Abstract
Mother's milk is the fundamental food for infants. It contains proteins, fat, carbohydrates and essential metals which are necessary to ensure correct functioning of the organism. Unfortunately, breast milk is a potential source of toxic metals, which are dangerous for a baby. In Poland, previous research concerning the content of metals in breast milk was very scarce or its results were unavailable. The present study aimed at assessing the content of Cd, Pb, Cu and Zn in human breast milk, as well as estimating the mean weekly intake of these metals by breast-fed infants from Poland. The average concentrations of Cd, Pb, Cu and Zn were 2.114 μg/l, 6.331 μg/l, 0.137 mg/l and 1.623 mg/l, respectively. The admissible levels of supply of these toxic metals has not been exceeded, but their contents were high, particularly in 6-month-old infants (nearly 85 % TWI for Cd and nearly 70 % BMDL01 for Pb). The daily intake of Cu and Zn did not fully satisfy the infant's requirements determined by Polish standards and WHO recommendations. Since the lifestyle of lactating women has a direct influence on the content of these elements in breast milk, women should be educated in this respect with particular focus on eliminating tobacco smoking, both by breastfeeding mothers and by their direct environment.
Keywords: Breast milk, Infant, Cadmium, Lead, Zinc, Copper, Intake
Introduction
Breastfeeding is the best method of nutrition for infants. The World Health Organisation (WHO) recommends breastfeeding as the exclusive method of nutrition for newborns and infants at least up to 6 months of age [1]. Previous research by this author [2] carried out in the region of Lublin (eastern Poland) demonstrated that as many as 87 % of women were breastfeeding for at least 6 months. The components of mother's milk, such as lactoferrin, lysozyme and α-lactalbumin, create a barrier protecting the baby against harmful environmental factors, enhancing the body's defence mechanisms and stimulating the development of the immune system [3]. Breast milk also has an influence on the development of intestinal microflora [3] and structural and functional maturity of mucous membranes and reduces the risk of allergies and autoimmune diseases [4]. However, mother's milk can also be a source of cadmium (Cd) and lead (Pb) for a baby. The content of these elements in breast milk reflects the level of environmental pollution and the mother's diet [5–8]. The presence of these metals in food products has become a global problem [5, 9, 10]. In Poland, the highest content of Cd and Pb is found in food derived from plants including but not limited to vegetables, in particular, potatoes, and grains and cereals [11]. Mothers who smoked tobacco create an additional source of toxic trace elements in the body, thus increasing the content of Cd and Pb in the breast milk [12, 13]. Research by Öhrvik et al. [14] revealed that Cd leads to a dysfunction of the mammary glands in mice, which is the reason for developmental disorders among sucklings. Since ‘heavy metals’ is a poor scientific term [15], in the present study, the term ‘toxic metals’ is used with reference to Cd and Pb, and ‘essential metals’ with reference to copper (Cu) and zinc (Zn).
The cycle of metals in the environment is linked with the food chain: soil–plant–animal–man. The transfer of toxic metals to the higher link results in a cumulative increase in their content [16]. Cd is primarily deposited in the liver and kidneys, but in infants, it poses the greatest hazard to their dynamically developing nervous system, also affecting bone-formation processes, and is a significant carcinogen [17–20]. Tests on rats demonstrate that Cd supplied with milk affects the serotonin level in the brain of a growing animal [21]. The highest Cd accumulation levels are recorded during the first 3 years of human life [22]. In children, it can inhibit intellectual development and causes anaemia and rickets [23]. Significantly, a positive correlation between the concentration of Cd and Pb in children and the occurrence of autism has been observed [16]. Neurological effects of Pb observed in children include motor skills disorder and behavioural problems [24]. Exposure to this metal for several years after birth is particularly detrimental to the future intellectual potential of children [2, 25]. Children are exposed to accumulation of Pb due to a slower excretion process and lower body weight and also because of reduced immunity [26].
Essential metals, such as Cu and Zn, are necessary to ensure the correct functioning of the body but, in excessive amounts, have a harmful effect [27]. Cu is an element necessary for synthesising haemoglobin, forming myelin sheaths in the nervous system, and for bone tissue formation processes [27, 28]. Zn is an important element at the foetal stage [27]. A deficit of this element can lead to defects in the nervous system and cause retardation of the foetus' growth and development [29].
In Poland, previous research concerning the content of Pb, Cd, Cu and Zn in breast milk was very scarce or its results were unavailable. No detailed evaluations of the effect of age and lifestyle of Polish women on the content of metallic elements in breast milk are available. The study is aimed to evaluate the content of Cd, Pb, Cu and Zn in their breast milk and to estimate the intake of these metals by breastfed infants.
Materials and Methods
Materials
Breast milk samples (~25 ml) were collected by manual expression from 320 healthy lactating women from the Lublin region (eastern Poland) during 7 days of sampling. All women were informed about the purpose of the study and voluntarily consented to provide breast milk samples for analysis. Contact with the subjects was established during the medical check-up of their children in paediatric clinics.
The samples were collected in the period from August to December 2010. Each sample was sealed in a polyethylene bag and frozen at -20 °C for further analysis.
