Abstract
Background
We investigated the differences in HBsAg kinetics at different levels of viremia in hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB).
Methods
We compared HBsAg levels among HBeAg-negative CHB patients with persistently undetectable HBV DNA (≤20 IU/mL; Group A, n = 100), HBV DNA 20–2,000 IU/mL (Group B, n = 100), and HBV DNA >2,000 IU/mL (Group C, n = 100). HBsAg and HBV DNA levels were measured at three consecutive time points during follow-up (median 21.4 months).
Results
Median HBsAg levels were significantly lower in Group A than in Groups B and C at all time points (p < 0.001). HBV DNA and HBsAg levels were weakly correlated (r = 0.180 and 0.151 for Groups B and C, respectively). Among patients with HBsAg <100 IU/mL, Group A patients had the greatest median serum HBsAg reduction (0.341 log IU/mL/year; Group B, 0.122 log IU/mL/year; Group C, 0.057 log IU/mL/year; p = 0.002). Among Group A patients with HBsAg <100 IU/mL, baseline HBsAg achieved an AUROC of 0.876 in predicting >1 log annual HBsAg reduction; 10–100 IU/mL HBsAg was the optimal level for prediction (sensitivity 90 %; specificity 74.6 %). Serum HBsAg/HBV DNA ratios were significantly higher in Group B than in Groups A and C (p < 0.05).
Conclusions
HBV DNA and HBsAg were weakly correlated. Only patients with undetectable HBV DNA showed decline in HBsAg levels during follow-up. The greatest reduction in HBsAg levels occurred in patients with baseline HBsAg <100 IU/mL.
Keywords: Chronic hepatitis B, HBsAg, HBeAg, HBV DNA, Kinetics
Introduction
With the gradual aging of the hepatitis B virus (HBV)-infected population, hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) has become the predominant form of CHB worldwide [1–3]. Traditionally, HBeAg-negative CHB patients are classified as inactive ‘healthy’ carriers, indicating a quiescent form of the disease [4]. However, recent studies have shown that disease progression still occurs in a substantial proportion of HBeAg-negative patients [5, 6], with more than 73 % of Asian CHB patients being HBeAg-negative when cirrhosis or hepatocellular carcinoma (HCC) develops [7]. With improvements in the sensitivity of HBV DNA assays, serum HBV DNA levels are found to fluctuate markedly throughout the natural history of HBeAg-negative CHB [8, 9], with elevated levels associated with the development of cirrhosis and HCC [10–12]. The presence of a core promoter mutation, found mainly in HBeAg-negative CHB, is also associated with an increased risk of HCC development [13].
Serum hepatitis B surface antigen (HBsAg) quantification has been recently advocated as an additional marker in the monitoring of CHB [14]. Serum HBsAg titer was initially developed as a surrogate marker for intrahepatic HBV and closed covalently circular DNA (cccDNA) [15, 16]. However, a recent study found that HBsAg levels correlated with serum HBV DNA and intrahepatic cccDNA in only HBeAg-positive disease, and not in HBeAg-negative patients [17]. A recent longitudinal study has shown serum HBsAg to decrease at a slow rate of 0.04 log IU/mL per year, although >1 log reduction in HBsAg has been associated with good immune control [18].
The lack of correlation between serum HBsAg and HBV DNA levels in HBeAg-negative CHB was also observed in recent studies investigating the natural history of changes in serum HBsAg levels [19, 20]. These studies divided the HBeAg-negative cohort into only two groups: ‘inactive carriers’ (serum HBV DNA <2,000 IU/mL) and ‘HBeAg-negative hepatitis’ [serum HBV DNA ≥2,000 IU/mL with elevated alanine aminotransferase (ALT)]. The use of single-point serum HBsAg and HBV DNA has been shown to be able to identify inactive carriers [21], while a low HBV DNA level was predictive of subsequent HBsAg seroclearance [22]. These findings suggest a possible association between the two serum markers in HBeAg-negative CHB, but probably only after more detailed stratification of different viral loads.
In our present study, we propose studying serum HBsAg kinetics in three groups of HBeAg-negative patients stratified by their serum HBV DNA levels, including a group of patients with persistently undetectable serum HBV DNA (≤20 IU/mL).
Methods
The Liver Clinic, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong is a tertiary referral center for the management of chronic liver diseases. Between January 2005 and April 2011, 100 HBsAg-positive, HBeAg-negative CHB patients (Group A) were noted to have persistently undetectable serum HBV DNA (≤20 IU/mL) over three consecutive time points during their follow-up. The median duration of follow-up for these patients was 19.0 months (range 10.8–45.0 months). All patients remained HBsAg-positive and were treatment-naive. All serum collected was stored at −20 °C until testing.
