Abstract
The rate of obesity in Japan, defined as having a body mass index (BMI) of 25 kg/m2 or greater, is reportedly at 24 %, a lower level of severe obesity than in the EU and US. However, the incidence of obesity-related health problems is reportedly higher among Asians. Laparoscopic sleeve gastrectomy (LSG) is the most frequently performed bariatric surgery in Japan and accounted for 54 % of such surgeries in 2011; procedures such as laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass (LRYGB), practiced frequently worldwide, were uncommon. Possible reasons include concern over delayed postoperative discovery of gastric cancer in LRYGB, and rapid adoption of the comparatively simple LSG procedure. In type 2 diabetes mellitus (T2DM) patients, where continued pursuit of medical treatment is difficult and a potential exists for future deterioration of diabetes-complicated diseases, the criterion for surgical indication in the EU and US is a BMI of 30–35 kg/m2, with priority given to BMI >35 kg/m2. For Asian patients, the recommendation is to lower this indication criterion by 2.5 kg/m2. Efficacy of metabolic surgery is anticipated particularly among T2DM patients with obesity complication, a short history of insulin treatment, and intact insulin secreting ability, and in these cases bariatric surgery should be contemplated.
Keywords: Obesity, Diabetes mellitus, Bariatric surgery, Metabolic surgery, Sleeve gastrectomy, Gastric bypass
Introduction
At the 33rd Annual Session of the United Nations Standing Committee on Nutrition held in 2006, the World Health Organization (WHO) reported that nearly 300 million adults worldwide were severely obese. While the definition of obesity in the EU and US as having a body mass index (BMI) of 30 kg/m2 or greater places more than one-third of the adult population within the category of obesity, some 6–10 million individuals are said to be severely obese, with a BMI of 40 kg/m2 or greater. Based on current trends, the rate of obesity in the US is projected to reach approximately 40 % by 2025 [1]. In Japan, the Japan Society for the Study of Obesity (JASSO) defines obesity as a BMI of 25 kg/m2 or greater. According to recent surveys, BMI values are 25 or greater in 24 % of the population, 30 or greater in 3 %, 35 or greater in 0.5 %, and 40 or greater in 0.2 %, reflecting a lower level of severe obesity than in the EU or US. However, a WHO Expert Committee reported that the incidence of health problems stemming from obesity is higher among Asians [2]. While the general treatment for obesity in Japan centers on medical treatments such as dietary, behavioral, and exercise therapy, surgical treatments termed “metabolic surgery” have been practiced more recently. Our review article discusses the current status of bariatric surgery in Japan and its effectiveness in the treatment of obesity and diabetes.
Worldwide trends in bariatric surgery and current status in Japan
Based on questionnaire survey results from 33 countries, Buchwald et al. [3, 4] reported that more than 340,000 bariatric surgeries were performed worldwide in 2008. The proportion of laparoscopic procedures among bariatric surgeries was 63 % in 2003 but had reached 92 % in 2008. Types of bariatric surgery are shown in Fig. 1. The relative shares of various procedures in 2008 included 43 % laparoscopic Roux-en-Y gastric bypass (LRYGB), 42 % laparoscopic adjustable gastric banding (LAGB), and 5 % laparoscopic sleeve gastrectomy (LSG). LAGB allows adjustment of the band tightener after surgery and removal of the band in reoperation and is also a comparatively simple procedure. For these reasons, LAGB has increased rapidly in North America and is predicted to become the most frequently performed weight loss surgery in the near future.
Fig. 1.
