Abstract
Many insignificant events in our daily life are forgotten quickly but can be remembered for longer when other memory-modulating events occur before or after them. This phenomenon has been investigated in animal models in a protocol in which weak memories persist longer if exploration in a novel context is introduced around the time of memory encoding. This study aims to understand whether other types of rewarding or novel tasks, such as rewarded learning in a T-maze and novel object recognition, can also be effective memory-modulating events. Rats were trained in a delayed matching-to-place task to encode and retrieve food locations in an event arena. Weak encoding with only one food pellet at the sample location induced memory encoding but forgetting over 24 h. When this same weak encoding was followed by a rewarded task in a T-maze, the memory persisted for 24 h. Moreover, the same persistence of memory over 24 h could be achieved by exploration in a novel box or by a rewarded T-maze task after a “non-rewarded” weak encoding. When the one-pellet weak encoding was followed by novel object exploration, the memory did not persist at 24 h. Together, the results confirm that place encoding is possible without explicit reward, and that rewarded learning in a separate task lacking novelty can be an effective memory-modulating event. The behavioral and neurobiological implications are discussed.
Memories for the trivial daily events of life fade away quickly while those of emotionally significant or surprising events are more likely to last. Sometimes, the memory of significant events can be accompanied by a “halo” of remembering for what would normally be considered unimportant details—a phenomenon captured by the metaphor of “flashbulb memory” (Brown and Kulik 1977). A recent series of prospective experiments (Moncada and Viola 2007; Ballarini et al. 2009; Wang et al. 2010; Almaguer-Melian et al. 2012) have created an animal model of this flashbulb memory phenomenon with a view to understanding the underlying neurobiology. In our own study using an appetitively motivated task for rats (Wang et al. 2010), we observed that spatial memories that typically decay within a day could still be observed after 24 h if the initial encoding was followed or preceded by exploration in a novel context. Novelty was also used as a successful modulator in the studies of the Viola group. Collectively, this series of studies has established that novelty-induced enhancement of the persistence of memory is sensitive to pharmacological blockade of dopamine D1/D5 receptors in the hippocampus and local inhibition of protein synthesis (Moncada and Viola 2007; Wang et al. 2010). The theoretical basis of this work, in particular its link to Lisman and Grace's model of novelty-associated modulation of hippocampal-dependent memory (Lisman and Grace 2005) and to the synaptic tagging and capture (STC) hypothesis of Frey and Morris (1998), is outlined in the discussion below. The relevance of work on memory modulation by post-training events (Cahill and McGaugh 1998; McGaugh 2000) and stress (Diamond et al. 2007) is also considered.
The aim of the present study was to investigate further, at a strictly behavioral level, the determinants of this memory persistence. The protocols that we developed can be thought of as consisting of: (1) an “everyday” spatial memory task in which new memories are formed each day; and (2) occasional modulating experiences that are scheduled at a separate time from memory encoding (typically 30 min later). The spatial memory task involves training hungry animals to search in a test arena for a single sandwell in which they can dig to secure food; the location of the sandwell varies from day to day. This memory-encoding trial is followed by a daily choice trial in which the animal is exposed to five sandwells in different locations of which only one contains food—this being the sandwell that occupies the same location in the arena as that used for encoding (win-stay). The “correct” location of the encoding trial varies from day to day, and the protocol deployed enables training to continue over days, weeks, and even months (i.e., a repeated-measures, within-subject design across conditions). Good performance in this task is displayed by minimal errors in searching for the correctly located sandwell on the daily choice trial. This choice measure is supplemented by occasional probe tests in which the daily choice trial is replaced by an extinction trial in which none of the five sandwells of the second trial of the day contains food, and the time spent digging in each sandwell serves as an index of memory. This probe trial is scheduled at varying times after the encoding trial, varying from 30 min to even 24 h (i.e., the next day). Successful application of the win-stay principle predicts that more time will be spent digging in the “correct” rather than the “wrong” sandwells, with a significant difference between these constituting evidence for memory of that day's encoding location. In contrast to the main task, the modulating experience used so far has consisted of no more than occasionally placing the animal inside a Perspex box that is put inside the arena, and allowing the animals to explore this box. Rats are very sensitive to the floor substrate on which they walk, and so varying the substrate of the Perspex box (sand, feathers, carpet, etc.) enables repeated novel experiences for the animals (provided the protocol is not repeated too frequently). This 5-min period of exploration is typically scheduled 30 min after the encoding trial. The supposition is that novelty exploration triggers neuromodulatory events that enable consolidation of the otherwise decaying memory trace of the daily location of the sandwell used in the earlier encoding trial.