Methods
The breast milk samples were shaken manually before the analysis. Also, three samples were taken (2 ml) from each of the bags. Samples were dried at 65°C for 12 h, next at 105°C for 24 h, and mineralised in a muffle furnace. The samples were treated by dry mineralisation at a temperature of 450°C for 12 h, and the ash obtained was suffused with 2 ml of hydrogen peroxide, evaporated until dry and once more burnt at a temperature of 450°C for 12 h. Ashed samples were diluted in spectrally pure 1 M HNO3. The content of Cd and Pb was determined by GF AAS technique in a Varian Spectr AA 880 apparatus, including atomisation in a graphite furnace and using the Zeeman background correction. Argon was used as the pure gas. The content of Cd was determined at λ = 228.8 nm, with 4 mA and 0.5 nm spectral bandpass (LOD 0.01 mg/kg, LOQ 0.02 mg/kg). The deviation of duplicate measurement was below 5.3 %. The content of Pb was determined at wavelength λ = 217.0 nm, with 10 mA and 1 nm spectral bandpass (LOD 0.209 mg/kg, LOQ 0.419 mg/kg). The content of Zn and Cu was determined using the FAAS flame technique in a Varian SpectrAA 280 FS apparatus (with SPS3 autosampler). Zn was detected at λ = 213.9 nm, with 5 mA and 1 nm spectral bandpass (LOD 0.01 mg/kg, LOQ 0.02 mg/kg), Cu at λ = 324.8 nm, with 4 mA and 0.5 nm spectral bandpass (LOD 0.013 mg/kg, LOQ 0.016 mg/kg). In order to make the calibration line, standard solutions of Cd, Pb, Zn and Cu were procured from Merck (Germany). A series of solutions of varying concentrations were prepared for all the examined ions by diluting the standards: 0.00, 0.10, 0.20, 0.40, 1.00 and 2.00 ng/ml. Quality control of analytical measurements was performed using blank samples and certified reference materials: IRMM-804 Rice Flour (Cd) and BCR-063R Skimmed Milk Powder (Pb, Zn and Cu). The certified reference materials contained Cd 1.16 mg/g, Pb 0.815 mg/g, Zn 49.0 μg/g and Cu 0.602 μg/g. The mean recovery rate of Cd from the reference materials was 96 %, of Pb 95 %, of Zn 117 % and of Cu 121 %.
Calculation of Metals Intake
The mean intake of Cd, Pb, Cu and Zn in breast milk was calculated for infants aged 1, 3, 6 and 12 months. Since it is difficult to calculate the daily intake of milk by a breast-fed infant, the supply of powdered milk, as recommended in Poland, was used as a reference value [30]. The calculation of the tolerable intake of toxic metals was based on mean infant body weight (Table 1). The tolerable weekly intake (TWI) for Cd was determined at 2.5 μg/kg of body weight/week [31]. The benchmark dose lower confidence limit (BMDL01) for Pb was determined at 0.5 μg/kg of body weight/day (3.5 μg/kg of body weight/week) [32].
Table 1.
Average body weight of infants from Poland and daily milk consumption
| Age of children | ||||
|---|---|---|---|---|
| 1 month | 3 months | 6 months | 12 months | |
| Average body weighta relevant for Poland, g | 4,500 | 6,250 | 7,750 | 10,500 |
| Daily consumption of milkb, ml | 700 | 780 | 900 | 660 |
aThe average body weight relevant for Poland was estimated according to a centile chart for infants. The values for boys and girls were averaged
bThe supply of milk formulas, as recommended in Poland, was used as a reference value, adopter from Ref. [30]
Statistical Analysis
The results were analysed using statistical methods. Arithmetic mean values, standard deviation (SD), median, maximum and minimum values and percentile values (25 and 75) were calculated using STATISTICA 6.0 software. The p values < 0.05 were considered significant. Full factorial analysis of variance (ANOVA) was used to test for significant effects of the women's age, stage of lactation and tobacco smoking on Cd, Pb, Cu and Zn levels in breast milk. The significance of differences between mean values was estimated using Tukey's test.
Profile of the Analysed Population of Women
During the first meeting the women were interviewed to determine the profile of the studied population (age, number of lactations, period of current lactation). The questions also concerned exposure of the women to factors likely to affect the content of toxic metals in milk (occupational exposure, smoking). The data was presented in Table 2.
Table 2.
The profile of the analysed population of women
| Age of mothers, years | Total | ||||
|---|---|---|---|---|---|
| 20–25 | 26–30 | 31–35 | 36–40 | ||
| Number of women | 35 | 158 | 70 | 60 | 323 |
| Place of residence | |||||
| Village | 25 | 59 | 30 | 9 | 123 |
| City < 25,000 habitants | 10 | 28 | 22 | 0 | 60 |
| City > 25,000 habitants | 0 | 71 | 18 | 51 | 140 |
| Number of lactation | |||||
| I | 35 | 143 | 25 | 10 | 213 |
| II | 0 | 15 | 45 | 0 | 60 |
| III | 0 | 0 | 0 | 40 | 40 |
| IV | 0 | 0 | 0 | 10 | 10 |
| Lactation stage | |||||
| 1 month | 0 | 40 | 10 | 9 | 59 |
| 2–3 months | 11 | 52 | 9 | 36 | 108 |
| 4–6 months | 14 | 36 | 36 | 0 | 86 |
| 7–12 months | 10 | 30 | 15 | 15 | 70 |
| Smoking habit | |||||
| Current smoker | 0 | 20 | 20 | 0 | 40 |
| Past smoker | 0 | 23 | 0 | 3 | 26 |
| Never | 35 | 115 | 50 | 57 | 257 |
| Passive smoking | |||||
| Yes | 12 | 34 | 8 | 7 | 61 |
| No | 23 | 124 | 62 | 53 | 262 |
| Occupational exposure to Cd or Pb | 0 | 0 | 0 | 0 | 0 |
Results
Content of Cd and Pb in the Breast Milk
Table 3 presents the content of Cd, Pb, Cu and Zn covered by the research in the breast milk and effects of women's age. The average content of Cd in the milk slightly exceeded 2 μg/l (0.215–7.355), while that of Pb amounted to 6.3 μg/l (0.486–12.01). The highest content of Cd was found in the breast milk of women aged 31 to 35 (more than 3 μg/l), while most Pb was discovered in the breast milk of women aged 36 to 40 and 26 to 30 (approx. 7–7.4 μg/l). The lowest content of both Cd and Pb was characteristic of the milk of women aged 20 to 25.
Table 3.