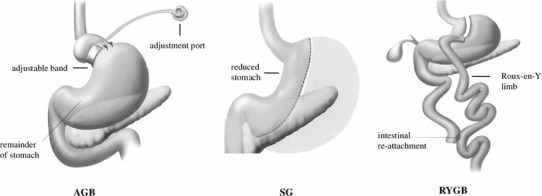
Types of bariatric surgery. AGB adjustable gastric banding, SG sleeve gastrectomy, RYGB Roux-en-Y gastric bypass
Bariatric surgery was first performed in Japan by Kawamura et al. in 1982 [5]. The bariatric restrictive procedure became eligible for insurance reimbursement in 1988 but did not achieve widespread dissemination for reasons of technique and indication. However, the increasing number of obese patients, growing societal needs in response to obesity, and progress in laparoscopic-assisted surgical techniques have produced remarkable developments in the treatment of obesity. Kitano et al. [6] introduced the endoscopic intragastric balloon (EIGB) to Japan in December 2004 and LAGB in August 2005 and have reported good results. But as of 2012, only 10 facilities are performing bariatric surgery, and currently there are few bariatric surgeons. According to the results from the 1st questionnaire survey of the Japan Research Society for Endoscopic and Laparoscopic Treatment of Obesity (JELTO), the number of bariatric surgeries performed between 2000 and 2009 was 340, while 228 procedures were performed in 2010 and 2011, showing a nascent awareness of obesity pathology and a wider discussion of indications for surgical treatment [7]. However, the most widely performed bariatric surgery in Japan is LSG, accounting for 54 % in 2011, while LAGB, LRYGB, and other such procedures performed frequently worldwide are uncommon. Possible reasons include a concern over the delayed postoperative discovery of gastric cancer in LRYGB and rapid adoption of the comparatively simple LSG procedure; another issue bearing mention is consideration of the improving effect on diabetes and other obesity-related diseases.
Indications for bariatric surgery
The objective of treatment for obesity is to improve obesity-related diseases, without requisite attainment of a standard body weight. Medical treatments such as dietary, exercise, behavioral, and drug therapy are the treatments of first choice for obesity, but many cases of severe obesity are resistant to medical treatment and also present difficulties in achievement of adequate weight loss and maintenance of body weight after weight loss. The US National Institutes of Health (NIH) reports that more than 90 % of patients on a medically managed weight loss program ultimately regain weight within 1 year. For these patients with severe obesity resistant to medical treatment, or patients in end-stage obesity syndromes that require urgent intervention for obesity-related diseases, surgical treatment must be considered. In 1991, the NIH stated that severe obesity patients (patients with a BMI of 40 kg/m2 or greater, or those with a BMI of 35 kg/m2 or greater and obesity-related disease) presenting a low likelihood of positive results from any treatment other than surgical treatment were indicated for bariatric surgery.
In 2006, a joint committee of the JASSO, the Japan Gastroenterological Endoscopy Society (JGES), and the Japan Society for Endoscopic Surgery created guidelines for indication of obesity treatment by endoscopy and endoscopic surgery [7]. One point of agreement was that treatments such as EIGB and LAGB were approved for use in Japan. However, the use of devices not yet medically approved in both treatments led to a policy of restricting clinical use to then-current advanced treatment hospitals. Indication of bariatric surgery was restricted to patients with a BMI of 35 kg/m2 or greater and obesity-related disease resistant to medical treatment [8]. Thereafter, the Japanese Society for the Surgery of Obesity and Metabolic Disorders (JSSO) prepared a “2010 Statement for Safe and Exceptional Surgical Treatment for Severe Obesity in Japan.” The statement defined surgical indication as BMI ≥35 kg/m2 for a primary objective of weight loss and, with reference to clinical research, as BMI ≥32 kg/m2 for a primary objective of treatment for obesity-related disease [9]. In 2011, the International Diabetes Federation (IDF) called for a BMI of 30–35 kg/m2 to represent surgical indication for type 2 diabetes mellitus (T2DM) unresponsive to medical treatment, with priority to be given to BMI >35 kg/m2 [9]. Surprisingly, the IDF also released a statement recommending that this indication criterion be lowered by 2.5 kg/m2 for Asian individuals [10].
In research carried out in France among more than 1,000 subjects undergoing LAGB in a 2-month period, outcomes were interpreted as successful if the percentage of excess weight loss (%EWL) at 2 years postoperatively was 50 % or greater. Results were good among patients age 40 years or younger, those with a BMI of <50 kg/m2 at initial examination, and those demonstrating a return to physical activity and changes in dietary habits. These were reported as crucial factors for indication of LAGB and prediction of weight loss effect. Good results were also reported in facilities with abundant experience including two or more weight loss surgeries in a 1-week period, and the adoption of a team treatment system was recommended [11]. Pontiroli et al. [12] stated that periodic outpatient visits were an important positive factor for prediction of LAGB outcomes, and in contrast, self-centered personality was an important negative factor. These factors are regarded as crucial for achieving successful bariatric surgery.