The determinants of persistence of this daily spatial memory are likely to include (1) events happening at the time of memory encoding, and (2) events happening at the time of the modulating experience. Accordingly, we first investigated both the necessity for food reward and variation in the magnitude of reward at the time of memory encoding (Experiment 1). We then turned to the modulating experience itself and asked whether a rewarding task in a familiarized T-maze, with or without procedural novelty, would be sufficient to modulate the persistence of the spatial memory (Experiment 2). Finally, we examined the impact of novel object exploration on the persistence of the spatial memory (Experiment 3). More generally, we can think of the daily spatial memory experiences as relatively trivial “events,” and the later, albeit occasional novelty and/or reward experiences, as the surprising or emotionally significant events which happen from time to time that may create a halo of better memory for other closely timed events.
Results
Male Lister-Hooded rats were trained in an event arena to perform the everyday memory task described above. We describe first the outcome of the initial training protocol and levels of asymptotic performance that were sustained through the subsequent within-subject experiments. We then describe Experiments 1–3 in turn.
Training and asymptotic performance
The animals (n = 16) were first habituated to the event arena and taught to dig for food in a single sandwell located at the central location of the arena (row 4, column 4 [see Fig. 1A]). After 6 d in which the animals were effectively rewarded for returning to the same location to secure additional food pellets, daily training commenced for the main spatial memory task. This involved an encoding trial (one sandwell) and a retrieval choice trial (five sandwells) (Fig. 1A). During the encoding trial of each day, one sandwell was located in a specific but varying location in the arena. Rats entered the arena from one of four start boxes (North, East, South, West—varied across days), explored the arena, encountered the sandwell, dug in it, and so retrieved a food pellet hidden in the sand that they typically carried back to eat in the start box. The animals then returned to the sandwell to collect individually two further food pellets, whereupon they were returned to the home cage. After a 30- to 40-min delay, they would be put back into the same start box for 30 sec, the door separating it from the arena would open, and the animals would be confronted by five sandwells as a retrieval-choice trial. Only the sandwell that matched the location of the encoding trial would again contain food pellets. Rats would dig in the sandwells and find more pellets in the matching location, with their choices of which sandwells at which they dug recorded by the experimenter with “correct” and “wrong” sandwell choices noted. Daily training concluded after the rats had collected and eaten three further food pellets. Across animals, different baited locations were used within a day to avoid animals using cryptic traces from other animals in the arena to solve the task. Across days, different baited locations were also used for individual rats so rats would learn to update the information based on the baited location in the encoding trial.
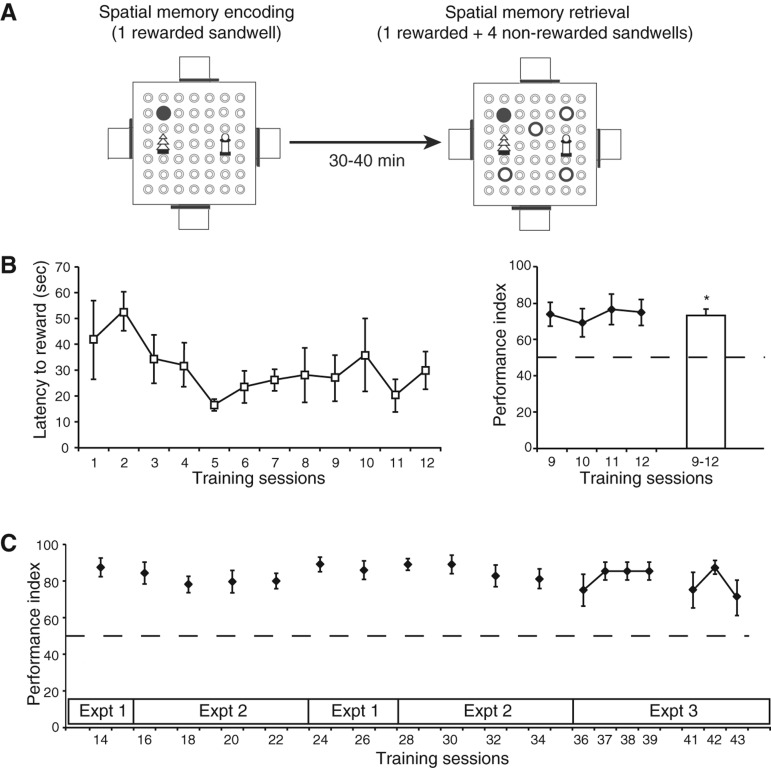
Figure 1.