Content of Cd, Pb, Cu, and Zn in the breast milk and effect of the mother's age, μg/l
| Age of mothers, years | n | Cd | Pb | Cu | Zn |
|---|---|---|---|---|---|
| 20–25 | 35 | 0.723a | 4.762a | 0.088a | 0.701a |
| 26–30 | 158 | 2.151b | 6.932c | 0.154c | 2.504c |
| 31–35 | 70 | 3.031d | 6.221b | 0.137b | 1.674b |
| 36–40 | 60 | 2.552c | 7.411d | 0.169d | 1.612b |
| Mean | 2.114 | 6.331 | 0.137 | 1.623 | |
| Standard deviation | 2.112 | 4.614 | 0.092 | 1.763 | |
| Minimum value | 0.215 | 0.486 | 0.025 | 0.043 | |
| Maximum value | 7.355 | 12.01 | 0.455 | 8.160 | |
| Median | 1.260 | 1.951 | 0.106 | 1.240 | |
| 25th percentile | 0.557 | 1.602 | 0.069 | 0.448 | |
| 75th percentile | 3.017 | 9.188 | 0.158 | 1.941 | |
| Effects of women's age | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
a,b,c,dIn the same column, values with different superscripts differ significantly (p < 0.05)
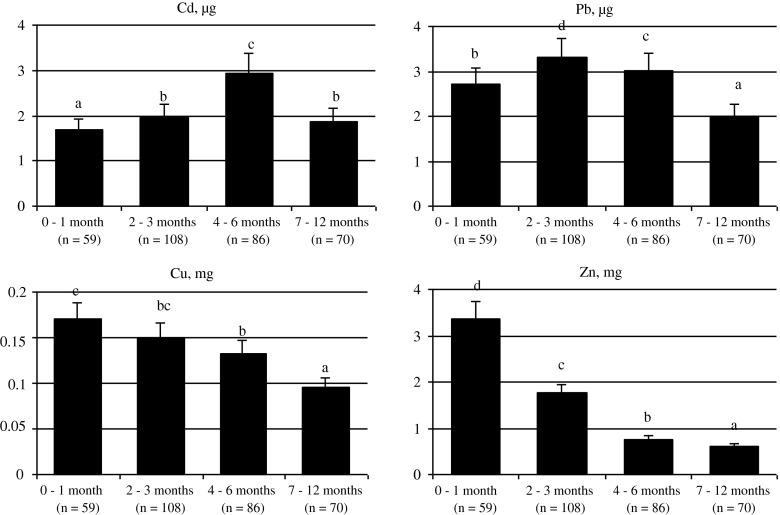
The stage of lactation had a significant effect on the content of Cd (ANOVA; F = 9.76; p < 0.05) and Pb (ANOVA; F = 2.35; p < 0.05) in the breast milk (Fig. 1). The highest content of Cd was characteristic of breast milk between 4 and 6 months of lactation (2.94 μg/l), while the lowest value was recorded for the first month's milk, i.e. the colostrum (less than 1.7 μg/l). The highest content of Pb was found in milk between 2 and 3 months of lactation (nearly 3.3 μg/l), while it was definitely lower in milk between 7 and 12 months of lactation (less than 2 μg/l).
Fig. 1.
Effects of the lactation stage (months) on Cd, Pb, Cu and Zn levels in breast milk, per 1 L. a,b,c,d p < 0.05
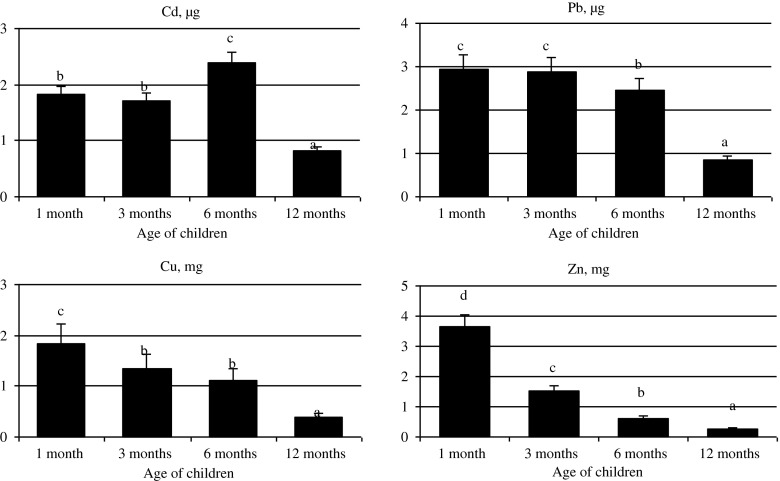
Figure 2 presents the mean weekly intake of metals by the infant in the first, third, sixth and 12th months of life per 1 kg of body weight. The results were calculated based on the mean content of the analysed components in milk, depending on the stage of lactation. The body weight of an infant and the estimated daily intake of milk is presented in Table 1. Table 4 compares the % TWI and % BMDL01 for infants aged 1, 3, 6 and 12 months with the average body weight recorded in Poland, assuming the value of 2.5 μg Cd [31] and 3.5 μg Pb [32] per 1 kg of body weight/week. It was determined that the admissible levels of Cd and Pb supply were not exceeded; they were elevated though. For Cd, this was from 32.8 % TWI (in 12-month-old babies) to 84.8 % TWI (in 6-month-old infants), whereas for Pb from 23.9 % BMDL01 (in 12-month-old babies) to 84 % BMDL01 (in 1-month-old infants).
Fig. 2.
Mean weekly intake of Cd, Pb, Cu and Zn from breast milk, per 1 kg of infant's body weight. a,b,c,d p < 0.05
Table 4.
% TWI (for Cd) and % BMDL01 (for Pb) for infants aged 1, 3, 6, and 12 months with the average body weight relevant for Poland (see Table 1), μg/kg of body weight/week
| Cd | Pb | |
|---|---|---|
| EFSA norm | 2.5 μg [31] | 3.5 μg [32] |
| Infant's age | % TWI | % BMDL01 |
| 1 month | 72.72b | 84.00c |
| 3 months | 67.70b | 82.37c |
| 6 months | 84.84c | 69.91b |
| 12 months | 32.84a | 23.89a |
a,b,cIn the same column, values with different superscripts differ significantly, p < 0.05
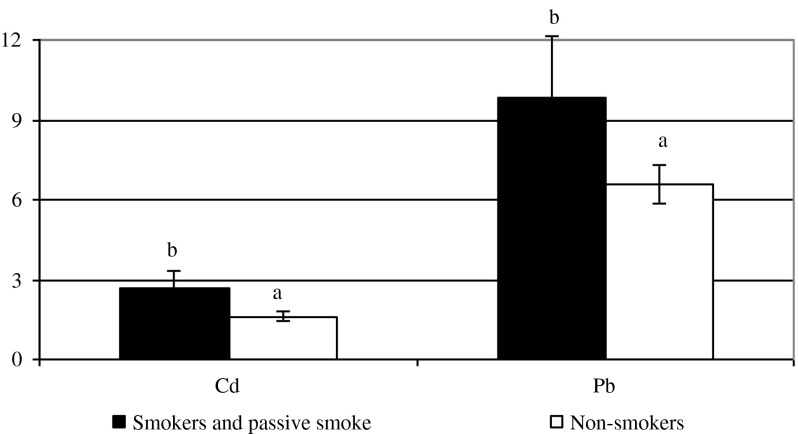
Figure 3 presents the content of Cd and Pb in the breast milk of smoking women compared to non-smokers. ANOVA indicated that this factor had a significant effect on the levels of Cd (F = 27.08; p < 0.05) and Pb (F = 18.22; p < 0.05). As expected, more toxic metals were found in the milk of women who smoked tobacco.
