Abstract
The long-term use of hypotensive drugs may cause side effects, including impaired glucose metabolism and mineral status. This study tested the hypothesis that some hypotensive drugs can affect tissular chromium levels and indices of glucose metabolic and antioxidant potential in rats. The experiment was performed on 40 male spontaneously hypertensive rats (SHRs), which were assigned to five groups: control (C), with perindopril (PR), with metoprolol (MT), with indapamide (ID), and with amlodipine (AM). All rats were provided ad libitum standard diet (with or without drugs) and distilled water for 45 days. Glucose and insulin levels, along with total antioxidant status (TAS) and concentrations of TNF-alpha and C-reactive protein, were assayed in serum. Chromium concentrations in the liver and kidney were determined using the flame atomic absorption spectrometry method. Detailed statistical analysis was performed using Statistica for Windows 10.0 (StatSoft, Poland). One-way analysis of variance (ANOVA), followed by a post hoc Tukey test, was used to compare the data between groups. Treatment with indapamide and amlodipine resulted in significantly higher chromium concentrations in the liver and kidney (AM) of the rats, compared with the control group. A markedly higher concentration of glucose was found in the ID group. Treatment with amlodipine significantly increased TAS levels in serum and decreased TNF-alpha concentration in serum of the rats. A significant positive correlation between chromium concentration in tissues and serum TAS level was observed, as was a significant negative correlation between chromium concentration in the kidneys, and TNF-alpha and glucose levels in serum. In conclusion, the administration of amlodipine may lead to an increase in chromium accumulation in the internal organs, which is associated with increased antioxidant status and suppression of the inflammatory response of cells in SHRs.
Keywords: Antihypertensive drugs, Chromium, Glucose metabolism, Inflammation, Antioxidant status
Introduction
Recently, the interaction of active components of drugs with certain nutrients has been a matter of increasing interest. Due to their complex chemical structures and properties, the hypotensive drugs used in the treatment of hypertension may have broader biological activity, unrelated to their therapeutic target actions. Of particular interest is the possibility of interaction between hypotensive drugs and essential minerals, leading to the increase or decrease of mineral availability, storage, and excretion, which in consequence may affect the mineral status of related systems and of the body as a whole. Antihypertensive drugs could influence the metabolism of minerals in many ways, from intestinal absorption to bioavailability and elimination. In clinical and experimental studies, it has been observed that treatment with angiotensin-converting enzyme inhibitors (ACE-I) and some diuretics may result in deficits of magnesium, potassium, and zinc [1–3]. Moreover, treatment with certain antihypertensive drugs, such as thiazides and β-blockers, may impair carbohydrate metabolism and lead to an increased incidence of diabetes mellitus in hypertensive patients [4, 5]. On the other hand, it has also been found that amlodipine (a long-acting calcium-channel blocker) improved insulin sensitivity in essential hypertensive patients and exhibited antioxidant and anti-inflammatory properties [6, 7]. However, it is not known whether the effects of antihypertensive drugs on glucose metabolism are associated with the level of chromium in the body. Chromium(III) has a documented role in the carbohydrate, lipid, and protein metabolisms. Trivalent chromium has been shown to lower oxidative stress and to improve glucose and lipid metabolism, but the mechanisms of its action on the molecular level are not fully understood [8]. There are no reliable biomarkers of the body’s Cr status [9]. Since Cr is stored mainly in the liver and the kidneys, these tissues are often used to estimate the major reserves of the element in laboratory animals.
Considering the above facts, we tested the hypothesis that hypertensive drugs can affect chromium status, glucose metabolism, and antioxidant and related indices in spontaneously hypertensive rats (SHRs).
Materials and Methods
Animals
This study was approved by the local bioethics committee for animal studies (approval no. 49/2009).
The experiment was performed on male SHRs (8 weeks old), derived from Wistar Kyoto rats with elevated blood pressure at the Kyoto School of Medicine. The rats were purchased from Charles River Laboratories, Germany. The rats adapted to laboratory conditions during the first 5 days. The mean body mass of the rats was 195 ± 21 g. The animals were housed individually in stainless steel cages coated with metal-free enamel and kept under controlled room conditions: temperature (21 °C), humidity (55–65 %), and 12/12 h light/dark cycle.