One-trial spatial memory task in the event arena. (A) Schematic representation of the basic protocol and training procedure showing the separate encoding and retrieval choice trials. (B) Performance during acquisition of the task. Latency to the first reward in the retrieval trial gradually declined over the first 12 training sessions. The performance index (PI), computed from the number of choice errors, over the last four of these sessions was consistently above chance level. (C) The PI was maintained at a stable, above chance level, across the whole study.
The latency to locate the rewarded sandwell in the retrieval-choice trial gradually declined over the course of 12 sessions of daily training (F(1,15) = 4.53, P = 0.05, linear trend) (Fig. 1B). Over the last four of these sessions, choice errors were low resulting in a performance index (PI = 100 × (1 − (errors/4))) that was consistently above the chance level (all t(15) > 2.42, P < 0.05; random search is two errors/trial which equals a performance index of 50%) (Fig. 1C). After this initial training, numerous modulatory conditions were introduced over Experiments 1–3 (Fig. 1C) interspersed by further training days. This took an additional 31 sessions and the performance index on retrieval-choice trials throughout was well above chance (session 14–35, all t(15) > 4.14, P < 0.005; session 36–42, all t(11) > 9.9, P < 0.005; session 43, t(11) = 2.15, P = 0.053). These data indicate that the animals had learned this “matching-to-place” task and sustained above chance levels of performance throughout the various conditions and probe tests of the three experiments described below. Broadly speaking, these experiments were conducted and are reported in order; however, some studies are reported in their logical rather than their chronological order (see Fig. 1C and Materials and Methods for details; in brief, Experiment 1 was done before Experiment 2 and Experiment 3 with the exception that the 1-h memory test in Experiment 1 [Fig. 2B] was done after confirming the effectiveness of the T-maze task in Experiment 2). The use of counterbalancing of conditions within these experiments and the stability of retrieval-choice performance indicates that this was a reasonable manner of conducting and comparing conditions.
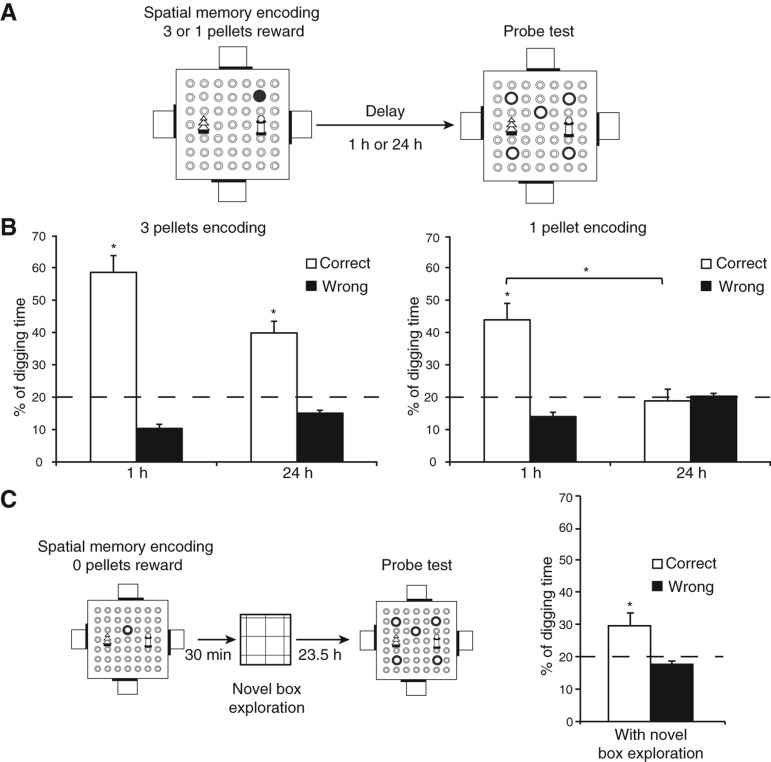
Figure 2.