An additional 200 HBsAg-positive, HBeAg-negative treatment-naive patients with follow-up between June 2006 and July 2011 were recruited for comparison. They were divided into two groups: 100 patients with baseline serum HBV DNA between 20 and 2,000 IU/mL (Group B) and 100 patients with baseline HBV DNA >2,000 IU/mL (Group C). The median duration of follow-up for Groups B and C was 21.7 months (range 12.2–51.9 months) and 22.5 months (range 11.2–35.8 months), respectively. Serum HBsAg and HBV DNA levels were measured at three consecutive time points within the follow-up period; no significant difference was noted in the median duration of follow-up among all three groups (p = 0.573). Patients from all three groups were age- and sex-matched at a ratio of 1:1:1 at baseline, with all patients being HBsAg-positive and HBeAg-negative for at least 6 months prior to enrollment.
Patients with concomitant hepatitis C and D infection, evidence of Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, and significant intake of alcohol (30 g per day for males, 20 g per day for females) were excluded. In addition, patients requiring antiviral therapy, or encountering HBeAg seroreversion or HBsAg seroclearance were also excluded. This study was approved by the institutional review board of the University of Hong Kong and West Cluster of Hospital Authority, Hong Kong.
Laboratory assays
Serum HBsAg, antibody to HBsAg (anti-HBs), HBeAg, and antibody to HBeAg (anti-HBe) were measured using commercially available immunoassays (Abbott Laboratories, Chicago, IL, USA). Serum HBV DNA was measured using Cobas Taqman assay (Roche Diagnostics, Branchburg, NJ, USA), with a linear range of 20–1.98 × 108 IU/mL. Serum HBsAg titer was measured using the Elecsys HBsAg II assay (Roche Diagnostics, Gmbh, Mannheim), with a linear range of 0.05–13,000 IU/mL. Samples with HBsAg levels higher than 13,000 IU/mL were retested at a dilution of 1:100, according to the manufacturer’s instructions. The upper limit of normal for serum ALT was set at 40 U/L.
Statistical analysis
All continuous values were expressed in median (range). Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), with a two-sided p value of <0.05 being considered statistically significant. For patients with undetectable serum HBV DNA or HBsAg, the results were taken as the lower limit of detection (20 and 0.05 IU/mL, respectively). The HBsAg (log IU/mL)/HBV DNA (log IU/mL) ratio, which reflects the percentage of subviral particles over virions, was measured. The annual