Experimental Design
Forty animals were randomly assigned to five groups of eight rats each: the control group (C), a group with perindopril (PR), a group with metoprolol (MT), a group with indapamide (ID), and a group with amlodipine (AM). All rats were fed a standard diet (maintenance diet for rats 1320, Altromin), whose full composition is presented in Table 1. In the diet of the noncontrol groups, perindopril, metoprolol, indapamide, and amlodipine were added at a rate of 0.2, 3.0, 0.03, and 0.2 mg/kg body mass of rat, respectively. The drug was administered in the diet, and fresh solutions were prepared every day. The drug concentrations were adjusted so that the doses (calculated as milligrams per kilogram per day) were kept constant, regardless of dietary intake and body weight. The intake of the diet was monitored daily. The rats were weighed once a week.
Table 1.
The composition of the diet
| Ingredient | Amount | Ingredient | Amount |
|---|---|---|---|
| Total energy (kcal/kg) | 2,844 | Biotin (μg/kg) | 60 |
| Total protein (% of energy) | 24 | Nicotinic acid (mg/kg) | 36 |
| Total fat (% of energy) | 11 | Pantothenic acid (mg/kg) | 21 |
| Total carbohydrate (% of energy) | 65 | Choline chloride (mg/kg) | 600 |
| Protein (g/100 g) | 19 | Calcium (g/kg) | 9 |
| Fat (g/100 g) | 4 | Phosphor (g/kg) | 7 |
| Fiber (g/100 g) | 6 | Magnesium (g/kg) | 3 |
| Vitamin A (IU) | 1,500 | Sodium (g/kg) | 2 |
| Vitamin D3 (IU) | 600 | Potassium (g/kg) | 1 |
| Vitamin B1 (mg/kg) | 18 | Iron (mg/kg) | 165 |
| Vitamin B2 (mg/kg) | 12 | Manganium (mg/kg) | 75 |
| Vitamin B6 (mg/kg) | 9 | Zinc (mg/kg) | 70 |
| Vitamin B12 (μg/kg) | 24 | Copper (mg/kg) | 13 |
| Vitamin C (mg/kg) | 36 | Iodium (mg/kg) | 1.5 |
| Vitamin K3 (mg/kg) | 3 | Selenium (mg/kg) | 0.6 |
| Vitamin E (mg/kg) | 75 | Cobalt (mg/kg) | 0.3 |
| Folic acid (mg/kg) | 2 | Chromium (mg/kg) | 4.5 |
The animals are allowed to eat diet and drink distilled water for 45 days “ad libitum”.
Tissue and Serum Collection
At the end of the experimental period, the animals were weighed and anesthetized with a sodium thiopental injection (40 mg/kg body weight). Blood and tissues were collected from the rats following 12 h of fasting. The liver and kidney were dissected, weighed, and stored frozen (−80 °C) for analysis for chromium content. The blood samples were collected by cardiac puncture in serum-separated tubes to obtain serum. The coagulated blood was left to clot at room temperature for 30 min and then centrifuged for 15 min at 2,000 rpm at 4 °C; the supernatant fluid was then separated and stored frozen (−80 °C) for analysis.
Biochemical Measurements
The concentration of glucose in the blood serum was estimated using the glucose oxidase method [10]. Serum insulin was determined using the radioimmunoassay method with a rat insulin RIA kit (Insulin RIA Kit, Linco Research, USA). Serum TNF-alpha was measured by enzyme immunoassay (enzyme-linked immunosorbent assay; R&D Systems, Inc., Minneapolis, MN, USA). Total antioxidant status (TAS) was measured using a TAS Randox kit (Randox Laboratories, Ltd, Crumlin, UK) and spectrophotometry (SPECORD M40; Carl Zeiss, Jena, Germany). Serum C-reactive protein (CRP) level was determined by ELISA (R&D system, USA).