Experiment 1: The impact of manipulating the amount of reward during encoding of the spatial memory task. (A) Schematic representation of the protocol in which the rats dug one sandwell to find three pellets or one pellet as reward (memory encoding) and received a nonrewarded probe trial 1 h or 24 h later (retrieval). (B) Memory was effective with three-pellet reward at 1 h and 24 h. When only one pellet was available at encoding, memory persistence decayed from above chance at 1 h to chance at 24 h. (C) Spatial memory after encoding with no reward (i.e., no pellets) was maintained at 24 h by the exploration of a novel box 30 min after encoding.
Experiment 1: The impact of varying reward during encoding of the spatial memory task
The first step of our analysis was to establish that variation in the conditions prevailing at the time of spatial/event-memory encoding could alter the duration for which such a memory could be detected. Interspersed training always took place with three food pellets available at encoding, as in baseline training. Reducing this reward from three to one pellet on the encoding trial (Fig. 2A) may still allow a spatial memory to be encoded, but this unexpected reduction in reward may cause faster decay, with memory no longer detectable 1 d (24 h) after the encoding trial. Although a choice trial might allow some assessment of the impact of reward reduction, our experience in this and other delayed matching-to-place tasks, i.e., in the water maze task (Steele and Morris 1999), is that a nonrewarded probe trial offers a more sensitive and graded measure of memory within and across animals. In such a trial, no food reward is available at any of the five available sandwells of the choice trial and the time spent digging at each well is measured. The use of three- or one-pellet encoding was counterbalanced across animals, with two successive probe tests interspersed by further baseline training, and the probe trial concluded by the experimenters placing food into the correct sandwell after 60 sec and allowing the animal to retrieve this food (so avoiding extinction).
The results showed effective spatial memory with three-pellet reward at 1 h and 24 h whereas spatial memory declined to chance over 24 h with one-pellet reward at encoding (Fig. 2B). Analyses showed that, at 24 h, memory was above chance only for the three-pellet condition (t(15) = 3.97, P < 0.005) but not the one-pellet condition (t(15) = 0.4, P = 0.7). Importantly, memory declined between 1 h and 24 h for the one-pellet condition as shown by a significant interaction between time (1 h or 24 h) and digging type (correct or wrong; F(1, 15) = 47.7, P < 0.001).
In a further investigation of the impact of reward during initial encoding, we asked whether reward was actually necessary. Clearly this is a task in which the daily protocol was ordinarily one in which three pellets were available on both encoding and retrieval-choice trials. The “event” of finding a sandwell, encoding its location, and retrieving food reward can, however, be broken up into components each of which may contribute to the totality of an event memory. Accordingly, one session was given in which the encoding trial had no food available, a modulatory event scheduled, and a subsequent probe trial conducted to see whether the animals could remember that location. In pilot work, we found that such encoding of space without reward does not result in good memory when the probe trial is conducted at 24 h (correct digging = 19.67 ± 3.03%, indifferent from chance, t(15) = 0.1, P = 0.92) (SH Wang and RGM Morris, unpubl.). Interestingly, here we found that performance was significantly above chance at 24 h (t(15) = 2.38, P < 0.05) (Fig. 2C) when novel box exploration, a method that we reliably showed to facilitate the 24-h memory persistence of encoding one-pellet location (Wang et al. 2010), was introduced at 30 min after this type of nonrewarded encoding.
