2009;73(4):1082–1087. doi: 10.1016/j.ijrobp.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 34.Hefferan MP, Johe K, Hazel T, Feldman EL, Lunn JS, Marsala M. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant. 2011;20(8):1153–1161. doi: 10.3727/096368910X564553. [DOI] [PubMed] [Google Scholar]
- 35.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10(24):3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87(3):279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 37.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 39.Maisano X, Carpentino J, Becker S, Lanza R, Aaron G, Grabel L, Naegele JR. Embryonic stem cell-derived neural precursor grafts for treatment of temporal lobe epilepsy. Neurotherapeutics. 2009;6(2):263–277. doi: 10.1016/j.nurt.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park) 2000;14(1):75–79. discussion 79, 81-72, 85. [PubMed] [Google Scholar]
- 41.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 42.Meyers CA, Geara F, Wong PF, Morrison WH. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46(1):51–55. doi: 10.1016/s0360-3016(99)00376-4. [DOI] [PubMed] [Google Scholar]
- 43.Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14(4):629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 44.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 45.Peister A, Zeitouni S, Pfankuch T, Reger RL, Prockop DJ, Raber J. Novel object recognition in Apoe(-/-) mice improved by neonatal implantation of wild-type multipotential stromal cells. Exp Neurol. 2006;201(1):266–269. doi: 10.1016/j.expneurol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Peper M, Steinvorth S, Schraube P, Fruehauf S, Haas R, Kimmig BN, Lohr F, Wenz F, Wannenmacher M. Neurobehavioral toxicity of total body irradiation: a follow-up in long-term survivors. Int J Radiat Oncol Biol Phys. 2000;46(2):303–311. doi: 10.1016/s0360-3016(99)00442-3. [DOI] [PubMed] [Google Scholar]
- 47.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 48.Riess P, Molcanyi M, Bentz K, Maegele M, Simanski C, Carlitscheck C, Schneider A, Hescheler J, Bouillon B, Schafer U, Neugebauer E. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J Neurotrauma. 2007;24(1):216–225. doi: 10.1089/neu.2006.0141. [DOI] [PubMed] [Google Scholar]
- 49.Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, Taub J, Kesner V, Feldman EL, Boulis NM. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71(2):405–416. doi: 10.1227/NEU.0b013e31825ca05f. discussion 416. [DOI] [PubMed] [Google Scholar]
- 50.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 52.Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68(23):9763–9770. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saury JM, Emanuelson I. Cognitive consequences of the treatment of medulloblastoma among children. Pediatr Neurol. 2011;44(1):21–30. doi: 10.1016/j.pediatrneurol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO Europ. Org. Res. Treat. Canc. Brain Tumor & Radiat Oncol Groups; Natl. Canc. Inst. Can. Clin. Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 56.Surma-aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, Paetau A, Kallio M, Pyykkonen J, Jaaskelainen J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56(10):1285–1290. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 57.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usvald D, Vodicka P, Hlucilova J, Prochazka R, Motlik J, Kuchorova K, Johe K, Marsala S, Scadeng M, Kakinohana O, Navarro O, Santa R. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 2010;19(9):1103–1122. doi: 10.3727/096368910X503406. [DOI] [PubMed] [Google Scholar]
- 59.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 60.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514(4):297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82(7):865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci. 2007;27(44):11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24(8):1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 64.Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4(2):e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou Y, Corniola R, Leu D, Khan A, Sahbaie P, Chakraborti A, Clark DJ, Fike JR, Huang TT. Extracellular superoxide dismutase is important for hippocampal neurogenesis and preservation of cognitive functions after irradiation. Proc Natl Acad Sci U S A. 2012;109(52):21522–21527. doi: 10.1073/pnas.1216913110. [DOI] [PMC free article] [PubMed] [Google Scholar]