Fig. 3.
Mean levels of Cd and Pb in breast milk of smoking and non-smoking women, μg/l. a,b p < 0.05
Content of Cu and Zn in the Breast Milk
The average content of Cu in the milk slightly exceeded 0.137 mg/l (0.025–0.455), while that of Zn amounted to 1.623 mg/l (0.043–8.160; Table 3). The highest content of Cu was recorded in the milk of women aged 26–30 and 36–40 (approx. 0.15–0.17 mg/l). The breast milk of women aged 20–25 was found to contain half as much Cu. The highest content of Zn was characteristic of the breast milk of women aged 26–30 (more than 2.5 mg/l), while the lowest value was recorded for the milk of mothers aged 20–25 aged (slightly above 0.7 mg/l).
The content of Cu and Zn in breast milk was analysed depending on the stage of lactation (Fig. 1). The highest content of Cu was recorded in the breast milk of women in the first month of lactation (0.17 mg/l), while the lowest value of this element was noted in milk between 7 and 12 months of breastfeeding (less than 0.1 mg/l). The highest content of Zn was also found in the first month's milk (more than 3.3 mg/l), while the lowest value was recorded for milk between 7 and 12 months of lactation (approx. 0.61 mg/l).
The calculated % of coverage of the infant's requirement of Cu and Zn supplied in mother's milk is presented in Table 5. It was revealed that the daily supply of Cu did not fully cover the infant's demand for this element determined in the Polish standard [33]. About 60 % of the infants' requirement was covered during the first 6 months of life compared with less than 30 % during the second 6 months. Breast milk in the first month of lactation was rich in Zn–the supply of this element exceeded the infant's requirement by approximately 17 %. Afterwards, the concentration of this element decreased and after the first 6 months of life mother's milk covered only 20 % of the Zn requirement.
Table 5.
The calculated % of coverage of an infant's requirement of Cu and Zn from breast milk
| Cu | Zn | |
|---|---|---|
| Polish norm, mg/day [33] | 0.2–0.3 | 2.0 |
| % of Polish norm | ||
| Infant's age | ||
| 1 month | 59.39b | 117.6d |
| 3 months | 60.18b | 68.52c |
| 6 months | 61.69b | 34.43b |
| 12 months | 29.11a | 20.13a |
| WHO norm, mg/day [40] | 0.33–0.62 | 5.33–5.6 |
| % of WHO norm | ||
| Infant's age | ||
| 1 month | 36.00bc | 44.36d |
| 3 months | 36.47c | 25.86c |
| 6 months | 33.35b | 22.21b |
| 12 months | 9.701a | 7.189a |
a,b,c,dIn the same column, values with different superscripts differ significantly, p < 0.05
Discussion
Content of Cd, Pb, Cu and Zn in the Breast Milk
The content of Cd and Pb in breast milk is a global problem. It is primarily related to the common presence of these toxic metals in the environment and thus inevitable exposure to their effect [26, 34]. The present study showed no excessive admissible levels of toxic metals supply. However, the high content of Cd and Pb in milk consumed by infants at the age of 6 months is a disturbing fact since breast milk is not the only source of these metals for them. Research revealed that toxic metals are also present in other infant food, both in Poland [35] and in other countries [36, 37]. Admittedly, the studies referred above revealed no excessive standard limits; still, there is a risk of excessive supply of Cd and Pb in infants fed with breast milk and, at the same time, given complementary foods, particularly when in 2012 the admissible intake levels of Cd and Pb were radically reduced [31, 32]. In Table 6, the levels of Cd and Pb in breast milk recorded in this study were compared with those of other selected countries. The recorded Cd milk levels were considerably lower than those reported in Spain, Brazil and Nigeria and were higher than those of women from Sweden and Italy. The highest content of Cd—within the range of 0.6–1.3 μg/l—was recorded in the milk of mothers from Spain. The content of Pb resulting from the present study was comparable to data from Austria—the results referred to the maximum levels of Pb determined for this country—and was significantly lower than in women from Spain, Iran, Saudi Arabia and China (Table 6).
Table 6.