Determination of Chromium
The chromium content of the tissues was determined following digestion in 65 % (w/w) spectra pure HNO3 (Merck) in a Microwave Digestion System (MARS 5, CEM Corp., USA). Thereafter, the concentrations of chromium in the mineral solutions were measured using the atomic absorption spectrometry method (AAS-5, EA, Jenoptic). Chromium content was determined at a wavelength of 357.9 nm. The accuracy of the method was verified with certified reference materials (bovine liver-trace elements, NIST-1577C, CERT) and proved to be 93 %.
Statistical Analysis
Detailed statistical analysis was performed using Statistica for Windows 10.0 (StatSoft, Poland). The results were expressed as arithmetic means with standard errors. One-way analysis of variance (ANOVA) and a post hoc Tukey test were used to compare the data between groups. The correlations between biochemical variables were calculated using Pearson’s test (with Pearson’s r coefficient). The significance was set at the p < 0.05 level.
Results
The results of the experiment are shown in Tables 2, 3, and 4. The average intake of diet and chromium was comparable across groups (Table 2). As can be seen from Table 3, the treatment of the SHRs with hypotensive drugs (PR, MT, ID, and AM) did not affect major glucose metabolism indices in serum, such as glucose, insulin, and homeostasis model of assessment—insulin resistance (HOMA IR), except in the case of ID, where glucose levels increased (by 11.5 %).
Table 2.
Daily diet and chromium intake in rats
| Groups | |||||
|---|---|---|---|---|---|
| C (n = 8) | PR (n = 8) | MT (n = 8) | ID (n = 8) | AM (n = 8) | |
| Diet (g/day/rat) | 23.5 ± 1.1 | 24.2 ± 1.0 | 24.0 ± 0.9 | 24.3 ± 0.9 | 23.9 ± 1.1 |
| Cr (mg/day/rat) | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.03 |
C control group, PR group with perindopril, MT group with metoprolol, ID group with indapamide, AM group with amlodipine, n number of rats in the group
Table 3.
Biochemical parameters in rats
| Parameter | Groups | ||||
|---|---|---|---|---|---|
| C (n = 8) | PR (n = 8) | MT (n = 8) | ID (n = 8) | AM (n = 8) | |
| Glucose (mmol/l) | 6.1 ± 0.3a | 6.2 ± 0.5a | 5.9 ± 0.6a | 6.8 ± 0.3b | 6.1 ± 0.3a |
| Insulin (pmol/l) | 132.4 ± 41.0 | 137.5 ± 48.2 | 122.0 ± 36.6 | 108.1 ±21.2 | 134.6 ±48.3 |
| HOMA | 5.01 ± 1.43 | 5.60 ± 2.31 | 4.32 ± 1.47 | 4.47 ± 0.85 | 5.15 ± 1.77 |
| TAS (mmol/l) | 1.16 ± 0.32a | 1.12 ± 0.20a | 0.96 ± 0.17a | 1.59 ± 0.24a | 2.99 ± 0.26b |
| TNF-alpha (ng/ml) | 2.24 ± 0.25b | 2.17 ± 0.24b | 1.97 ± 0.18b | 2.31 ± 0.16b | 1.05 ± 0.09a |
| CRP (ng/ml) | 84.9 ± 5.26 | 80.5 ± 7.74 | 79.8 ± 7.2 | 82.3 ± 11.6 | 76.3 ± 12.1 |
C control group, PR group with perindopril, MT group with metoprolol, ID group with indapamide, AM group with amlodipine, HOMA homeostasis model of assessment—insulin resistance index, n number of rats in the group
a,bSignificant differences between five groups (ANOVA test, p < 0.05)
Table 4.