Types of bariatric surgery and effects
EIGB represents an endoscopic treatment (in general terms, a surgical treatment) for severe obesity. Surgical treatments are classified as restrictive procedures, in which the stomach is reduced in size to restrict food intake, malabsorptive procedures, in which nutrient absorption capacity is reduced by application of a surgical procedure to the small intestine, and procedures combining both of these techniques. Examples of restrictive procedures include LAGB and LSG, an example of a malabsorptive procedures is biliopancreatic diversion (BPD), which shortens the small intestine, through which food transits; and examples of treatments combining both a restrictive procedure and a malabsorptive procedure include LRYGB and LSG with duodenojejunal bypass (LSG/DJB). Though there are as of yet few reports of weight loss results from bariatric surgery in Japan, good weight loss outcomes equivalent to those among Europeans and Americans have been realized (Table 1) [7, 13, 14]. In contrast, rates of bariatric surgical complication are 25.3 % in LRYGB, 5.1 % in LAGB, 15.3 % in LSG, and 15.5 % in LSG/DJB. These rates are somewhat higher than those outside Japan due to a lack of quantitative experience, indicating that practiced technique and team treatment are crucial (Table 2) [15].
Table 1.
Outcomes of major bariatric procedures
| Source | Procedure | No. of patients | %EWL 12 months | %EWL 24 months | %EWL 36 months |
|---|---|---|---|---|---|
| JELTO [7] | LRYGB | 147 | 76 | 77 | 74 |
| LSG | 102 | 66 | 68 | – | |
| LAGB | 55 | 43 | 58 | 55 | |
| Deitei et al. [13] | LSG | 19,605 | 62.7 | 64.7 | 64.0 |
| Garb et al. [14] | LRYGB | 1,615 | 61.5 | 69.7 | 71.2 |
| LAGB | 5,768 | 42.6 | 50.3 | 55.2 |
JELTO Japan Research Society for Endoscopic and Laparoscopic Treatment of Obesity, LRYGB laparoscopic Roux-en-Y gastric bypass, LSG laparoscopic sleeve gastrectomy, LAGB laparoscopic adjustable gastric banding, %EWL percentage of excess weight loss
Table 2.
Morbidity rates after bariatric procedure in Japan
| Morbidities | LRYGB (n = 91) | LAGB (n = 59) | LSG (n = 215) | LSG/DJB (n = 84) |
|---|---|---|---|---|
| Total (%) | 25.3 | 5.1 | 15.3 | 15.5 |
| Intraoperative (%) | 1.1 | 0 | 0.9 | 0 |
| Postoperative (%) | ||||
| Bleeding (reoperation) | 1.1 | 1.7 | 1.9 | 3.6 |
| Leakage | 0 | 0 | 1.9 | 1.2 |
| Anastomotic stenosis | 15.4 | 0 | 0 | 0 |
| Reoperation (%) | 9.9 | 5.1 | 5.1 | 7.1 |
LRYGB laparoscopic Roux-en-Y gastric bypass, LAGB laparoscopic adjustable gastric banding, LSG laparoscopic sleeve gastrectomy, LSG/DJB laparoscopic sleeve gastrectomy with duodenojejunal bypass
EIGB
EIGB as reported in 1982 involved numerous material-related complications, and long-term implantation was problematic. The debut of the BIB® (Orbera™) system in the US allowed 6-month implantation, leading to worldwide use. The most recent materials prepared by the manufacturer of the Orbera™ system (Allergan, Inc.) state that EIGB is optimally indicated for patients with a BMI of approximately 27–35 kg/m2 [16]. EIGB is also well-indicated as a preoperative treatment intended to mitigate perioperative risk in Class 2–3 obesity (40 > BMI ≥ 30 kg/m2) accompanied by severe health problems or among extremely obese patients (BMI ≥50 kg/m2). EIGB is a treatment performed under gastroscopic observation in which physiological saline including 400–700 ml of methylene blue is injected into a gastric balloon inserted transorally as a technique to reduce gastric capacity and the amount of food intake. Removal is also possible