Cd, Pb, Cu and Zn concentrations in breast milk of women from Poland and from other countries
| Cd, μg/l | Pb, μg/l | Cu, mg/l | Zn, mg/l | References | |
|---|---|---|---|---|---|
| Poland | 0.21–7.35 | 0.49–12.0 | 0.03–0.46 | 0.04–8.16 | This study |
| Poland | 5.4 | [60] | |||
| Poland | 0.27–0.45 | 1.4–8.2 | [48] | ||
| Slovakia | 0.43 | 4.7 | [61] | ||
| Sweden | 0.07 | 0.5 | [62] | ||
| Sweden | 0.028–0.27 | 0.74–6.4 | 0.32–0.67 | 1.24–5.71 | [50] |
| Spain | 0.6–11.3 | 0.1–32.3 | [63] | ||
| Austria | 0.02–11.17 | [64] | |||
| Austria | 0.22–0.26 | [65] | |||
| Portugal | 0.07–4.03 | 0.33–0.97 | 0.39–5.09 | [47] | |
| Croatia | 0.27–1.35 | 0.62–15.0 | [38] | ||
| Greece | 0.19 | 0.48 | 0.38 | 4.90 | [46] |
| Brazil | 1.0–8.0 | [34] | |||
| Nigeria | 9.7 | 8.7 | 0.83 | 0.7 | [66] |
| Kuwait | 0.41–0.71 | 1.7–3.2 | [67] | ||
| Japan | 0.07–1.22 | 0.12–0.70 | 2.73–11.6 | [51] | |
| Japan | 0.35 | 1.45 | [68] | ||
| Libya | 0.39–0.84 | 4.95–16.1 | [39] | ||
| Iran | 0.62–6.35 | 3.18–24.67 | [13] | ||
| Iran | 0.45–5.87 | 3.06–19.47 | [69] | ||
| Iran | 0.36 | 2.95 | [44] | ||
| Saudi Arabia | 1.73 | 31.67 | 3.10 | [70] | |
| Guatemala | 0.09–0.60 | 0.47–6.19 | [71] | ||
| China | 0.67 | 40.6 | [72] | ||
| Italy | <LOQ | 0.85–1.07 | 0.35–0.42 | 0.70–0.90 | [73] |
Mandić et al. [38] found that the breast milk of Croatian women contained nine times more Zn than the milk analysed during the present study (Table 6). The content of Zn in the analysed samples amounted to 1–4.95 mg/l. The highest content of Zn was recorded in the breast milk of women from Libya and the lowest in women from Venezuela [39]. According to Hannan et al. [39], the daily intake of Cu and Zn from the Libyan mothers' milk is higher than the recommended daily amount (RDA) value, whereas this study shows that the daily supply of Cu and Zn did not fully satisfy the infant's demand for those elements as defined by the Polish standard [33] and the WHO standard [40]. A low level of Cu causes no proven risk of nutritional deficiencies of Cu in the child during the first 6 months of lactation. Babies are born with hepatic reserves of Cu to balance low concentrations of these elements in breast milk [41]. Additionally, Zn and Cu in breast milk are characterised by high bioavailability compared with infant milk formulas [42–44], and it may be supposed that the body's resources of these trace elements are sufficient for breast-fed babies. On the other hand, a low content of Cu is a condition for bacteriostatic properties of breast milk [45]. However, it must be remembered that mother's milk is the sole source of food for an infant only during the initial 5–6 months of life, so a deficiency of Cu and Zn after the first 6 months of life should not cause alarm since the infant is also supplied with this element in other food.
Effects of Lactation Stage
The present study findings suggest a relationship between the stage of lactation and the content of Cd and Pb in milk. Leotsinidis et al. [46] found that the colostrum of Greek women contained much more Cd and Pb (0.19 and 0.48 μ/l) than the post-colostral milk (0.14 and 0.15 μ/l). By contrast, in our study, the lowest value for Cd was recorded for the first month's milk.
The content of Zn and Cu in breast milk depends on the stage of lactation. Almeida et al. [47] recorded 500 μg (0.5 mg) of Cu and nearly 2300 μg (2.3 mg) of Zn per 1 l of breast milk on the 30th day of lactation. According to Wasowicz et al. [48], the highest content of Zn and Cu is found in colostrum. The present study also demonstrated that the milk of mothers in the first 6 months of lactation contained more Zn and Cu than in the second 6 months. A statistical analysis carried out by Rydzewska and Król [49] revealed a correlation between the concentration of Zn and the day of the postpartum period and increased content of Zn and reduced level of Cu in the colostrum. Leotsinidis et al. [46] found that the colostrum of Greek women contained much more Zn than the post-colostral milk (4.9 vs. 2.99 mg/l). However, no significant difference was found in the content of Cu in the colostrum and the post-colostral milk. A different content of essential trace elements in the colostrum and the post-colostral milk can occur due to genetic conditions of the lactation process [50].
Effects of Women's Age
According to Rahimi et al. [13] the average concentration of Cd and Pb in women aged under 30 years was lower than in women aged over 30 years. In this study, the lowest content of both Cd and Pb was characteristic of the milk of women aged 20 to 25. The present study shows that the age of the women is also a factor affecting the concentration of essential trace elements in milk. Honda et al. [51] show that a woman's age is a factor affecting the concentration of essential trace elements in her breast milk. Picciano [52] found that the breast milk of women over 30 contains more Zn and Cu than that of women under 30. By contrast, Koyashiki et al. [34] and Khaghani et al. [44] found no statistically significant effect of the woman's age on the content of Zn and Cu.
Effects of Lifestyle
Cd and Pb in breast milk reflected maternal exposure. Nishijo et al. [7] observed a positive correlation between maternal Cd burden and Cd concentration in breast milk. Gundacker et al. [6] found a correlation between the content of Pb in the breast milk of Austrian women and their body weight, duration of pregnancy as well as diet and lifestyle. These authors have found the highest accumulation of Pb in the milk of women weighing less than 60 kg and women who gave birth after week 37 of pregnancy. Also the content of this element was significantly higher than in the milk of women who often consumed fish and food supplements.
Smoking had a great influence on the Pb value in breast milk. The milk of women who have never smoked tobacco contained 1.57 μg of Pb, while the milk of currently smoking women–2.4 μg/l (5). Research by Kwapuliński et al. [53] revealed the highest content of Cd in the breast milk of active and passive smokers—0.27 μg/g. In the group of non-smokers, the content of Cd was lower—approximately 0.20 μg/g. The present study also showed that the content of Cd and Pb in the milk of women who smoked was significantly higher than in non-smokers. According to Rahimi et al. [13], the concentration of Cd and Pb in breast milk significantly increased in mothers who were exposed to smoking compared to non-smokers (3.39 vs. 2.12 μg/l of Cd and 12.22 vs. 9.78 μg/l of Pb). The present study showed that the content of Cd and Pb in the breast milk of women who smoked tobacco was significantly higher than in non-smoking mothers.