HBsAg log reduction was calculated using the reduction achieved between the first and third time points with reference to the total period of follow-up. To compare the characteristics between the three patient groups, the Mann–Whitney U test, or the Kruskal–Wallis test when appropriate, was used for continuous variables with a skewed distribution; the Chi-square test was used for categorical variables. Correlation between serum HBsAg levels and HBV DNA levels was tested using Spearman’s bivariate correlation. The predictions of reduction in serum HBsAg were first examined by the construction of corresponding receiver operating characteristic (ROC) curves, followed by the assessment of overall accuracy by areas under the curves (AUCs).
Results
The baseline clinical parameters of all three groups are depicted in Table 1. Patients in Group C had the highest median HBsAg levels, followed by patients in Group B. Patients in Group B had a significantly higher HBsAg/HBV DNA ratio than those in Group A (p = 0.002) and Group C (p < 0.001).
Table 1.
Baseline parameters of all three patient groups
| Group A (n = 100) | Group B (n = 100) | Group C (n = 100) | p value | |||
|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | ||||
| Males | 61 | 61 | 61 | 1.000 | ||
| Age (years) | 47.5 (18.5 to 77.2) | 49.6 (21.4 to 79.8) | 48.9 (22.5 to 77.4) | 0.609 | 0.391 | 0.910 |
| Cirrhosis | 3 | 2 | 2 | – | ||
| Albumin (g/L) | 45 (36 to 51) | 44 (34 to 50) | 44 (37 to 50) | 0.101 | 0.136 | 0.095 |
| Bilirubin (μmol/L) | 10 (2 to 99) | 10 (4 to 50) | 10 (4 to 39) | 0.079 | 0.581 | 0.247 |
| ALT (U/L) | 26 (9 to 102) | 26 (10 to 97) | 34.5 (8 to 89) | 0.317 | 0.016 | <0.001 |
| HBV DNA (IU/mL) | 20 | 407 (27 to 1,921) | 17,520 (2,503 to 3.59 × 106) | <0.001 | ||
| HBsAg (IU/mL) | 7.14 (0.061 to 10,860) | 514 (0.085 to 57,530) | 1,729 (0.47 to 28,920) | <0.001 | ||
| HBsAg/HBV DNA ratio | 0.655 (−0.980 to 3.10) | 1.045 (−0.728 to 2.651) | 0.713 (−0.568 to 1.168) | 0.002 | 0.372 | <0.001 |
All continuous variables expressed in median (range). HBsAg/HBV DNA ratio expressed in logarithm
ALT alanine aminotransferase, HBsAg hepatitis B surface antigen, Group A HBV DNA persistently ≤20 IU/mL, Group B baseline HBV DNA 20–2,000 IU/mL, Group C baseline HBV DNA >2,000 IU/mL
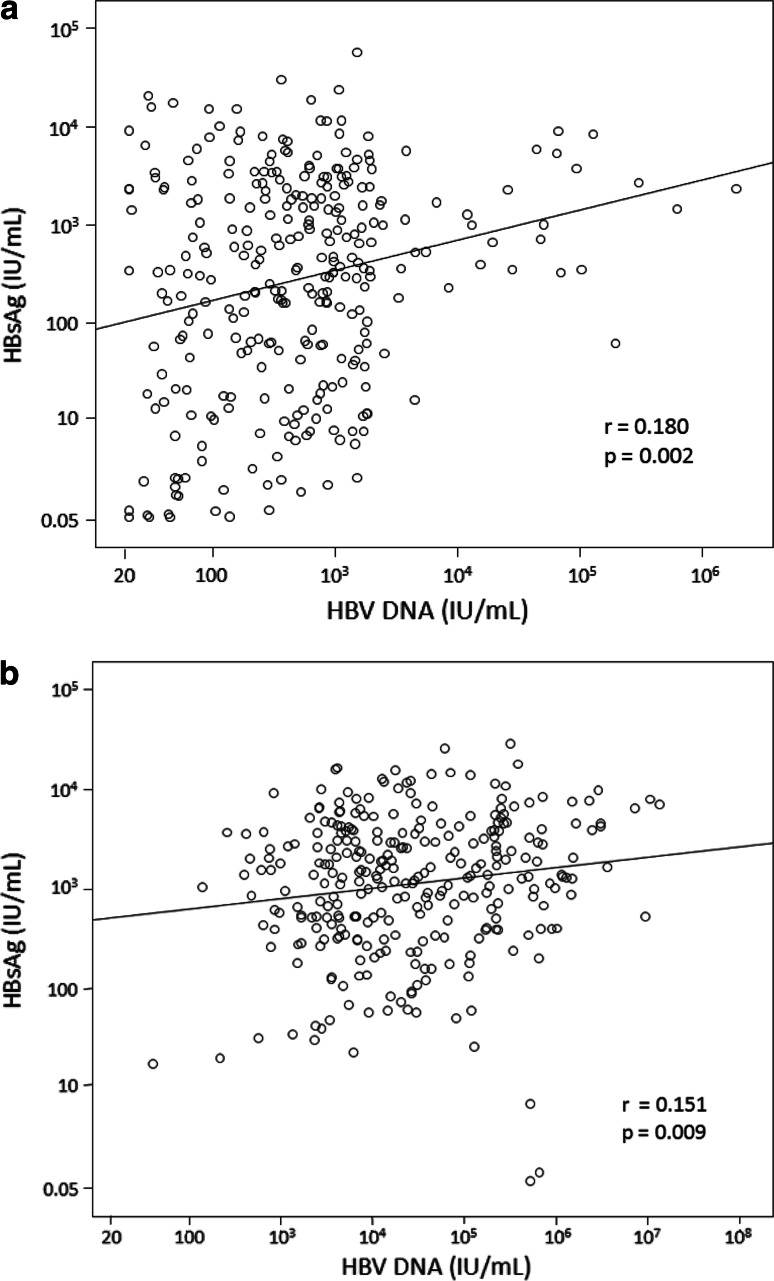
The correlations between serum HBsAg and HBV DNA for Groups B and C are depicted in Fig. 1a, b, respectively. In both groups, serum HBsAg correlated poorly with serum HBV DNA (r = 0.180 and 0.151, respectively).
Fig. 1.