and is accomplished by transoral endoscope, using a specialized remover kit. In Japan, the BIB® system was introduced by Kitano et al. [6] in 2004, and Ohta et al. [17] reported the first Japanese clinical results for 21 cases in which the BIB® system was used. No serious complications were found in this research, and weight loss (17 cases) at 5 months after balloon implantation was 12 kg, while %EWL was 27 %. The improvement rate for obesity-related disease was 80 % (8/10) in hypertension, 86 % in dyslipidemia (12/14), 100 % (9/9) in liver dysfunction, 100 % (10/10) in metabolic syndrome, and 100 % (5/5) in diabetes, reflecting good results. However, they reported that two patients (9.5 %) required early removal (within 1 week) of the balloon due to continuous abdominal discomfort. They also reported that four patients maintained a %EWL >20, and that the remaining four patients returned to their pretreatment weight (%EWL at 12 months after balloon removal: 27 ± 12 vs 0.2 ± 2.1, P < 0.01). Genco et al. [18] investigated 2,515 cases of intragastric balloon implantation in multicenter collaborative research and reported that %EWL 6 months postoperatively was 34 %, while the rate of complications was 2.8 % (0.76 % gastric obstruction, 0.19 % gastric rupture, 0.36 % balloon rupture).
LAGB
LAGB is a restrictive procedure in which a silicone band is wrapped around the upper portion of the stomach and restricts food intake. An adjustable band was developed in 1986, and use in laparoscopic surgery began in 1993. Food is digested and absorbed along the physiological route. Today, a tool (Lap-Band®; INAMED Co.) allowing adjustment of band tightness has been developed, and adjustment is accomplished through an access point embedded subcutaneously. Typically, the device is implanted by the pars flaccida technique (an approach from the avascular portion of the lesser omentum), and several gastric–gastric sutures are made to prevent band slippage. Benefits include the ability to adjust band tightness postoperatively, to remove the band by reoperation and return to the preoperative state, and to perform postoperative endoscopy in normal fashion. The difficulty of the surgical technique in LAGB is lower than that in LRYGB, and complications are also less frequent, leading to performance in many countries. However, the weight loss effect and the improvement rate in obesity-related diseases are slightly lower than those in LRYGB, and depending on postoperative symptoms, several band adjustments may be necessary. Surgical indication must be considered carefully.
According to a systematic review reported by Buchwald et al. [19], mean postoperative %EWL by surgical technique was 47.5 % in LAGB and 61.6 % in LRYGB, reflecting significance for LRYGB versus LAGB. Similar results have also been reported in multicenter, randomized clinical research. Nguyen et al. [20] compared 86 cases of LAGB and 111 cases of LRYGB and reported that 4-year postoperative %EWL was 45.4 % in LAGB and 68.4 % in LRYGB. Himpens et al. [21] compared 40 cases of LAGB and 40 cases of LSG and reported that the 3-year postoperative %EWL was 48 % in LAGB and 66 % in LSG.
As of yet there are few reports of long-term results for LAGB in Japan. Ohta et al. [22] performed LAGB in 27 Japanese cases of severe obesity with a mean BMI of 41 kg/m2 and reported that the 5-year postoperative %EWL was 56 %. Kasama et al. [23] performed LAGB in 13 cases of severe obesity with a mean BMI of 37.5 kg/m2 and reported that 18-month postoperative reduction of excess BMI was 69.6 %.