Experiment 2: The impact of reward in a separate task on the persistence of memory in the event arena
Experiment 1 had confirmed that memory persistence is sensitive to reward magnitude at the time of memory encoding. We now asked whether variation in reward magnitude in a separate rewarded learning task, given as modulatory experience 30 min after spatial memory encoding, could facilitate memory persistence. The task we chose was a simple T-maze choice task in which the rats were rewarded for leaving a start box and turning left or right to a goal (Fig. 3A). Such a task can be acquired in parallel with the DMP task in the event arena without causing interference, it can be acquired in a similar timeframe, and it allows changes of the rewarding scheme that may cause “novelty or surprise” on the critical days for assessing an impact on memory persistence.
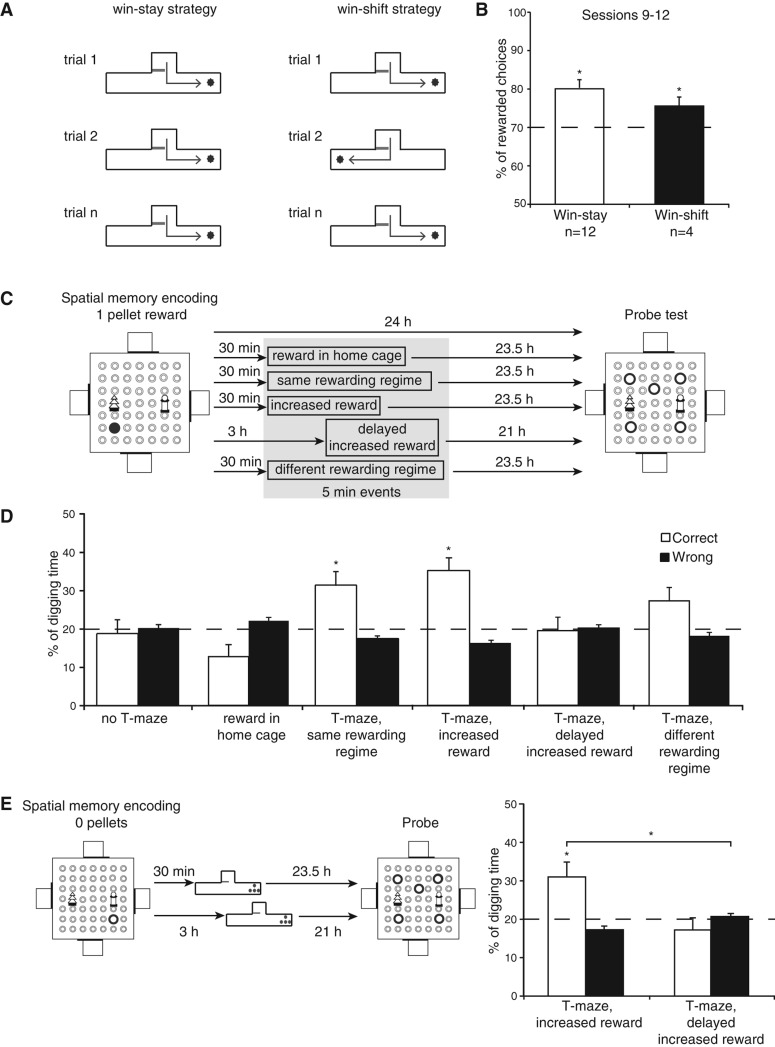
Figure 3.
Experiment 2: The impact of a rewarded learning in the T-maze, with or without procedural novelty, on spatial memory persistence. (A) Identification of the preferred strategy by individual animals in the T-maze task. Some animals always visited the right (shown) or the left (not shown) arm after leaving the start box; these are indicated as “win-stay” animals. Other animals switched between right and left arms in successive trials; these are indicated as “win-shift” animals. (B) Performance of the animals in the two groups (i.e., win-stay and win-shift) over the last four sessions was consistently above chance level. (C) Schematic representation of the procedures. Rats received an encoding trial with one pellet, followed by various modulating events, and tested at 24 h for the spatial memory. The modulating events include rewards in the home cage or rewarded tasks in a T-maze. (D) The 24-h spatial memory of one-pellet encoding was at chance level (see Fig. 2C, right) and was not enhanced by rewards in the home cage. However, this 24-h spatial memory was above chance when the T-maze was introduced 30 min, not 3 h, after the encoding. (E) Spatial memory produced by encoding with no reward (i.e., no pellets) was rescued by the introduction of a T-maze task with increased reward 30 min after encoding, but not when the task was given 3 h after encoding.