Many researchers found no influence of Cu and Zn in the diet on the content of Cu and Zn in breast milk [54, 55], while single researchers demonstrated that the level of Zn slightly increased after receiving Zn supplements [56]. Domellöf et al. [57] found no correlation between the content of Cu and Zn in milk and the mineral status of a woman. On the other hand, however, since breast milk of women from different regions of the world, who due to climatic, economic and cultural differences have different diet routines, contains different amounts of Zn and Cu (Table 6), the effect of the diet routine on the content of these essential metals in milk cannot be completely excluded. It is possible that a diet deficient in Zn and Cu has an influence on the low content of these elements in milk. Hambidge [29] found that Zn deficiency was a problem affecting approximately 50 % of the population. Some authors found that the diet of Polish girls and women is deficient in Zn [58]. According to Szymelfejnik et al. [59], 50 % of female students are at a high risk of Zn deficiency and approximately 90 % are likely to have Cu deficiency.
To sum up, the admissible levels of Cd and Pb supply were not exceeded but their content was high, particularly in 6-month-old infants (nearly 85 % TWI for Cd, nearly 70 % BMDL01 for Pb). It is significant that the analysed milk contained insufficient amounts of Zn and Cu compared with an infant's requirements of these elements. The reasons for low levels of these essential metals in breast milk must be carefully reviewed. Perhaps these could be attributed to nutritional mistakes of lactating women. The analysed milk should be regarded as safe for infants; however, as breast milk is the basic food for infants, it must be continuously monitored for the level of toxic and essential metals. Since the lifestyle of lactating women has a direct influence on the content of these elements in breast milk, women should be educated in this respect with particular focus on eliminating tobacco smoking, both by breastfeeding mothers and by their direct environment.
References
- 1.WHO . Global strategy for infant and young child feeding. Geneva: WHO Library Cataloguing-in-Publication Data; 2003. [Google Scholar]
- 2.Winiarska-Mieczan A. Assessment of infant exposure to lead and cadmium content in infant formulas. J Elementol. 2009;14:573–581. [Google Scholar]
- 3.Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77(Suppl):1537–1543. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 4.Matson AP, Thrall RS, Rafti E, Lingenheld EG, Puddington L. Research IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin Mol Allergy. 2010;8:9. doi: 10.1186/1476-7961-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong S, Schirnding YE, Prapamontol T. Environmental lead exposure a public health problem of global dimensions. Bull WHO. 2000;78:1068–1077. [PMC free article] [PubMed] [Google Scholar]
- 6.Gundacker C, Pietschnig B, Wittmann JK, Lischka A, Salzer H, Hohenauer L, Schuster E. Lead and mercury in breast milk. Pediatrics. 2002;110:873–878. doi: 10.1542/peds.110.5.873. [DOI] [PubMed] [Google Scholar]
- 7.Nishijo M, Nakagawa H, Honda R, Tanabe K, Saito S, Teranishi K, Tawara K. Effects of maternal exposure to cadmium on pregnancy and breast milk. Occup Environ Med. 2002;59:394–397. doi: 10.1136/oem.59.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koizumi N, Murata K, Hayashi C, Nishio H, Goji J. High cadmium accumulation among humans and primates: comparison across mammalian species—a study from Japan. Biol Trace Elem Res. 2008;121:205–214. doi: 10.1007/s12011-007-8048-9. [DOI] [PubMed] [Google Scholar]
- 9.Yüzbaşi N, Sezgin E, Yıldırım M, Yıldırım Z. Survey of lead, cadmium, iron, copper and zinc in Kasar cheese. Food Addit Contam, Part A. 2003;20:464–469. doi: 10.1080/0265203031000094654. [DOI] [PubMed] [Google Scholar]
- 10.Iwegbue CMA, Nwajei GE, Arimoro FO, Eguavoen O. Characteristic levels of heavy metals in canned sardines consumed in Nigeria. Environment. 2009;29:431–435. [Google Scholar]
- 11.Kot A, Zaręba S, Wyszogrodzka-Koma L. Assessment of cadmium contamination in cereals, cereal products and potatoes. Bromat Chem Toksykol. 2009;3:537–542. [Google Scholar]
- 12.Kutlu T, Karagozler AA, Gozukara EM. Relationship among placental cadmium, lead, zinc, and copper levels in smoking pregnant women. Biol Trace Elem Res. 2006;114:7–17. doi: 10.1385/BTER:114:1:7. [DOI] [PubMed] [Google Scholar]
- 13.Rahimi E, Hashemi M, Torki Baghbadorani Z. Determination of cadmium and lead in human milk. Int J Environ Sci Technol. 2009;6:671–676. doi: 10.1007/BF03326108. [DOI] [Google Scholar]
- 14.Öhrvik H, Yoshioka M, Oskarsson A, Tallkvist J. Cadmium-induced disturbances in lactating mammary glands of mice. Toxicol Lett. 2006;3:207–213. doi: 10.1016/j.toxlet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Duffus JH. “Heavy metals”—a meaningless term? Pure Appl Chem. 2002;74:793–807. doi: 10.1351/pac200274050793. [DOI] [Google Scholar]
- 16.Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR. A case-control study of mercury burden in children with autistic spectrum disorders. J Am Phys Surg. 2003;3:76–79. [Google Scholar]
- 17.IARC . Cadmium and cadmium compounds. CAS No. 7440-43-9. International Agency for Research on Cancer. Lyon: Monographs. IARC Press; 1993. [Google Scholar]