Correlation of serum HBsAg and HBV DNA levels in Groups B (a) and C (b)
HBsAg levels
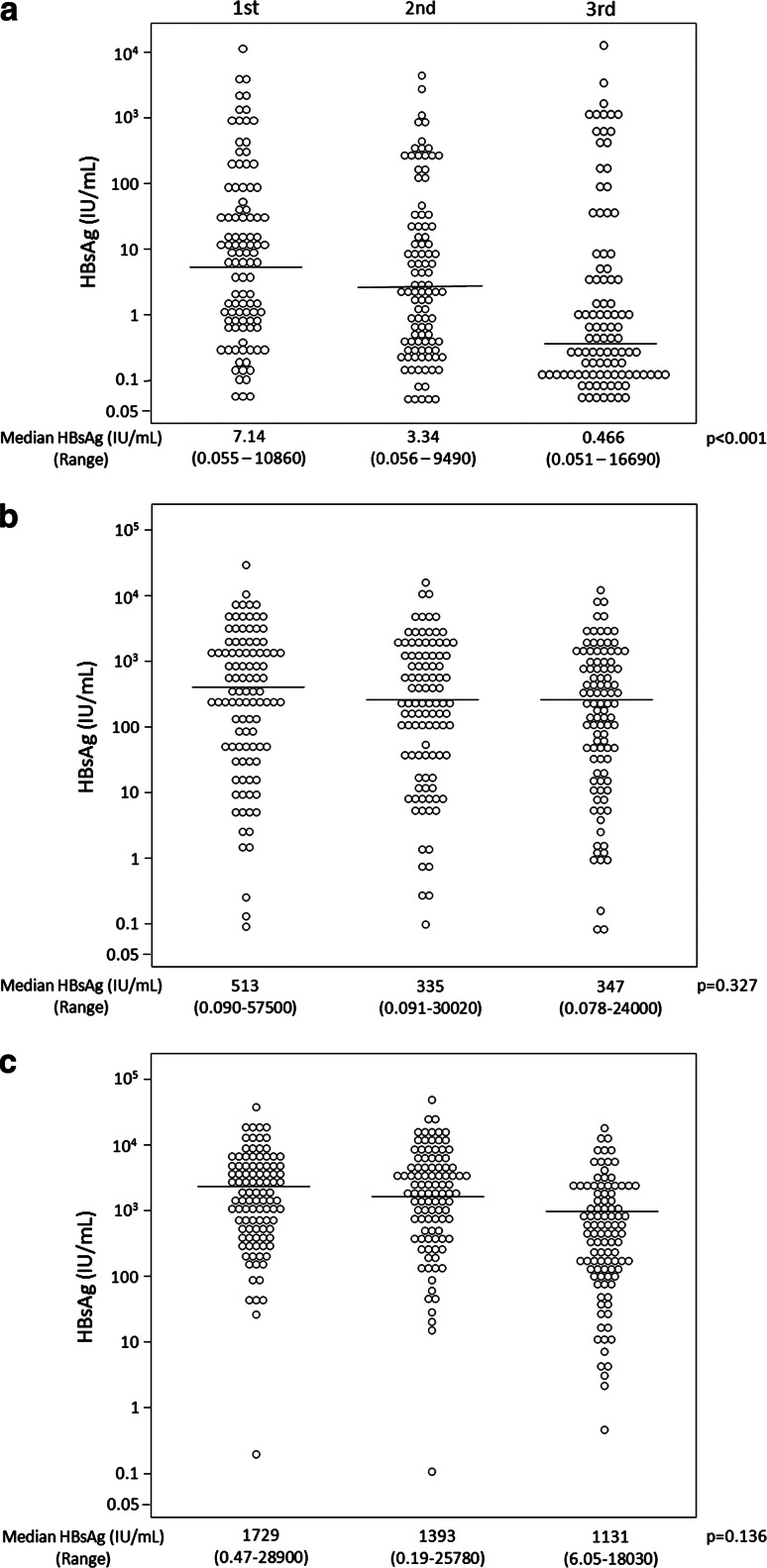
The changes in serum HBsAg kinetics in all three patient groups are depicted in Table 2 and Fig. 2. For all time points, median HBsAg levels increased significantly from Group A to Group B to Group C (p < 0.001; Tables 1, 2). During the follow-up period, median serum HBsAg levels significantly decreased in Group A (p < 0.001) and remained similar in Groups B and C (p = 0.327 and 0.136, respectively).
Table 2.
Serum HBsAg levels, HBV DNA levels, and HBsAg/HBV DNA ratio throughout the study period
| 2nd time point | p value | 3rd time point | p value | |
|---|---|---|---|---|
| HBsAg levels (IU/mL) | ||||
| Group A | 3.34 (0.056 to 9,490) | 0.466 (0.051 to 16,690) | ||
| Group B | 335 (0.091 to 30,020) | <0.001 | 347 (0.078 to 24,000) | <0.001 |
| Group C | 1,393 (0.19 to 25,780) | 1,131 (6.05 to 18,030) | ||
| HBV DNA levels (IU/mL) | ||||
| Group A | 20 | 20 | ||
| Group B | 473 (20 to 1.89 × 106) | <0.001 | 558 (20 to 6.23 × 105) | <0.001 |
| Group C | 19,200 (40 to 1.06 × 107) | 20,700 (140 to 1.35 × 107) | ||
| HBsAg/HBV DNA ratio | ||||
| Group A | 0.403 (−0.993 to 3.057) | <0.001 | −0.255 (−0.993 to 4.014) | <0.001 |
| Group B | 0.872 (−0.484 to 2.950) | 0.004 | 0.865 (−0.822 to 3.044) | 0.005 |
| Group C | 0.699 (−0.125 to 1.478) | 0.687 (0.137 to 1.407) | ||
All continuous variables expressed in median (range). HBsAg/HBV DNA ratio expressed in logarithm
HBsAg hepatitis B surface antigen, Group A HBV DNA persistently ≤20 IU/mL, Group B baseline HBV DNA 20–2,000 IU/mL, Group C baseline HBV DNA >2,000 IU/mL
Fig. 2.