Chromium concentration in tissues of rats (ng/g d.w.)
| Tissue | Groups | ||||
|---|---|---|---|---|---|
| C (n = 8) | PR (n = 8) | MT (n = 8) | ID (n = 8) | AM (n = 8) | |
| Liver | 603.5 ± 86.3a | 612.0 ± 96.1a | 613.5 ± 95.5a | 812.8 ± 117.8b | 881.7 ± 126.7b |
| Kidney | 369.3 ± 38.0a | 489.2 ± 98.6a | 468.2 ± 105.9a | 414.6 ± 94.9a | 673.9 ± 92.6b |
C control group, PR group with perindopril, MT group with metoprolol, ID group with indapamide, AM group with amlodipine, n number of rats in group
a,bSignificant differences between five groups (ANOVA test, p < 0.05)
The treatment of the rats with the hypotensive drugs also had no influence on the indices of antioxidant status and proinflammatory factor (TNF-alpha) and CPR status in serum. On the other hand, treatment of rats with AM brought about a significant increase in serum TAS (of 158 %) accompanied by a decline in TNF-alpha levels (of 53 %).
The treatment of rats with these drugs affected the tissular chromium contents in a drug-dependent manner (Table 4). While PR and MT brought about a slight (though not significant) increase in the hepatic and renal Cr contents, the effect of ID and AM was appreciable. The ID and AM treatment significantly increased hepatic Cr levels by 34 and 46 %, respectively, whereas AM also increased the renal Cr content by 83 % over the control value.
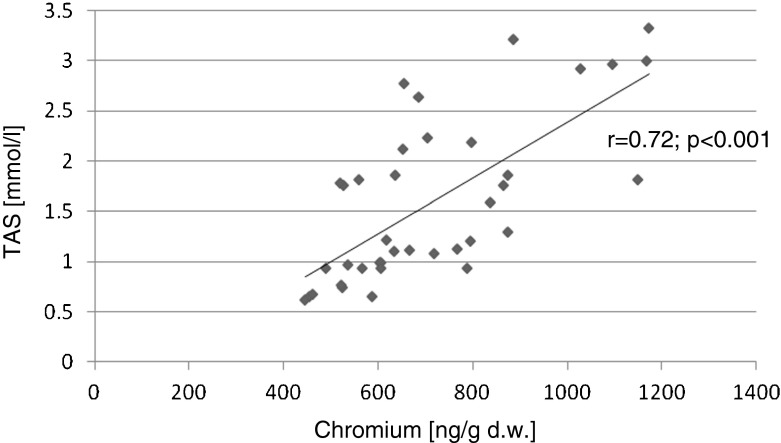
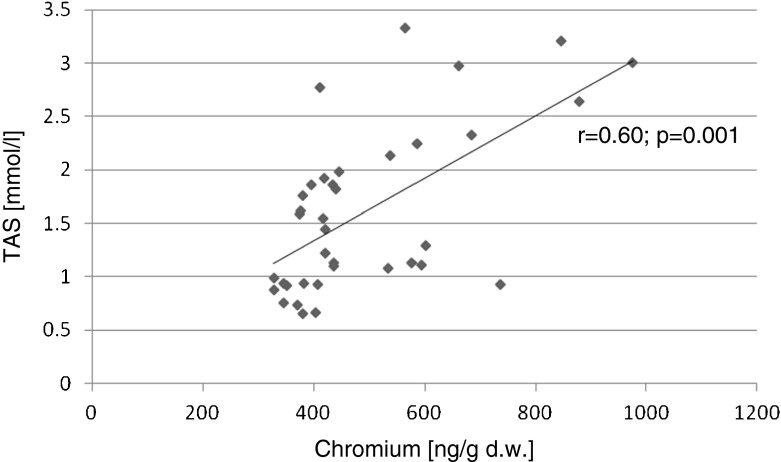
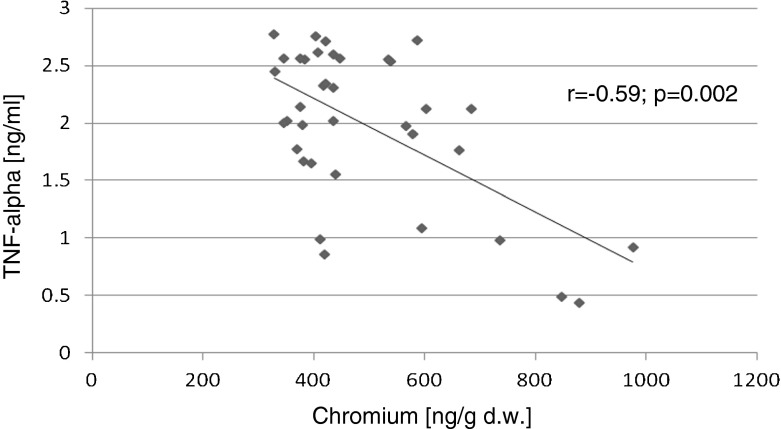
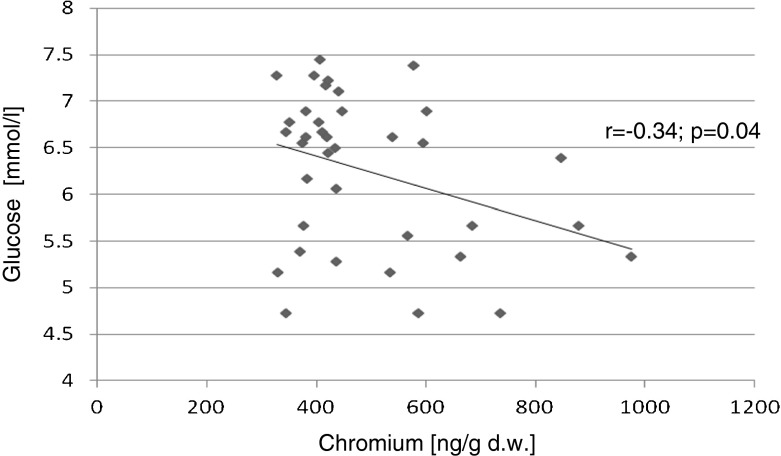
The relationships between the biochemical variables were evaluated using Pearson’s correlation test (with Pearson’s r coefficient). Statistically significant positive correlations were found for the following parameters: liver and kidney Cr contents and serum TAS (r = 0.72, p < 0.001; r = 0.60, p = 0.001), respectively. Significant negative correlations were found between kidney Cr content and serum glucose levels (r = −0.34, p = 0.04) and the kidney Cr content and serum TNF-alpha (r = −0.59 p = 0.002; Figs. 1, 2, 3, and 4).