We anticipated that the rats would spontaneously alternate between the two T-maze choice options to collect food reward (Ainge et al. 2007; Bett et al. 2012). Hence, we initially trained the rats to collect food rewards (with a flavor that was different from the pellets used in the event arena) with both arms rewarded for the first trial. However, for the remaining trials, reward would be in the arm that was not rewarded in the previous trial (i.e., alternation). On Day 1, the T-maze session stopped after the rats collected three rewards from the left arm and three rewards from the right arm; on Days 2–5 training continued until five rewards had been secured in each arm.
This alternating rewarding scheme is similar to the “win-shift” schedule that is commonly used in the radial arm maze, but clearly opposite to the “win-stay” principle that governed performance in the event arena. Not surprisingly, half of our animals (n = 8 of 16 animals) alternated naturally and secured 73.3 ± 0.8% rewarded choices in the T-maze; the other half (n = 8 of 16 animals) preferentially repeated previous choices (rewarded choices dropped to 65.3 ± 1.4%; group differentiation criterion, chance = 70%, calculated by 10 rewards collected in 10 choices = 100% rewarded choices, 10 rewards in 11 trials = 90.9% rewarded choices, …, 10 rewards in 20 trials = 50% rewarded choices; average of 100%, 90.9%, …, 50% = 70%). Given that it was important to match the reward regime to the rats’ natural or trained preferences, we continued with win-shift for the first half of the group, but switched the others to win-stay over sessions 6–8. During these sessions, the win-shift subgroup displayed further heterogeneity such that half of these animals (n = 4 of the eight win-shift animals) started to adopt a win-stay rule also. By the end (sessions 9–12) (Fig. 3B), we had 12 animals who preferred and were rewarded according to a win-stay principle (rewarded choices significantly better than chance, 70% as described above, t(11) = 4.57, P < 0.05) and four animals who continued on a win-shift reward protocol (rewarded choices significantly better than chance, t(15) = 2.85, P < 0.05; no between group difference, t(15) = 1.6, P = 0.12).
These 12 d of training, conducted in parallel with continued training in the event arena, always used one-pellet reward in the goal arms of the T-maze. There were five types of modulatory conditions introduced 30 min after the encoding of one-pellet location in the event arena (Fig. 3C). The first modulatory condition was to give rats “rewards” in their home cage that were normally only obtained in the T-maze. This made the 24-h spatial memory in the event arena slightly worse than chance (t(15) = −2.38, P = 0.03) (Fig. 3C,D), but no different from the 24-h memory of one-pellet spatial encoding without modulatory events (t(15) = 1.44, P = 0.17). The second modulatory condition was a regular T-maze training session to which the rats were already familiarized. This made the 24-h spatial memory significantly better than chance (t(15) = −2.99, P < 0.01). The third condition was to introduce procedural novelty by increasing the reward magnitude per choice. We baited the goal arm of the T-maze with two double-sized pellets (four times larger than the usual single reward). This also made the 24-h spatial memory significantly better than chance (t(15) = 4.39, P < 0.001). The fourth condition was similar to condition 3 but the procedure was delayed by 3 h, a time point that we have previously shown to be ineffective for memory modulation. As expected, this had no effect on memory persistence, with 24-h spatial memory no different to chance (t(15) = −0.15, P = 0.88). The fifth condition was to introduce a different type of procedural novelty based on reward contingency. We switched the reward regime in a way that win-stay rats now received rewards baited in the alternating arm and win-shift rats now had rewards baited in the fixed arm. This modulatory condition made the 24-h spatial memory above chance with a marginal trend toward significance (t(15) = 2.07, P = 0.056), but it did not differ from the spatial memory in condition 2 (t(15) = 0.67, P = 0.51) (Fig. 3C,D).