Distribution of serum HBsAg levels in Groups A (a), B (b), and C (c)
The annual log reduction in HBsAg for Groups A, B, and C were 0.316 (range −1.11 to 2.05), 0.138 (range −1.04 to 1.46), and 0.109 (range −1.42 to 0.66) log IU/mL per year, respectively. The annual log reduction in HBsAg for Group A was significantly greater than that for Groups B (p = 0.001) and C (p < 0.001). There was no significant difference in the annual log reduction in HBsAg between Group B and Group C (p = 0.089).
HBV DNA levels
The changes in serum HBV DNA levels are depicted in Table 2. Seventy-nine patients (79 %) in Group B had serum HBV DNA persistently <2,000 IU/mL throughout the follow-up period. For Groups B and C, there were no significant changes in HBV DNA levels throughout the study period (p = 0.945 and 0.851, respectively).
Prediction of HBsAg reduction
The median annual HBsAg log reduction in patients stratified by baseline HBsAg levels is depicted in Table 3. Among patients with serum HBsAg <100 IU/mL, Group A patients experienced greater HBsAg reduction than Group B and C patients (p = 0.002). In contrast, in patients with baseline serum HBsAg 100–1,000 or ≥1,000 IU/mL, the difference in HBsAg reduction for different viral loads was not significant.
Table 3.
Association of baseline HBsAg and HBV DNA levels with HBsAg reduction
| HBsAg (IU/mL) | Groups | Number of patients (%) | Annual HBsAg log reduction (log IU/mL per year) | p value | |
|---|---|---|---|---|---|
| ≥1,000 | A | 7 (6.2 %) | 0.192 (−1.429 to 1.493) | 0.533 | |
| B | 42 (36.8 %) | 0.153 (−0.214 to 1.010) | |||
| C | 65 (57.0 %) | 0.112 (−0.180 to 0.659) | |||
| 100–1,000 | A | 12 (17.1 %) | 0.082 (−0.686 to 1.897) | 0.527 | |
| B | 28 (40.0 %) | 0.130 (−1.039 to 0.484) | |||
| C | 30 (42.9 %) | 0.105 (−1.418 to 0.405) | |||
| <100 | A | 81 (69.8 %) | 0.341 (−1.114 to 2.049) | 0.002 0.321 |
0.002 |
| B | 30 (25.8 %) | 0.122 (−0.478 to 1.468) | |||
| C | 5 (4.3 %) | 0.057 (−0.622 to 0.213) | |||
Continuous variables expressed in median (range)
The increased HBsAg reduction in patients with HBsAg <100 IU/mL and undetectable HBV DNA is highlighted in italics
HBsAg hepatitis B surface antigen, Group A HBV DNA persistently ≤20 IU/mL, Group B baseline HBV DNA 20–2,000 IU/mL, Group C baseline HBV DNA >2,000 IU/mL
In Group A, patients with baseline serum HBsAg <100 IU/mL (n = 81) had a larger median annual reduction in HBsAg than patients with serum HBsAg ≥100 IU/mL (n = 19) (0.341 and 0.116 log IU/mL/year, respectively), although the difference was not significant (p = 0.230).