Fig. 1.
Correlation (Pearson’s r) between TAS level in serum and chromium concentration in liver of rats
Fig. 2.
Correlation (Pearson’s r) between TAS level in serum and chromium concentration in kidney of rats
Fig. 3.
Correlation (Pearson’s r) between TNF-alpha level in serum and chromium concentration in kidney of rats
Fig. 4.
Correlation (Pearson’s r) between glucose level in serum and chromium concentration in kidney of rats
Discussion
In this study, an association between amlodipine in the diet of SHR and increased levels of chromium in their tissues was observed. Higher concentration of chromium in the liver and kidney in the AM group was associated with higher TAS and low TNF-alpha levels in serum of the rats. To our knowledge, this is the first study evaluating the effects of antihypertensive drugs on the level of chromium in SHRs.
The results of this study confirm the antioxidant and anti-inflammatory effects of amlodipine. Some studies have found that amlodipine has an anti-inflammatory effect by inhibiting the production of TNF-alpha and NO [11]. It is suggested that this beneficial activity of amlodipine is dependent on its interaction with cholesterol and oxidants, and/or the mechanism by which amlodipine regulates NO production and its implications [12].
Koh et al. [13] found that amlodipine therapy significantly reduced biomarkers of oxidant stress and improved glucose metabolism, including increases in insulin sensitivity in hypertensive patients. The antioxidative, anti-inflammation, and antidiabetic effect of chromium(III) supplementation has been also observed in experimental studies in rats [14–16].
These results have demonstrated that the administration of amlodipine increases kidney and liver Cr stores, and that indapamide elevates liver Cr stores alone, in comparison with baseline values. The mechanism responsible for this phenomenon is unknown. There are various possibilities that could alter the accumulation of this element in the internal organs.