To confirm that conditions 3 and 5 indeed produced behavioral changes reflecting novelty, we examined the behavioral performance changes in the T-maze task in condition 3 and condition 5 in comparison with the familiarized T-maze task of condition 2. We found that in condition 2, rats averagely collected 9.4 ± 0.4 pellets in 11.2 ± 0.7 choices in an ∼5 min session. This resulted in a reward/choice ratio of 0.86 ± 0.02, which reflected the behavioral efficacy in obtaining rewards. This efficacy was significantly increased in condition 3 (1.82 ± 0.1, t(15) = 6.54, P < 0.001) when more rewards were baited per goal arm. This efficacy was significantly decreased in condition 5 (0.71 ± 0.02, t(15) = 4.19, P < 0.001) when the rewarding regime did not match the familiarized regime.
Finally, we asked whether modulatory condition 3 (i.e., increased reward) described above could also extend the persistence of memory of a nonrewarded spatial encoding in the event arena (similar to that in Fig. 2C). A 3-h delay of such condition (similar to condition 4 described above) was conducted in parallel. We found that while a T-maze task with increased reward enabled 24-h spatial memory in the event arena significantly above chance (t(15) = 2.86, P = 0.01), no such effect was seen with a 3-h delay (t(15) = 0.88, P = 0.39). The interaction between these two conditions and digging types (correct or wrong) was also significant (F(1,15) = 8.3, P = 0.01) (Fig. 3E).
Experiment 3: The impact of post-encoding novel object experience on the persistence of spatial memory
Training continued in the event arena, with the same animals, with habituation to a box over three consecutive days that would later be used for novel object recognition conducted in parallel with the main task. Daily box habituation lasted 10 min for each animal. On the next pair of sessions (interspersed with regular training), the animals received a one-pellet encoding trial in the event arena followed, after 30 min, by a 5-min session in the now habituated box with or without two identical novel objects (i.e., the novel object and control conditions, respectively). A probe trial in the event arena was given 24 h after each of the encoding trials (Fig. 4A) to test whether novel object exploration facilitated the persistence of spatial memory. Spatial memory of one-pellet encoding was at chance level at 24 h, with no difference between correct and wrong digging (t(11) < 1, P > 0.05) (Fig. 4C, placed graphically under the “probe test” of Fig. 4A). Exploration of the novel objects 30 min after encoding failed to enhance this 24-h memory (no significant difference between correct and wrong digging, t(11) = 1.19, P > 0.05). There was also no significant interaction between the conditions and digging type (F(1,11) = 0.91, P = 0.35) (Fig. 4C). Within-subject comparison of conditions also indicated no difference between performance on days with or without novel objects in the familiarized box (t(11) < 1, P > 0.05).
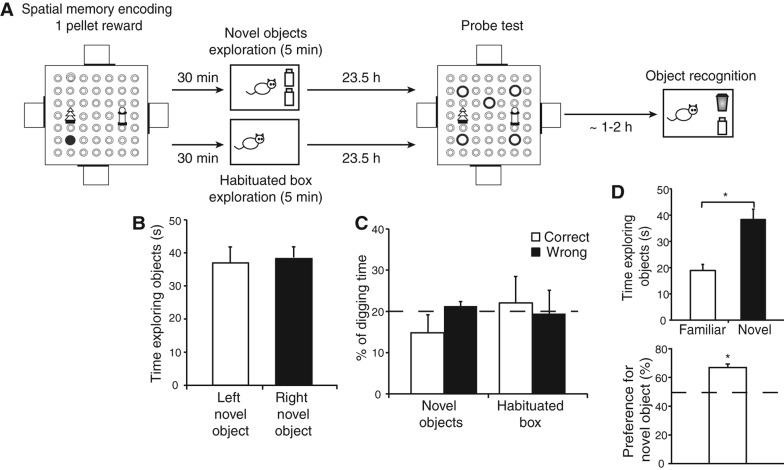
Figure 4.
Experiment 3: The impact of the post-encoding exploration of two novel objects. (A) Schematic representation of the protocol. Rats were placed in a familiarized box with or without two identical novel objects, 30 min after spatial memory encoding with one-pellet reward in the event arena. The probe test was conducted 24 h later. After probe test in the event arena, animals were placed in the same box with one of the previously experienced object (now familiar) and one novel. This served as a measure of recognition memory for the novel object encoding scheduled the previous day. (B) Time spent exploring was comparable between the two novel objects during first exposure. (C) Persistence of spatial memory in the arena was not facilitated by the exposure to the novel objects. (D) Exploration of the objects during the object recognition test. Animals explored significantly more the novel object than the familiar one, indicating that object recognition memory persisted for 24 h.