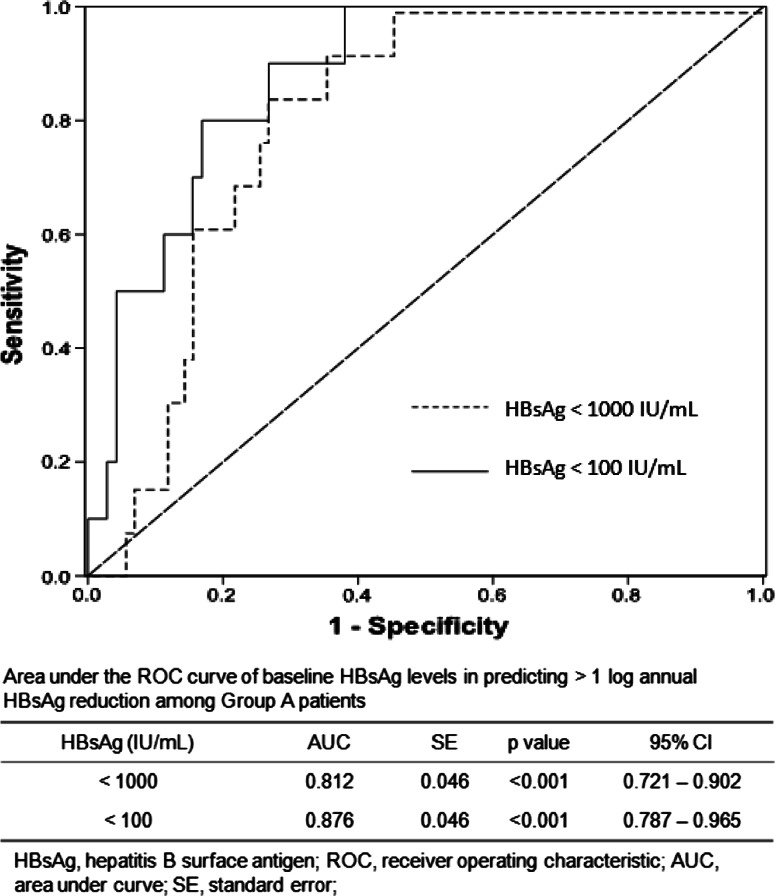
Among our whole study population, 17 (5.67 %) patients achieved an annual HBsAg reduction >1 log, of which 14 (82.4 %) were from Group A, significantly more than the number of such patients in Groups B and C combined (p < 0.001). The ROC curves and the AUC values of baseline serum HBsAg levels among Group A patients in predicting >1 log reduction in serum HBsAg are depicted in Fig. 3. Among patients with serum HBsAg <100 IU/mL, baseline serum HBsAg levels achieved an AUC of 0.876, better than that in patients with serum HBsAg <1,000 IU/mL (AUC 0.812). The optimal HBsAg range for predicting >1 log HBsAg reduction was found to be between 10 and 100 IU/mL (sensitivity 90 %; specificity 74.6 %).
Fig. 3.
ROC curves of baseline HBsAg in predicting >1 log annual HBsAg reduction among Group A patients
HBsAg/HBV DNA ratio
The changes in HBsAg/HBV DNA ratio are depicted in Table 2. In Group A, there was a significant reduction in HBsAg/HBV DNA ratio (p < 0.001), but in Groups B and C, there were no significant changes in HBsAg/HBV DNA ratios during the study period (p = 0.144 and 0.458, respectively). For all three time points, the HBsAg/HBV DNA ratio of Group B was significantly higher than those of Groups A and C (p < 0.05).
ALT levels
The median serum ALT levels in Groups A, B, and C at the three study time points were 26, 22, and 21 U/L; 26, 26, and 25 U/L; and 34.5, 32, and 32.5 U/L, respectively. There were no significant changes in ALT for all three groups (all p > 0.05). In Group C, 50 patients had persistently normal ALT, and 50 patients had elevated ALT on at least one occasion. There was no difference in median annual HBsAg reduction in these two groups (0.115 log IU/mL per year and 0.106 log IU/mL per year, respectively; p = 0.939).
Discussion
Depending on the sensitivity of the HBV DNA assay used, patients with undetectable HBV DNA constitute a minority (11–28 %) of the total HBeAg-negative CHB population in Asian patients [1, 10, 11]. Serum HBV DNA levels, however, are known to fluctuate with time [8]. The clinical implications of measuring HBV DNA levels have been readily demonstrated in studies associating lower HBV DNA levels with favorable outcomes, including HBsAg seroclearance [22] and reduced incidences of cirrhosis [10] and HCC [11]. Defining the role of HBsAg levels in this area requires further studies. The first step of investigation should be studies examining the longitudinal changes in HBsAg levels in patients with different HBV DNA levels. The natural disease course of HBeAg-negative CHB can be hugely variable and often unpredictable. The present study investigated the longitudinal profile of serum HBsAg levels at different viral loads in treatment-naïve HBeAg-negative CHB patients, including patients with persistently undetectable HBV DNA (≤20 IU/mL). To our knowledge, the present study is also the first to report the changes in HBsAg kinetics and their implications in patients achieving a persistently undetectable viral load.
The weak correlation found between HBV DNA and HBsAg (Fig. 1) can be explained by the fluctuating nature of serum HBV DNA levels and the excess production of HBsAg by the virus. The lowest serum HBsAg levels were found in patients with persistently undetectable viremia (Group A), followed by those with intermediate HBV DNA levels (Group B), while patients having high HBV DNA levels (Group C) had the highest HBsAg titers. The findings of the present study imply that a positive relationship between serum HBsAg and HBV DNA does exist, but in a broader sense and not in a purely monotonic manner. Hence, patients with low HBV DNA levels tend to have low HBsAg levels and vice versa, but among patients with small HBV DNA differences, e.g., 1–2 logs, the variations in serum HBsAg would be minimal.