In some studies, it has been found that antihypertensive drugs disturb the homeostasis of electrolytes and other minerals through alterations in kidney or intestine mineral reabsorption, as well as by effecting changes in the reabsorption of minerals from the system blood to the tissues [1, 3, 17]. The mechanism involved in the change in the tissue chromium concentration as a result of amlodipine treatment may be similar. In the previous study, it was found that amlodipine and indapamide affected the potential bioavailability of minerals from food in in vitro enzymatic digestion [18, 19]. The increased concentration of Cr in the liver and kidneys may result from increased gastrointestinal absorption of this element in the presence of amlodipine (and indapamide, to a lesser extent). Generally, dietary Cr is absorbed with very low efficiency (0.4–2 %) [20], and the rate of its uptake depends on the coupling ligand [21]. Commonly used forms of Cr in supplements—including CrCl3, Cr(III) nicotinate, and CrPic—are absorbed at only 0.5–1.3 % of the dose, while the Cr complex with propionic acid (chemical formula [Cr3O(O2CCH2CH3)6(H2O)3]+, also called Cr3) is absorbed with a very high efficiency of 40–60 % [22]. The difference in the degree of absorption is readily explained by the stability and solubility of the cation in the physiological milieu. The dietary Cr used in this experiment was present in an inorganic form (CrCl3 × 6H2O), so its absorption was presumed to be low (<1 %). It is possible that an active component of amlodipine might somehow chelate dietary Cr, thus improving its absorption and further storage in the internal organs. Another possible explanation of the increased hepatic and renal Cr levels found in this study is the formation of Cr-drug complexes that are trapped in the tissular matrix.
Amlodipine may have also reduced Cr urinary excretion, thus leading to higher retention in the body. Finally, the drugs tested here (amlodipine and indapamide) might affect the distribution of chromium in the body. In a recent report [23], the effects of prednisolone (a glucocorticoid) on Cr distribution in mice fed a high-fat diet was demonstrated. In that study, prednisolone treatment lead to reduced Cr levels in insulin-sensitive tissues (liver, muscle, and fat), while tending to elevate Cr levels in the thigh bone.
Whichever of these mechanisms occurred in this study, the higher hepatic and renal Cr levels were associated with increased serum TAS values. Significant moderate correlations were found for hepatic and renal Cr levels and serum TAS, suggesting that Cr plays some role in supporting antioxidant potential. The mechanisms of its action are not fully understood.
Some reports indicate that Cr(III) supplementation can decrease oxidative stress and proinflammatory cytokines (such as TNF-alpha, IL-6, and CRP) in animal and human studies [14, 24]. In this study, the possible antioxidant properties of chromium might have been enhanced by treatment with amlodipine, because of the antioxidant and anti-inflammatory activities of amlodipine.
The effect of chromium on inflammation may indirectly affect glucose metabolism. It is known that TNF-alpha, IL-6, and CRP play an important role in insulin resistance and the vascular inflammation process through their multiple actions [25, 26]. Rui et al. [27] found that TNF-alpha reduces insulin-stimulated receptor tyrosine kinase activity at low concentrations and may also decrease the expression of the insulin receptor IRS-1 and Glut-4 at higher concentrations, while also increasing the phosphorylation of serine 307 in IRS-1, thus impairing its ability to bind to the insulin receptor and initiate downstream signaling.
In this study, a moderate negative correlation was observed for the kidney Cr level and the serum glucose level, supporting the opinion that Cr is involved in the regulation of glucose homeostasis. A number of studies in both diabetic animals and diabetic human patients have reported that chromium supplementation may be beneficial, as demonstrated by decreased blood glucose, glycosylated hemoglobin, and cholesterol values, or by decreased insulin requirements following chromium supplementation [15, 28, 29].
Conclusions
This study showed that the administration of hypotensive drugs, and in particular amlodipine, significantly increased hepatic and renal Cr levels and is associated with increased serum TAS value and decreased levels of the inflammatory marker TNF-alpha in serum in SHRs. In addition, significant positive correlations were observed between hepatic and renal Cr levels and serum TAS, and significant negative correlations were seen between the renal Cr level and serum TNF-alpha and serum glucose levels in SHRs treated with hypotensive drugs.