LRYGB
LRYGB is a surgical technique combining a restrictive procedure and a malabsorptive procedure, and accounted for 66 % of bariatric surgeries (35 % laparoscopic surgeries, 31 % laparotomies) performed worldwide in 2002 and 2003 [3]. The surgical technique entails the separation of the upper portion of the stomach with an automatic suturing device to reduce the gastric capacity by 15–30 ml. The jejunum is separated with an automatic suturing device at a location approximately 50 cm from the Treitz ligament, and the jejunum is lifted on the anal side and mechanically anastomosed to the contracted stomach. The length of the Roux-Y leg is approximately 75 cm in the standard surgical technique and approximately 150 cm in extremely obese patients (BMI ≥50 kg/m2). The absorptive and digestive capacity of the alimentary system is thus reduced, providing an effective reduction of capacity.
At a %EWL of 62 %, better results have been reported than in EIGB and LAGB. Improvement in obesity-related disease was 86 % in diabetes, 79 % in hypertension, 79 % in hyperlipidemia, and 84 % in sleep apnea syndrome. The rate of postoperative complications was 5 %, and the rate of surgical deaths was 0.5 % [3]. LRYGB is the most frequently performed bariatric surgery worldwide, performed in approximately 80 % of cases in the US. Adams et al. [24] investigated death rates comparatively during a mean 7.1-year observational period among 7,925 age-, sex-, and BMI-matched subjects in an RYGB cohort and 7,925 subjects in a control cohort (BMI ≥35 kg/m2). The death rate in the RYGB cohort was 40 % lower than among controls. Death rates in obesity-related disease were reportedly reduced by 56 % for coronary artery disease, 92 % for diabetes, and 60 % for cancer. However, death rates from causes unrelated to obesity such as accidents and suicide were reportedly higher than in the control cohort, making future trends in RYGB of interest. Considering that the current incidence of gastric cancer in Japan is higher than that in the EU and US, the difficulty that RYGB presents to examination of the residual stomach calls for a prudent stance on indication of RYGB in Japan.
LSG
LSG, used as an initial bariatric surgery for severely obese patients, is a new technique for lowering rates of complication and surgical death. Though its efficacy has drawn attention, it was not recommended by the 2004 American Society for Bariatric Surgery Consensus Conference on Bariatric Surgery. The surgical technique involves insertion of a 40–60 Fr esophageal bougie into the stomach and gastric excision with an automatic suturing device from the greater curvature of the pyloric portion, approximately 5 cm proximally from the pyloric ring, toward the angle of His. The intent is to reduce gastric capacity to 60–200 ml, and if a sufficient capacity-reducing effect is not achieved, a secondary RYGB or other such treatment is pursued.
For this new surgical technique, further study of long-term results is needed, but 19,605 cases of LSG were investigated at the Third International Summit for LSG held in 2010. Overweight reduction rates at 1, 2, 3, 4, and 5 years postoperatively were 62.7, 64.7, 64.0, 57.3, and 60.0 %, respectively [13]. Reported complications were 1.3 % high leaks, 0.5 % lower leaks, and a 0.1 % rate of death, and the surgical technique was evaluated as safe. From studies in Asia, Moon Han et al. [25] reported a good weight reduction of 72 % mean %EWL at 6 months postoperatively and 83 % at 1 year in short-term results for 130 cases of LSG among obese patients with a mean BMI of 37.2 kg/m2. Sasaki et al. [26] reported good results for median %EWL, at 56 (42–70) % at 6 months postoperatively, and 68 (49–87) % at 1 year in short-term results for seven LSG cases among severe obesity patients with a median BMI of 47 kg/m2. Tagaya et al. [27] investigated short-term results comparatively in 30 cases among obese patients with a mean BMI of 49.1 kg/m2, comparing results for BMI of <50 kg/m2 and BMI of ≥50 kg/m2. They reported good results for patients with BMI of <50 kg/m2, i.e., mean BMI of 27.8 versus 52.2 kg/m2, at 12 months postoperatively and 29.7 versus 45.5 kg/m2 at 18 months postoperatively. These reports show that LSG alone has the potential to produce good results among Asian patients with severe obesity represented by a BMI of <50 kg/m2, and given that the procedure does not use specialized devices awaiting medical approval, further adoption is anticipated.