- 18.Silbergeld EK, Waalkes M, Rice JM. Lead as a carcinogen: experimental evidence and mechanisms of action. Am J Ind Med. 2000;38:316–323. doi: 10.1002/1097-0274(200009)38:3<316::AID-AJIM11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 20.Sughis M, Penders J, Haufroid V, Nemery B, Nawrot TS. Bone resorption and environmental exposure to cadmium in children: a cross-sectional study. Environ Health. 2011;10:104–109. doi: 10.1186/1476-069X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson H, Petersson-Grawe K, Lindqvist E, Luthman J, Oskarsson A, Olson L. Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol Teratol. 1997;19:105–115. doi: 10.1016/S0892-0362(96)00218-8. [DOI] [PubMed] [Google Scholar]
- 22.Sreedharan R, Mehta DI. Gastrointestinal tract. Pediatrics. 2004;113:1044–1049. [PubMed] [Google Scholar]
- 23.D'Souza HS, Menezes G, Venkatesh T. Role of essential trace minerals on the absorption of heavy metals with special reference to lead. Ind J Clin Biochem. 2003;18:154–160. doi: 10.1007/BF02867382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich KN, Berger OG, Succop PA. Lead exposure and the motor development status of urban six-year-old children in the Cincinnati prospective study. Pediatrics. 1993;91:301–307. [PubMed] [Google Scholar]
- 25.Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Patison N, Law AJ, Korsch MJ, Salter MA. Relationships of lead in breast milk to lead in blood, urine and diet of the infant and mother. Environ Health Perspect. 1998;106:667–674. doi: 10.1289/ehp.98106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants: a review. Afr J Food Sci. 2010;4:200–222. [Google Scholar]
- 28.Wu J, Ricker M, Muench J. Copper deficiency as cause of unexplained hematologic and neurologic deficits in patient with prior gastrointestinal surgery. J Am Board Family Med. 2006;19:191–194. doi: 10.3122/jabfm.19.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Hambidge M. Human zinc deficiency. J Nutr. 2000;130:1344–1349. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 30.Książyk J, Weker H. New feeding plan for infants in Poland, since 2007. Pediatr Współcz Gastroenterol Hepatol Żyw Dziecka. 2007;9:9–14. [Google Scholar]
- 31.EFSA Cadmium dietary exposure in the European population. EFSA J. 2012;10:2551–2588. [Google Scholar]
- 32.EFSA Lead dietary exposure in the European population. EFSA J. 2012;10:2831–2890. [Google Scholar]
- 33.Jarosz M, Bułhak-Jachymczyk B. Norms of human nutrition: essential of prevention of obesity and non-infectious diseases. Warsaw: PZWL; 2008. [Google Scholar]
- 34.Koyashiki GAK, Paoliello MMB, Matsuo T, de Oliveira MMB, Mezzaroba L, Carvalho MF, Sakuma AM, Turini C, Vannuchi MTO, Barbosa CSD. Lead levels in milk and blood from donors to the Breast Milk Bank in Southern Brazil. Environ Res. 2010;110:265–271. doi: 10.1016/j.envres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Winiarska-Mieczan A, Kiczorowska B. Determining the content of lead and cadmium in baby food from the Polish market. Int J Food Sci Nutr. 2012;63:708–712. doi: 10.3109/09637486.2011.644765. [DOI] [PubMed] [Google Scholar]
- 36.Dabeka R, Fouquet A, Belisle S, Turcotte S. Lead, cadmium and aluminum in Canadian infant formulae, oral electrolytes and glucose solutions. Food Addit Contam. 2011;28:744–753. doi: 10.1080/19393210.2011.571795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Khlifa AS, Ahmad D. Determination of key elements by ICP-OES in commercially available infant formulae and baby foods in Saudi Arabia. Afr J Food Sci. 2010;4(7):464–468. [Google Scholar]
- 38.Mandić Z, Mandić ML, Grgić J, Grgić Z, Klapec T, Primorac L, Hasenay D. Copper and zinc content in human milk in Croatia. Eur J Epidemiol. 1993;13:185–188. doi: 10.1023/A:1007337303724. [DOI] [PubMed] [Google Scholar]
- 39.Hannan AM, Dogadkin NN, Ashur AI, Markus MW. Copper, selenium, and zinc concentrations in human milk during the first three weeks of lactation. Biol Trace Elem Res. 2005;107:11–20. doi: 10.1385/BTER:107:1:011. [DOI] [PubMed] [Google Scholar]
- 40.WHO (2003) Feeding and nutrition of infants and young children. Guidelines for the WHO European Region, with emphasis on the former Soviet countries. European Series, No 87. WHO Regional Publication, Copenhagen
- 41.Donangelo CM, Trugo NMF, Dorea JG. Hepatic reserves of iron, copper and vitamin B12 in Brazilian fetuses and infants of different socioeconomic status. Nutrition. 1993;9:430–432. [PubMed] [Google Scholar]
- 42.Casey CE, Hambidge KM, Neville MC. Studies in human lactation: zinc, copper, manganese and chromium in human milk in the first month of lactation. Am J Clin Nutr. 1985;41:1193–1200. doi: 10.1093/ajcn/41.6.1193. [DOI] [PubMed] [Google Scholar]
- 43.Lönnerdal B, Bell JG, Keen CL. Copper absorption from human milk, cow's milk, and infant formulas using a suckling rat model. Am J Clin Nutr. 1985;42:836–844. doi: 10.1093/ajcn/42.5.836. [DOI] [PubMed] [Google Scholar]
- 44.Khaghani S, Ezzatpanah H, Mazhari N, Givianrad M-H, Mirmiranpour H, Sadrabadi FS. Zinc and copper concentrations in human milk and infant formulas. Iran J Pediat. 2010;20:53–57. [PMC free article] [PubMed] [Google Scholar]
- 45.Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12:2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 46.Leotsinidis M, Alexopoulos A, Kostopoulou-Farri E. Toxic and essential elements in human milk from Greek lactating women. Association with dietary habits and other factors. Chemosphere. 2005;61:238–247. doi: 10.1016/j.chemosphere.2005.01.084. [DOI] [PubMed] [Google Scholar]
- 47.Almeida AA, Lopes MPVC, Silva MSA, Barrado E. Trace elements in human milk; Correlation with blood levels, inter-element correlations and concentration during the first month of lactation. J Trace Elem Med Biol. 2008;22:196–205. doi: 10.1016/j.jtemb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Wasowicz W, Gromadzinska J, Szram K, Rydzyński K, Cieślak J, Pietrzak Z. Selenium, zinc, and copper concentrations in the blood and milk of lactating women. Biol Trace Elem Res. 2001;79:221–233. doi: 10.1385/BTER:79:3:221. [DOI] [PubMed] [Google Scholar]