It is hypothesized that the administration of hypertensive drugs, in particular of amlodipine, may lead to increases in chromium accumulation in the internal organs, which in turn can mediate (increasing antioxidant status) and suppress the inflammatory response of cells. However, elucidation of the exact mechanisms responsible for these effects requires further investigation.
Acknowledgment
The research was supported by grants from the National Science Centre, Poland (2669/B/P01/2011/40).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Braun LA, Rosenfeldt F. Pharmaco-nutrient interactions—a systematic review of zinc and antihypertensive therapy. Int J Clin Pract. 2012 doi: 10.1111/ijcp.12040. [DOI] [PubMed] [Google Scholar]
- 2.Pikilidou MI, Lasaridis AN, Sarafidis PA, Tziolas IM, Zebekakis PE, Dombros NV, Giannoulis E. Blood pressure and serum potassium levels in hypertensive patients receiving or not receiving antihypertensive treatment. Clin Exp Hypertens. 2007;29(8):563–573. doi: 10.1080/10641960701744103. [DOI] [PubMed] [Google Scholar]
- 3.Samaras D, Samaras N, Lang PO, Genton L, Frangos E, Pichard C. Effects of widely used drugs on micronutrients: a story rarely told. Nutrition. 2013;29(4):605–610. doi: 10.1016/j.nut.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Dunder K, Lind L, Zethelius B, Berglund L, Lithell H. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: population based cohort study. BMJ. 2003;326(7391):681. doi: 10.1136/bmj.326.7391.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manrique C, Johnson M, Sowers JR. Thiazide diuretics alone or with β-blockers impair glucose metabolism in hypertensive patients with abdominal obesity. Hypertension. 2010;55:15–17. doi: 10.1161/HYPERTENSIONAHA.109.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harano Y, Kageyama A, Hirose J, Asakura Y, Yokota T, Ikebuchi M, Suzuki M, Omae T. Improvement of insulin sensitivity for glucose metabolism with the long-acting Ca-channel blocker amlodipine in essential hypertensive subjects. Metabolism. 1995;44(3):315–319. doi: 10.1016/0026-0495(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 7.Toma L, Stancu CS, Sanda GM, Sima AV. Anti-oxidant and anti-inflammatory mechanisms of amlodipine action to improve endothelial cell dysfunction induced by irreversibly glycated LDL. Biochem Biophys Res Commun. 2011;411(1):202–207. doi: 10.1016/j.bbrc.2011.06.137. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JB, Love ST. The need for combined inorganic, biochemical, and nutritional studies of chromium(III) Chem Biodivers. 2012;9(9):1923–1941. doi: 10.1002/cbdv.201100440. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JB. Chromium: celebrating 50 years as an essential element? Dalton Trans. 2010;39:3787–3794. doi: 10.1039/b920480f. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute/NCCLS. Procedures for the Handling and Processing of Blood Specimens; Approved Guideline. Third Edition; 2004
- 11.Li XQ, Cao W, Li T, Zeng AG, Hao LL, Zhang XN, Mei QB. Amlodipine inhibits TNF-alpha production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway. Int Immunopharmacol. 2009;9(9):1032–1041. doi: 10.1016/j.intimp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka C, Egashira K, Ishibashi M, Inoue S, Ni W, Hiasa K, Kitamoto S, Usui M, Takeshita A. Novel anti-inflammatory actions of amlodipine in a rat model of arteriosclerosis induced by long-term inhibition of nitric oxide synthesis. Am J Physiol Heart Circ Physiol. 2004;286(2):H768–H774. doi: 10.1152/ajpheart.00937.2002. [DOI] [PubMed] [Google Scholar]
