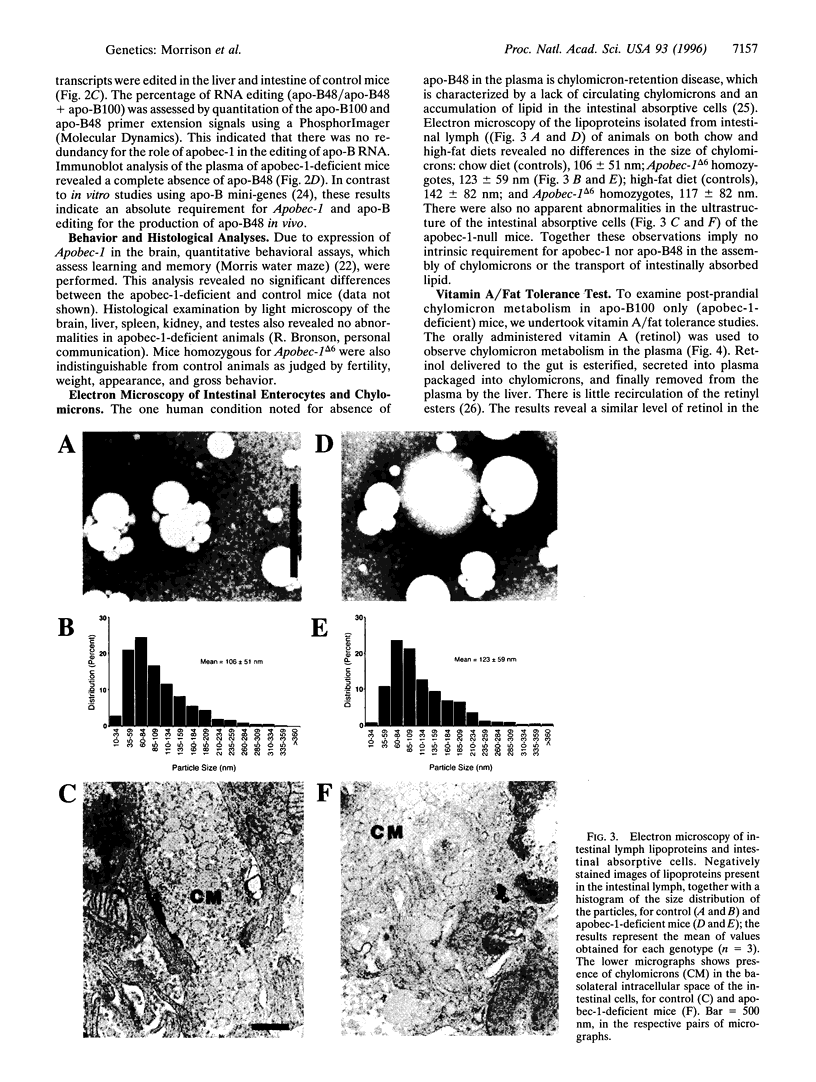
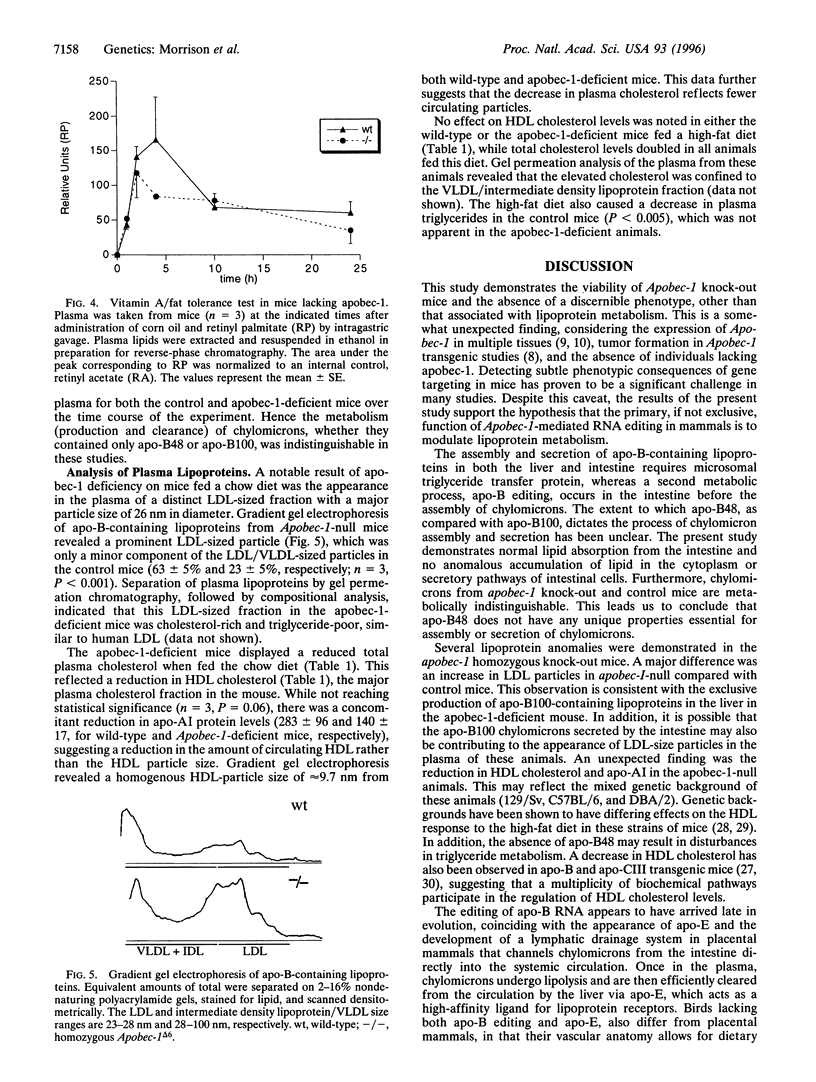
Abstract
RNA editing in the nucleus of higher eukaryotes results in subtle changes to the RNA sequence, with the ability to effect dramatic changes in biological function. The first example to be described and among the best characterized, is the cytidine-to-uridine editing of apolipoprotein B (apo-B) RNA. The editing of apo-B RNA is mediated by a novel cytidine deaminase, apobec-1, which has acquired the ability to bind RNA. The stop translation codon generated by the editing of apo-B RNA truncates the full-length apo-B100 to form apo-B48. The recent observations of tumor formation in Apobec-1 transgenic animals, together with the fact that Apobec-1 is expressed in numerous tissues lacking apo-B, raises the issue of whether this enzyme is essential for a variety of posttranscriptional editing events. To directly test this, mice were created with a null mutation in Apobec-1 using homologous recombination in embryonic stem cells. Mice, homozygous for this mutation, were viable and made apo-B100 but not apo-B48. The null animals were fertile, and a variety of histological, behavioral, and morphological analyses revealed no phenotype other than abnormalities in lipoprotein metabolism, which included an increased low density lipoprotein fraction and a reduction in high density lipoprotein cholesterol. These studies demonstrate that neither apobec-1 nor apo-B48 is essential for viability and suggest that the major role of apobec-1 may be confined to the modulation of lipid transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anant S., MacGinnitie A. J., Davidson N. O. apobec-1, the catalytic subunit of the mammalian apolipoprotein B mRNA editing enzyme, is a novel RNA-binding protein. J Biol Chem. 1995 Jun 16;270(24):14762–14767. [PubMed] [Google Scholar]
- Azrolan N., Odaka H., Breslow J. L., Fisher E. A. Dietary fat elevates hepatic apoA-I production by increasing the fraction of apolipoprotein A-I mRNA in the translating pool. J Biol Chem. 1995 Aug 25;270(34):19833–19838. doi: 10.1074/jbc.270.34.19833. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Rothfeld A. The form of absorption of lipids in the chicken, Gallus domesticus. Proc Soc Exp Biol Med. 1972 Dec;141(3):814–817. doi: 10.3181/00379727-141-36878. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Skrede B., Norum K. R. Uptake of chylomicron remnant retinyl ester via the low density lipoprotein receptor: implications for the role of vitamin A as a possible preventive for some forms of cancer. J Intern Med. 1990 Sep;228(3):207–210. doi: 10.1111/j.1365-2796.1990.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Callow M. J., Ferrin L. J., Rubin E. M. Single base, site-directed mutagenesis of a 90 kilobase-pair P1 clone. Nucleic Acids Res. 1994 Oct 11;22(20):4348–4349. doi: 10.1093/nar/22.20.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow M. J., Verstuyft J., Tangirala R., Palinski W., Rubin E. M. Atherogenesis in transgenic mice with human apolipoprotein B and lipoprotein (a). J Clin Invest. 1995 Sep;96(3):1639–1646. doi: 10.1172/JCI118203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Nordhausen R. W. Electron microscopy of negatively stained lipoproteins. Methods Enzymol. 1986;128:442–457. doi: 10.1016/0076-6879(86)28086-6. [DOI] [PubMed] [Google Scholar]
- Funahashi T., Giannoni F., DePaoli A. M., Skarosi S. F., Davidson N. O. Tissue-specific, developmental and nutritional regulation of the gene encoding the catalytic subunit of the rat apolipoprotein B mRNA editing enzyme: functional role in the modulation of apoB mRNA editing. J Lipid Res. 1995 Mar;36(3):414–428. [PubMed] [Google Scholar]
- Hayek T., Azrolan N., Verdery R. B., Walsh A., Chajek-Shaul T., Agellon L. B., Tall A. R., Breslow J. L. Hypertriglyceridemia and cholesteryl ester transfer protein interact to dramatically alter high density lipoprotein levels, particle sizes, and metabolism. Studies in transgenic mice. J Clin Invest. 1993 Sep;92(3):1143–1152. doi: 10.1172/JCI116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann T., Metzger S., Fisher E. A., Breslow J. L., Huang L. S. Alternative polyadenylation of apolipoprotein B RNA is a major cause of B-48 protein formation in rat hepatoma cell lines transfected with human apoB-100 minigenes. J Lipid Res. 1994 Dec;35(12):2200–2211. [PubMed] [Google Scholar]
- Hodges P., Scott J. Apolipoprotein B mRNA editing: a new tier for the control of gene expression. Trends Biochem Sci. 1992 Feb;17(2):77–81. doi: 10.1016/0968-0004(92)90506-5. [DOI] [PubMed] [Google Scholar]