- 49.Rydzewska A, Król I. Contents of zinc, copper and cadmium in milk of women living in Poznań. Ginekol Pol. 1996;67:125–128. [PubMed] [Google Scholar]
- 50.Björklund KL, Vahter M, Palm B, Grandér M, Lignell S, Berglund M. Metals and trace element concentrations in breast milk of first time healthy mothers: a biological monitoring study. Environ Health. 2012;11:92–99. doi: 10.1186/1476-069X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honda R, Tawara K, Nishijo M, Nakagawa H, Tanebe K, Satio S. Cadmium exposure and trace elements in human breast milk. Toxicology. 2003;186:255–259. doi: 10.1016/S0300-483X(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 52.Picciano MF. Human milk: nutritional aspects of a dynamic food. Biol Neonate. 1998;74:84–93. doi: 10.1159/000014015. [DOI] [PubMed] [Google Scholar]
- 53.Kwapuliński J, Wiechuła D, Fischer A. The influence of smoking and passive smoking to occurrence of metals in breast milk. Przegl Lek. 2004;61:1113–1115. [PubMed] [Google Scholar]
- 54.Vuori E, Makinen SM, Kara R, Kuitunen P. The effects of the dietary intakes of copper, iron, manganese, and zinc on the trace element content of human milk. Am J Clin Nutr. 1980;33:227–231. doi: 10.1093/ajcn/33.2.227. [DOI] [PubMed] [Google Scholar]
- 55.Lönnerdal B. Effects of maternal dietary intake on human milk composition. J Nutr. 1986;116:499–513. doi: 10.1093/jn/116.4.499. [DOI] [PubMed] [Google Scholar]
- 56.Krebs NF, Reidinger CJ, Hartley S, Robertson AD, Hambidge KM. Zinc supplementation during lactation: effects on maternal status and milk zinc concentrations. Am J Clin Nutr. 1985;61:1030–1036. doi: 10.1093/ajcn/61.4.1030. [DOI] [PubMed] [Google Scholar]
- 57.Domellöf M, Lönnerdal B, Dewey KG, Cohen RJ, Hernell O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am J Clin Nutr. 2004;79:111–115. doi: 10.1093/ajcn/79.1.111. [DOI] [PubMed] [Google Scholar]
- 58.Bojar I, Owoc A, Humeniuk E, Wierzba W, Fronczak A. Inappropriate consumption of vitamins and minerals by pregnant women in Poland. Ann Agric Environ Med. 2012;19:263–266. [PubMed] [Google Scholar]
- 59.Szymelfejnik EJ, Wadolowska L, Cichon R. Magnesium, zinc and copper intake by Polish university students. Pak J Nutr. 2008;7:436–443. doi: 10.3923/pjn.2008.436.443. [DOI] [Google Scholar]
- 60.Baranowska I. The concentration of some trace elements in human milk. Pol J Environ Stud. 1994;3:5–8. [Google Scholar]
- 61.Ursinyova M, Massanova V. Cadmium, lead and mercury in human milk from Slovakia. Food Addit Contam. 2005;22:579–589. doi: 10.1080/02652030500135201. [DOI] [PubMed] [Google Scholar]
- 62.Palminger Hallen I, Jorhem L, Lagerkvist BJ, Oskarson A. Lead and cadmium levels in human milk and blood. Sci Total Environ. 1995;166:149–155. doi: 10.1016/0048-9697(95)04523-4. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez Rodriguez EM, Delgado Uretra E, Diaz Romero C. Concentrations of cadmium and lead in different types of milk. Z Lebensm Unters Forsch A. 1999;208:162–168. doi: 10.1007/s002170050395. [DOI] [Google Scholar]
- 64.Gundacker C, Pietschnig B, Wittmann KJ, Lischka A. Human milk mercury (Hg) and lead (Pb) levels in Vienna. Adv Exp Med Biol. 2000;478:387–388. doi: 10.1007/0-306-46830-1_40. [DOI] [PubMed] [Google Scholar]
- 65.Rossipal E, Krachler M. Pattern of trace elements in human milk during the course of lactation. Nutr Res. 1998;18:11–24. doi: 10.1016/S0271-5317(97)00196-6. [DOI] [Google Scholar]
- 66.Adesiyan AA, Akiibinu MO, Olisekodiaka MJ, Onuegbu AJ, Adeyeye AD. Concentrations of some biochemical parameters in breast milk of a population of Nigerian nursing mothers using hormonal contraceptives. Pak J Nutr. 2011;10:249–253. doi: 10.3923/pjn.2011.249.253. [DOI] [Google Scholar]
- 67.Al-awadi FM, Srikumar TS. Trace-element status in milk and plasma of Kuwaiti and non-Kuwaiti lactating mothers. Nutrition. 2000;16:1069–1073. doi: 10.1016/S0899-9007(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 68.Yamawaki N, Yamada M, Kan-no T, Kojima T, Kaneko T, Yonekubo A. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol. 2005;19:171–181. doi: 10.1016/j.jtemb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Goudarzi MA, Parsaei P, Navebpour F, Rahimi E. Determination of mercury, cadmium and lead in human milk in Iran. Toxicol Ind Health. 2012 doi: 10.1177/0748233712445047. [DOI] [PubMed] [Google Scholar]
- 70.Al-Saleh I, Shinwari N, Mashhour A. Heavy metal concentrations in the breast milk of Saudi women. Biol Trace Elem Res. 2003;96:21–37. doi: 10.1385/BTER:96:1-3:21. [DOI] [PubMed] [Google Scholar]
- 71.Dhonukshe-Rutten RAM, Vossenaar M, West CE, Schümann K, Bulux J, Solomons NW. Day-to-day variations in iron, zinc and copper in breast milk of guatemalan mothers. J Pediatr Gastroenterol Nutr. 2005;40:128–134. doi: 10.1097/00005176-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Liu K-S, Hao J-H, Xu Y-Q, Gu X-Q, Shi J, Dai Ch-F XF, Shen R. Breast milk lead and cadmium levels in suburban areas of Nanjing, China. Chin Med Sci J. 2013;28:7–15. doi: 10.1016/S1001-9294(13)60012-7. [DOI] [PubMed] [Google Scholar]
- 73.Abballe A, Ballard TJ, Dellatte E, di Domenico A, Ferri F, Fulgenzi AR, Grisanti G, Iacovella N, Ingelido AM, Malisch R, Miniero R, Porpora MG, Risica S, Ziemacki G, De Felip E. Persistent environmental contaminants in human milk: concentrations and time trends in Italy. Chemosphere. 2008;73:220–227. doi: 10.1016/j.chemosphere.2007.12.036. [DOI] [PubMed] [Google Scholar]