- 13.Koh KK, Han SH, Ahn JY, Chung WJ, Lee Y, Shin EK. Amlodipine improves endothelial function and metabolic parameters in patients with hypertension. Int J Cardiol. 2009;133(1):23–31. doi: 10.1016/j.ijcard.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 14.Jain SK, Rains JL, Croad JL. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin treated diabetic rats. Free Radic Biol Med. 2007;43(8):1124–1131. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krol E, Krejpcio Z. Evaluation of anti-diabetic potential of chromium(III) propionate complex in high-fat diet fed and STZ injected rats. Food Chem Toxicol. 2011;49(12):3217–3223. doi: 10.1016/j.fct.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Sahin K, Tuzcu M, Orhan C, Agca CA, Sahin N, Guvenc M, Krejpcio Z, Staniek H, Hayirli A. The effects of chromium complex and level on glucose metabolism and memory acquisition in rats fed high-fat diet. Biol Trace Elem Res. 2011;143(2):1018–1030. doi: 10.1007/s12011-010-8905-9. [DOI] [PubMed] [Google Scholar]
- 17.Skrzypczak W, Dratwa-Chałupnik A, Ozgo M, Michałek K, Lepczyński A, Hejza K, Siwa J. Effect of converting enzyme inhibitor on copper and iron concentrations of blood plasma in calves during the neonatal period. Folia Biol (Krakow) 2010;58(1–2):119–124. doi: 10.3409/fb58_1-2.119-124. [DOI] [PubMed] [Google Scholar]
- 18.Suliburska J, Bogdanski P, Chiniewicz B. The influence of selected hypotensive drugs on the bioavailability of minerals from buckwheat groats in vitro enzymatic digestion. Acta Sci Pol Technol Aliment. 2011;10(4):507–513. [PubMed] [Google Scholar]
- 19.Suliburska J, Bogdanski P, Chiniewicz B. Antihypertensive drugs affect potential bioavailability of minerals from shelled pea. J Elem. 2013;18(1):127–134. [Google Scholar]
- 20.Vincent The bioinorganic chemistry of chromium(III) Polyhedron. 2001;20:1–26. doi: 10.1016/S0277-5387(00)00624-0. [DOI] [Google Scholar]
- 21.Herring BJ, Logsdon AL, Lockard JE, Miller BM, Kim H, Calderon EA, Vincent JB, Bailey MM. Long-term exposure to [Cr(3)O(O(2)CCH(2)CH(3))(6)(H(2)O)(3)](+) in Wistar rats fed normal or high-fat diets does not alter glucose metabolism. Biol Trace Elem Res. 2013;151(3):406–414. doi: 10.1007/s12011-012-9580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clodfelder BJ, Gullick BM, Lukaski HC, Neggers Y, Vincent JB. Oral administration of the biomimetic [Cr3O(O2CCH2CH3)6(H2O)3]+ increases insulin sensitivity and improves blood plasma variables in healthy and type 2 diabetic rats. J Biol Inorg Chem. 2005;10(2):119–130. doi: 10.1007/s00775-004-0618-0. [DOI] [PubMed] [Google Scholar]
- 23.Chen PW, Lin C, Chen CD, Chen WY, Mao FC. Chromium levels in insulin-sensitive tissues and the thigh bone are modulated by prednisolone and high-fat diets in mice. Biometals. 2013;26:347–354. doi: 10.1007/s10534-013-9621-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen YL, Lin JD, Hsia JH, Mao FC, Hsu CH, Pei D. The effect of chromium on inflammatory markers, 1st and 2nd phase insulin secretion in type 2 diabetes. Eur J Nutr. 2013 doi: 10.1007/s00394-013-0508-8. [DOI] [PubMed] [Google Scholar]
- 25.Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R. Effects of tumor necrosis factor-a on insulin action in cultured human muscle cells. Diabetes. 2001;50:1102–1109. doi: 10.2337/diabetes.50.5.1102. [DOI] [PubMed] [Google Scholar]
- 26.Saghizadesh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF-a by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF- stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krol E, Krejpcio Z. Chromium(III) propionate complex supplementation improves carbohydrate metabolism in insulin-resistance rat model. Food Chem Toxicol. 2010;48(10):2791–2796. doi: 10.1016/j.fct.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]