Abstract
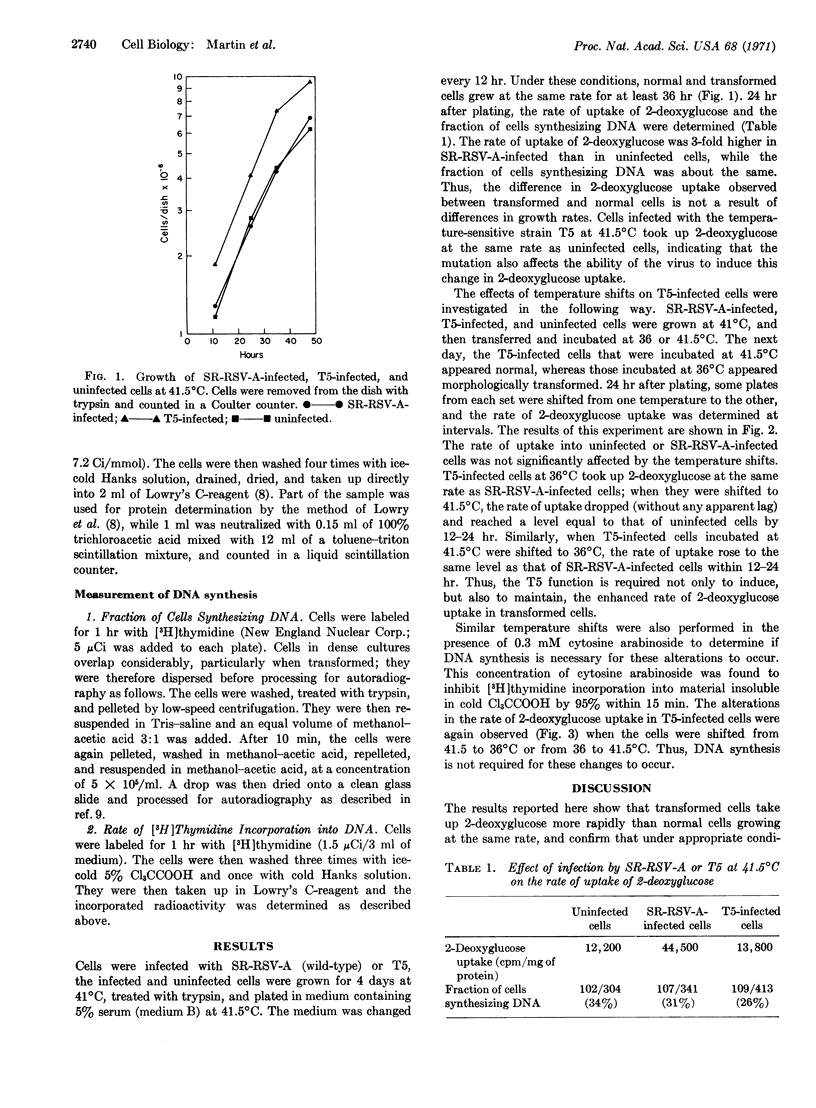
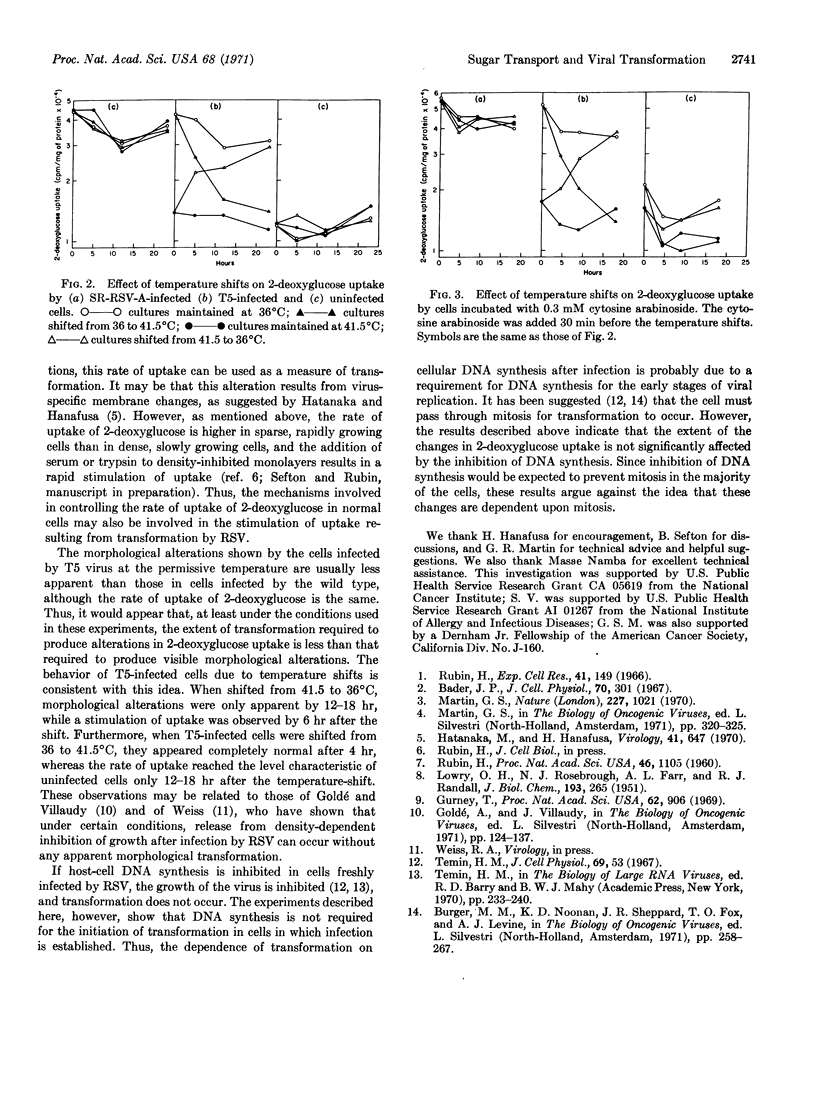
Cells transformed by Rous sarcoma virus take up 2-deoxyglucose at a faster rate than uninfected cells, under conditions where transformed and nontransformed cells grow at the same rate. In cells infected by a temperature-sensitive mutant, the stimulation of 2-deoxyglucose uptake is temperature dependent: the increase is observed at the permissive (36°C), but not at the nonpermissive (41.5°) temperature. When infected cells are shifted from the nonpermissive temperature to the premissive temperature, the uptake of 2-deoxyglucose increases from a rate equal to that of uninfected cells to a rate equal to that of cells infected by the wild-type Schmidt-Ruppin Rous sarcoma virus. The reverse change occurs when the infected cells are shifted from the permissive to the nonpermissive temperature. By the use of cytosine arabinoside, an inhibitor of DNA synthesis, it was possible to show that DNA synthesis is neither required for the transformation, which occurs when the infected cells are shifted from the nonpermissive to the permissive temperature, nor for the phenotypic reversion, which occurs in the reverse shift.
Keywords: 2-deoxyglucose uptake, transformation, cytosine arabinoside
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P. A change in growth potential of cells after conversion by Rous sarcoma virus. J Cell Physiol. 1967 Dec;70(3):301–308. doi: 10.1002/jcp.1040700310. [DOI] [PubMed] [Google Scholar]
- Gurney T., Jr Local stimulation of growth in primary cultures of chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1969 Mar;62(3):906–911. doi: 10.1073/pnas.62.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. The inhibition of chick embryo cell growth by medium obtained from cultures of Rous sarcoma cells. Exp Cell Res. 1966 Jan;41(1):149–161. doi: 10.1016/0014-4827(66)90555-6. [DOI] [PubMed] [Google Scholar]