- Hughes S. D., Rouy D., Navaratnam N., Scott J., Rubin E. M. Gene transfer of cytidine deaminase apoBEC-1 lowers lipoprotein(a) in transgenic mice and induces apolipoprotein B editing in rabbits. Hum Gene Ther. 1996 Jan;7(1):39–49. doi: 10.1089/hum.1996.7.1-39. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir J., Floresco S. B., O'Kusky J. R., Diewert V. M., Richman J. M., Zeisler J., Borowski A., Marth J. D., Phillips A. G., Hayden M. R. Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995 Jun 2;81(5):811–823. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- Navaratnam N., Bhattacharya S., Fujino T., Patel D., Jarmuz A. L., Scott J. Evolutionary origins of apoB mRNA editing: catalysis by a cytidine deaminase that has acquired a novel RNA-binding motif at its active site. Cell. 1995 Apr 21;81(2):187–195. doi: 10.1016/0092-8674(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Navaratnam N., Shah R., Patel D., Fay V., Scott J. Apolipoprotein B mRNA editing is associated with UV crosslinking of proteins to the editing site. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):222–226. doi: 10.1073/pnas.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina P. M., Wang J., Toyofuku W., Kuypers F. A., Ishida B. Y., Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993 Jul;28(7):599–605. doi: 10.1007/BF02536053. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Pászty C., Mohandas N., Stevens M. E., Loring J. F., Liebhaber S. A., Brion C. M., Rubin E. M. Lethal alpha-thalassaemia created by gene targeting in mice and its genetic rescue. Nat Genet. 1995 Sep;11(1):33–39. doi: 10.1038/ng0995-33. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. A place in the world for RNA editing. Cell. 1995 Jun 16;81(6):833–836. doi: 10.1016/0092-8674(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Teng B., Burant C. F., Davidson N. O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993 Jun 18;260(5115):1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J Lipid Res. 1986 Mar;27(3):236–250. [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Weintraub M. S., Eisenberg S., Breslow J. L. Dietary fat clearance in normal subjects is regulated by genetic variation in apolipoprotein E. J Clin Invest. 1987 Dec;80(6):1571–1577. doi: 10.1172/JCI113243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S., Balestra M. E., Ferrell L. D., Fan J., Arnold K. S., Taylor S., Taylor J. M., Innerarity T. L. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8483–8487. doi: 10.1073/pnas.92.18.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]