Improvement of obesity-related disease
The objective of bariatric surgery is not weight loss alone, but also improvement of prognosis in obesity-related disease. Sjöström et al. [28] compared long-term results prospectively among 2,010 cases undergoing weight loss surgery and a medically treated control cohort of 2,037 cases matched on 18 indices, including sex, age, and body weight. In the control cohort, no long-term weight loss effect was observed, while in the surgical cohort, change in body weight plateaued at approximately 10 years postoperatively, after which the weight loss effect was maintained. This study reported that the effect on survival rates from weight reduction and improvement of obesity-related diseases was a 29 % reduced risk of death in the surgical cohort versus controls.
Weight loss surgery has an improving effect on obesity-related disease additional to that of body weight reduction and has more recently been termed “metabolic surgery”. RYGB has a reportedly greater improvement effect on diabetes than food intake restriction surgery. Though the mechanism is not understood, two current theories exist. In one, the “hindgut hypothesis”, bypass of the upper small intestine diverts food directly to the lower small intestine, where glucagon-like peptide-1 (GLP-1) secretory cells exist, thus promoting GLP-1 secretion. In another, the “foregut hypothesis”, an as-yet unknown glucose tolerance complicating factor which inhibits GLP-1 secretion is secreted from the upper small intestine, and because bypass surgery prevents food from passing through the upper small intestine, this factor is no longer secreted [29].
Hutter et al. [30] reported that remission or improvement rates on T2DM were 79 % in LRYGB and 55 % in LSG, and outside Japan, there are many reports of better results in LRYGB versus LSG. In Japan, however, LSG, though a restrictive procedure, has been shown to have an improving effect on diabetes rivaling that of LRYGB. In a JELTO report, Ohta et al. [7] reported good results for remission of T2DM (fasting glucose <110 mg/dl and HbA1c <6 % without medication) from LSG, specifically, 91 % in LSG, 88 % in LRYGB, and 63 % in LAGB (Table 3). Sasaki [31] also reported achievement of complete remission (fasting glucose <100 mg/dl and HbA1c <5.8 % without medication) among seven T2DM patients after LSG. Though the mechanism of improved glucose tolerance after LSG is unknown, increased secretion of GLP-1 after surgery has been observed, as reported in LRYGB [31]. Lee et al. [32] reported metabolic surgery results in 200 cases of T2DM with a BMI of <35 kg/m2 in Taiwan, South Korea, India, Japan, and Hong Kong. Remission rates for T2DM were high in cases of gastric bypass surgery with a <5-year treatment history, BMI of 30–35 kg/m2, and 3 ng/ml or higher C-peptide. Kasama et al. [33] also reported rapid improvement in glucose tolerance over a period of several months and achievement of complete remission in 91 % of cases when LSG/DJB was performed. These results were realized primarily among patients with severe T2DM receiving insulin and manifesting low C-peptide levels, reduced initial insulin secreting capacity in oral glucose load testing, kidney disease, retinopathy, neuropathy, and other microangial complications. This indicates that even simple LSG and similar surgical procedures can provide remission of T2DM in attempts to reduce body weight by restriction of caloric intake among Japanese T2DM patients whose essential pathology is regarded as insulin resistance in conjunction with excess fat storage. At the same time, bypass-based surgical procedures acting directly on insulin secretion, combined with weight loss, are deemed to have an ample antidiabetic effect among T2DM patients whose essential pathology is regarded as impaired insulin secretion.
Table 3.