Another important observation in the present study was that significant HBsAg reduction during follow-up requires two important pre-requisites: low HBsAg levels, i.e., <100 IU/mL, and persistently undetectable HBV DNA. A >1 log annual HBsAg reduction occurred most frequently in patients possessing these two factors. Although HBsAg <100 IU/mL has been recently found to be predictive of subsequent HBsAg seroclearance [23, 24], the present study did not find any significant HBsAg reduction among patients with HBsAg <100 IU/mL and intermediate (Group B) or high (Group C) viral loads (Table 3). Serum HBsAg is usually produced in excess of viral need, and its kinetics in the presence of persistently undetectable viremia needs further investigation. Future studies could target the HBV surface gene messenger RNA (mRNA) transcripts in liver tissue among patients with different levels of viremia. In fact, measurements of HBV surface gene mRNA transcripts have been reported in studies investigating the histologic characteristics of patients achieving HBsAg seroclearance [25, 26].
Further insight concerning the relationship between HBsAg and HBV DNA can be gleaned from HBsAg/HBV DNA ratios. The HBsAg/HBV DNA ratio was higher in Group B than in Group C, suggesting that virion production was most efficient when viral replication was at intermediate levels. This suggests that the production of HBV DNA and HBsAg are under different kinetics. During intermediate levels of viral replication, the assembly of HBsAg exceeds that of virions, probably since viral integration, a non-essential aspect in the life cycle of HBV, produces HBsAg in the absence of viral replication [27]. Control of viral replication could also be at a post-transcriptional level, sparing the transcription and secretion of HBsAg [17].
Besides the difference in the kinetics of HBV DNA and HBsAg production, an intriguing regulation between intrahepatic rcDNA (the precursor of serum HBV DNA), cccDNA, and HBsAg levels may exist. Intrahepatic HBV DNA preferentially exists as cccDNA and not rcDNA during low viral replication [28], leading to a relative decrease in intrahepatic rcDNA for virion assembly and a relative increase in cccDNA for mRNA transcription, resulting in increased HBsAg production and an HBsAg/HBV DNA ratio, as seen in Group B. To further verify this speculation, it would be interesting to study the HBsAg/HBV DNA ratio in patients with very low HBV replication (Group A). However, there is a drawback in interpreting HBsAg/HBV DNA ratios in Group A, in which the value of serum HBV DNA was universally set as 20 IU/mL—the lower limit of detection by the assay. This group might not accurately reflect the true proportion of subviral particles versus virions. Future studies investigating the relationship between serum HBsAg and HBV DNA levels should consider the measurement of hepatitis B core-related antigen (HBcrAg), a proposed surrogate serological marker for cccDNA [29, 30].
The present study could provide a blueprint for future research investigating the usefulness of serum HBsAg in monitoring nucleoside analog therapy. Studies have shown that despite the profound suppression of serum HBV DNA to undetectable levels by nucleoside analogs, serum HBsAg levels remained static [31]. The present study provides a possible pointer to examine whether there will be further reduction of HBsAg in patients once they have achieved undetectable HBV DNA levels and an HBsAg level of less than 100 IU/mL. The prognostic significance of low HBsAg levels and >1 log annual HBsAg reduction in nucleoside analog therapy would also need further investigation.
A drawback of this study is the lack of HBV genotyping, especially since different HBV genotypes could have different HBsAg kinetics [32]. The present study also included few patients with established cirrhosis (2–3 % in each study group). Future studies should be performed to investigate HBsAg kinetics in cirrhotic patients and its relationship with clinical parameters that are associated with cirrhosis (e.g., thrombocytopenia and relevant ultrasonographic findings).
In conclusion, serum HBsAg <100 IU/mL in CHB patients with persistently undetectable HBV DNA was predictive of a greater subsequent reduction in HBsAg levels. Serial monitoring of HBsAg levels is thus useful in patients with both undetectable HBV DNA and low HBsAg titers. The changes in HBsAg kinetics described by the present study could provide a reference for future research investigating the use of serum HBsAg levels for response-guided management when sustained viral suppression is achieved with nucleoside analog therapy.
Acknowledgements
The assays used to determine serum HBV DNA levels (Cobas Taqman assay) and HBsAg levels (Elecsys HBsAg II assay) in our laboratory were supported by an unrestricted grant from Roche Diagnostics.
Conflict of interest
No conflicts of interest exist for all authors.