Remission and improvement rates of T2DM
| Source | Procedure | No. of patients | Remission (%) |
|---|---|---|---|
| JELTO [7] | LRYGB | 64 | 88 |
| LSG | 35 | 91 | |
| LAGB | 19 | 63 | |
| Buchwald et al. [19] | LRYGB | 989 | 83.8 |
| LAGB | 205 | 47.8 | |
| Hutter et al. [30] | LRYGB | 4,452 | 79a |
| LSG | 249 | 55a | |
| LAGB | 2,558 | 44a |
T2DM type 2 diabetes mellitus, JELTO Japan Research Society for Endoscopic and Laparoscopic Treatment of Obesity, LRYGB laparoscopic Roux-en-Y gastric bypass, LSG laparoscopic sleeve gastrectomy, LAGB laparoscopic adjustable gastric banding
aRemission and improvement rates
Conclusion
The principles of obesity treatment are based on the medical treatments of dietary and exercise therapy, which, combined with behavioral therapy allowing long-term maintenance, improve lifestyle habits. For patients with a BMI of 35 kg/m2 or greater and obesity-related disease resistant to medical treatment, bariatric surgery must be considered, and its outcomes among Japanese individuals are good, as in cases outside Japan. LRYGB and LSG also offer increased GLP-1 secretion postoperatively, suggesting a large potential for treatment of T2DM patients; however, the associated mechanisms are unknown, and further clinical research is needed. Bariatric surgery, which offers the effects of metabolic surgery, should be considered for T2DM patients with difficulty in continuing medical treatment and a potential for future deterioration and diabetic complications. Bariatric surgery is particularly well-indicated for T2DM patients with obesity complications, a short insulin treatment history, and intact insulin secreting ability. From the medical economics perspective of obesity and metabolic syndrome as well, minimally invasive bariatric surgery has substantial promise, and insurance coverage in the near term is awaited.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- WHO
World Health Organization
- NIH
National Institutes of Health
- IDF
International Diabetes Federation
- JGES
Japan Gastroenterological Endoscopy Society
- JASSO
Japan Society for the Study of Obesity
- JELTO
Japan Research Society for Endoscopic and Laparoscopic Treatment of Obesity
- JSSO
Japanese Society for the Surgery of Obesity and Metabolic Disorders
- BMI
Body mass index
- %EWL
Percentage of excess weight loss
- LRYGB
Laparoscopic Roux-en-Y gastric bypass
- LAGB
Laparoscopic adjustable gastric banding
- LSG
Laparoscopic sleeve gastrectomy
- EIGB
Endoscopic intragastric balloon
- BPD
Biliopancreatic diversion
- DJB
Duodenojejunal bypass
- T2DM
Type 2 diabetes mellitus
- GLP-1
Glucagon-like peptide-1
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2001;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H. Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. J Am Coll Surg. 2005;200:593–603. doi: 10.1016/j.jamcollsurg.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 5.Kasama K. Dr. Isao Kawamura, a pioneer in morbid obesity treatment in Japan. Obes Surg. 2012;22:1143. doi: 10.1007/s11695-012-0685-1. [DOI] [Google Scholar]
- 6.Kitano S, Shiromizu A, Endo Y, Ohta M, Yoshimatsu H. Intragastric balloon therapy for the treatment-resistant obesity (in Japanese) Gastroenterol Endosc. 2005;47:2197–2201. [Google Scholar]
- 7.Ohta M, Kitano S, Kasama K, Kawamura I, Inamine S, Wakabayashi G, et al. Results of a national survey on laparoscopic bariatric surgery in Japan, 2000–2009. Asian J Endosc Surg. 2011;4:138–142. doi: 10.1111/j.1758-5910.2011.00085.x. [DOI] [PubMed] [Google Scholar]
- 8.Japan Society for Endoscopic Surgery. Statement on surgical treatments for morbid obesity (in Japanese). http://www.asas.or.jp/jses/kitei/himan.html.
- 9.Japanese Society for the Surgery of Obesity and Metabolic Disorder. Statement on safety and excellent surgical treatments for morbid obesity in Japan (in Japanese). http://plaza.umin.ne.jp/~jsto/gakujyutsu/index.html.
- 10.Dixon JB, Zimmet P, Alberti KG, Rubino F, International Diabetes Federation Taskforce on Epidemiology and Prevention Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28:628–642. doi: 10.1111/j.1464-5491.2011.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevallier JM, Paita M, Rodde-Dunet MH, Marty M, Nogues F, Slim K, et al. Predictive factors of outcome after gastric banding: a nationwide survey on the role of center activity and patients’ behavior. Ann Surg. 2007;246:1034–1039. doi: 10.1097/SLA.0b013e31813e8a56. [DOI] [PubMed] [Google Scholar]
- 12.Pontiroli AE, Fossati A, Vedani P, Fiorilli M, Folli F, Paganelli M, et al. Post-surgery adherence to scheduled visits and compliance, more than personality disorders, predict outcome of bariatric restrictive surgery in morbidly obese patients. Obes Surg. 2007;17:1492–1497. doi: 10.1007/s11695-008-9428-8. [DOI] [PubMed] [Google Scholar]
- 13.Deitei M, Gagner M, Erickson AL, Crosby RD. Third international summit: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749–759. doi: 10.1016/j.soard.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Garb J, Welch G, Zagarine S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19:1447–1455. doi: 10.1007/s11695-009-9927-2. [DOI] [PubMed] [Google Scholar]
- 15.Finks JF, Kole KL, Yenumula PR, English WJ, Krause KR, Carlin AM, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan bariatric surgery collaborative. Ann Surg. 2011;254:633–640. doi: 10.1097/SLA.0b013e318230058c. [DOI] [PubMed] [Google Scholar]
- 16.Cobourn C, Cohen L, Genco A, Lope-Nava G, Caetano Marchesini J, Wahlen C, et al. Expert approaches to weight loss management, Issue 1. The Allergan Orbera™ managed weight loss system.
- 17.Ohta M, Kitano S, Kai S, Shiromizu A, Eguchi H, Endo Y, Masaki T, Kakuma T, Yoshimatsu H. Initial Japanese experience with intragastric balloon placement. Obes Surg. 2009;19:791–795. doi: 10.1007/s11695-008-9612-x. [DOI] [PubMed] [Google Scholar]
- 18.Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, et al. BioEnterics intragastric balloon: the Italian experience with 2,515 patients. Obes Surg. 2005;15:1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- 19.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250:631–641. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 21.Himpens J, Dapri G, Cadiere GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- 22.Ohta M, Kitano S, Kai S, Shiromizu A, Iwashita Y, Endo Y, et al. Initial Japanese experience with the LAP-BAND system. Asian J Endosc Surg. 2013;6:39–43. doi: 10.1111/j.1758-5910.2012.00156.x. [DOI] [PubMed] [Google Scholar]
- 23.Kasama K, Tagaya N, Kanahira E, Umezawa A, Kurosaki T, Oshiro T, et al. Has laparoscopic bariatric surgery been accepted in Japan? The experience of a single surgeon. Obes Surg. 2008;18:1473–1478. doi: 10.1007/s11695-008-9492-0. [DOI] [PubMed] [Google Scholar]
- 24.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 25.Moon Han S, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469–1475. doi: 10.1381/096089205774859227. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Umemura A, Nishizuka S, Nakajima J, Uesugi N, Wakabayashi G. Results of laparoscopic sleeve gastrectomy as a single stage bariatric procedure in Japanese patients. Asian J Endosc Surg. 2010;3:180–184. doi: 10.1111/j.1758-5910.2010.00057.x. [DOI] [Google Scholar]
- 27.Tagaya N, Kasama K, Kikkawa R, Kanahira E, Umezawa A, Oshiro T, et al. Experience with laparoscopic sleeve gastrectomy for morbid versus super morbid obesity. Obes Surg. 2009;19:1371–1376. doi: 10.1007/s11695-008-9774-6. [DOI] [PubMed] [Google Scholar]
- 28.Sjöström L, Narbo K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 29.Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270–2276. doi: 10.1007/s00125-009-1511-8. [DOI] [PubMed] [Google Scholar]
- 30.Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen MK, Merkow RP, et al. First report from the American College Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254:410–422. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki A. Metabolic surgery. In: Advances in diabetology, the 46th annual postgraduate course (in Japanese). Tokyo; 2012. p. 59–64.
- 32.Lee WJ, Hur KY, Lakadawaia M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16:45–51. doi: 10.1007/s11605-011-1740-2. [DOI] [PubMed] [Google Scholar]
- 33.Kasama K, Tagaya N, Kanehura E, Oshiro T, Seki Y, Kinouchi M, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg. 2009;19:1341–1345. doi: 10.1007/s11695-009-9873-z. [DOI] [PubMed] [Google Scholar]