References
- 1.Fung J, Seto WK, Lai CL, et al. Profiles of HBV DNA in a large population of Chinese patients with chronic hepatitis B: implications for antiviral therapy. J Hepatol. 2011;54(2):195–200. doi: 10.1016/j.jhep.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Chu CJ, Keeffe EB, Han SH, et al. Prevalence of HBV precore/core promoter variants in the United States. Hepatology. 2003;38(3):619–628. doi: 10.1053/jhep.2003.50352. [DOI] [PubMed] [Google Scholar]
- 3.Zarski JP, Marcellin P, Leroy V, et al. Characteristics of patients with chronic hepatitis B in France: Predominant frequency of HBe antigen negative cases. J Hepatol. 2006;45(3):355–360. doi: 10.1016/j.jhep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Di Bisceglie AM, Waggoner JG, Hoofnagle JH. Hepatitis B virus deoxyribonucleic acid in liver of chronic carriers. Correlation with serum markers and changes associated with loss of hepatitis B e antigen after antiviral therapy. Gastroenterology. 1987;93(6):1236–1241. doi: 10.1016/0016-5085(87)90250-2. [DOI] [PubMed] [Google Scholar]
- 5.Lai CL, Yuen MF. The natural history and treatment of chronic hepatitis B: A critical evaluation of standard treatment criteria and end points. Ann Intern Med. 2007;147(1):58–61. doi: 10.7326/0003-4819-147-1-200707030-00010. [DOI] [PubMed] [Google Scholar]
- 6.Chen JD, Yang HI, Iloeje UH, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138(5):1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 7.Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54(11):1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunetto MR, Oliveri F, Coco B, et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36(2):263–270. doi: 10.1016/S0168-8278(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 9.Feld JJ, Ayers M, El-Ashry D, et al. Hepatitis B virus DNA prediction rules for hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2007;46(4):1057–1070. doi: 10.1002/hep.21811. [DOI] [PubMed] [Google Scholar]
- 10.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Chen CF, Lee WC, Yang HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011;141(4):1240–1248.e2 [DOI] [PubMed]
- 13.Yuen MF, Tanaka Y, Shinkai N, et al. Risk for hepatocellular carcinoma with respect to hepatitis B virus genotypes B/C, specific mutations of enhancer II/core promoter/precore regions and HBV DNA levels. Gut. 2008;57(1):98–102. doi: 10.1136/gut.2007.119859. [DOI] [PubMed] [Google Scholar]
- 14.Fung J, Lai CL, Yuen MF. Hepatitis B virus DNA and hepatitis B surface antigen levels in chronic hepatitis B. Expert Rev Anti Infect Ther. 2010;8(6):717–726. doi: 10.1586/eri.10.45. [DOI] [PubMed] [Google Scholar]
- 15.Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44(3):675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 16.Volz T, Lutgehetmann M, Wachtler P, et al. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology. 2007;133(3):843–852. doi: 10.1053/j.gastro.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 18.Chan HL, Wong VW, Wong GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52(4):1232–1241. doi: 10.1002/hep.23803. [DOI] [PubMed] [Google Scholar]
- 19.Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52(4):514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52(4):508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Brunetto MR, Oliveri F, Colombatto P, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139(2):483–490. doi: 10.1053/j.gastro.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Yang HI, Lee MH, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139(2):474–482. doi: 10.1053/j.gastro.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 23.Tseng TC, Liu CJ, Su TH, et al. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141(2):517–525. doi: 10.1053/j.gastro.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Chan HL, Wong GL, Tse CH, et al. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204(3):408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 25.Yuen MF, Wong DK, Fung J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192–1199. doi: 10.1053/j.gastro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Kuhns M, McNamara A, Mason A, et al. Serum and liver hepatitis B virus DNA in chronic hepatitis B after sustained loss of surface antigen. Gastroenterology. 1992;103(5):1649–1656. doi: 10.1016/0016-5085(92)91191-6. [DOI] [PubMed] [Google Scholar]
- 27.Bill CA, Summers J. Genomic DNA double-strand breaks are targets for hepadnaviral DNA integration. Proc Natl Acad Sci USA. 2004;101(30):11135–11140. doi: 10.1073/pnas.0403925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong DK, Yuen MF, Yuan H, et al. Quantitation of covalently closed circular hepatitis B virus DNA in chronic hepatitis B patients. Hepatology. 2004;40(3):727–737. doi: 10.1002/hep.20353. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T, Rokuhara A, Sakamoto Y, et al. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol. 2002;40(2):439–445. doi: 10.1128/JCM.40.2.439-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka E, Matsumoto A, Suzuki F, et al. Measurement of hepatitis B virus core-related antigen is valuable for identifying patients who are at low risk of lamivudine resistance. Liver Int. 2006;26(1):90–96. doi: 10.1111/j.1478-3231.2005.01200.x. [DOI] [PubMed] [Google Scholar]
- 31.Fung J, Lai CL, Young J, et al. Quantitative hepatitis B surface antigen levels in patients with chronic hepatitis B after 2 years of entecavir treatment. Am J Gastroenterol. 2011;106(10):1766–1773. doi: 10.1038/ajg.2011.253. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama M, Tanaka Y, Kato T, et al. Influence of hepatitis B virus genotypes on the intra- and extracellular expression of viral DNA and antigens. Hepatology. 2006;44(4):915–924. doi: 10.1002/hep.21345. [DOI] [PubMed] [Google Scholar]