Before any interpretation of these data is possible, it was essential to establish that the novel object session in the familiarized box had itself created a memory that would last. This was tested by scheduling a further 5-min exposure to the box after the event arena probe trial, with one of the previously novel objects replaced with a new novel object. During the initial exposure to two novel objects, the absolute time spent examining the two objects within the 5-min session was ∼35 sec (Fig. 4B). During the second exposure to one of these same objects together with a new novel object, greater absolute time was spent exploring the new novel object than the now familiar one, indicating that a 24-h memory of the original two novel objects had been created (t(11) = 6.23, P < 0.01) (Fig. 4D). These absolute times of the second exposure to the box were converted into the percentage of time that a rat spent exploring the novel object (compared to the familiar one). A value of 50% indicates no preference (chance), whereas a value above 50% indicates a preferential exploration of the novel object. Using a one-sample t-test, percentage of exploration of the novel object was above chance (t(11) = 6.78, P < 0.01) (Fig. 4D).
Discussion
The main findings of this study are that conditions other than novelty exploration in a box 30 min after spatial memory encoding can also influence the persistence of memory. In Experiment 1, we found that an increase from one-pellet to three-pellet reward had the effect of rendering more stable a normally fragile memory of location in a task in which the correct location is constantly varying. However, reward at the time of memory encoding appears not to be necessary as novelty exploration 30 min after encoding with no reward still enables a 24-h memory of spatial location. In Experiment 2, we found that rewarded learning in a separate but parallel trained task, with or without changes in reward magnitude or procedural novelty, was successful in modulating the persistence of memory in the event arena. In Experiment 3, we observed that a different type of novelty—exploration of novel objects—could create a lasting 24-h memory of the objects, but was insufficient to successfully modulate memory of spatial location in the event arena. The implications of these findings will be discussed from a behavioral and a theoretical perspective.
Features of the task
It is important to recognize that the usual way in which behavioral experiments on learning are conducted in animals is with training of a single task at any one time. This is simple and analytically tractable. From this, numerous important principles have been derived, including the importance of the gradient of reinforcement (i.e., the deleterious impact of delaying reward for a correct action) and the impact of unexpected reinforcement on memory trace strength. However, while analytically more simple, this laboratory convenience is very unrealistic of everyday life in which a number of different things are typically happening within a short space of time.
Doing two things at once, or within a short period of time, opens the opportunity for interference—particularly if the information content of one task can affect information processing in the second. Equally, however, it opens the opportunity for synergistic interactions with the “behavioral tagging” phenomenon of novelty-associated enhancement of memory persistence being of this character (Moncada and Viola 2007). Specifically, there is no obvious way in which exploring a novel box can interfere in a strict informational sense with execution of an inhibitory avoidance response or making a choice of a sandwell in which to dig. Instead, as outlined below, a synergistic interaction may occur at the level of memory modulation in which neurobiological events are triggered by task 2 that affect and may support the stabilization of memory for task 1.
Behavioral interpretation
Our studies were motivated by a desire to establish whether novelty exploration in a box (as task 2) was the only kind of experience that could affect the persistence of memory of task 1—spatial memory encoding. Our results show clearly that it is not. Experiment 1 shows that persistence can be enhanced by events contained entirely within the main task (such as amount of reward), while Expts. 2 and 3 indicate that it can be affected by reward in a separate task, and unaffected by other forms of novelty. Thus, rewarded learning in task 2 in the T-maze, which is delivered 30 min after spatial memory encoding in the event arena (task 1), alters the persistence of memory for this main task. This period of time is much longer than the usual time intervals relevant to the concept of gradient of reinforcement, indicating that its likely effect is not on memory encoding per se but on a separate consolidation/stabilization process. The introduction of procedural novelty by increasing reward magnitude per choice or by changing rewarding regime in the T-maze did produce observable changes in behavior efficacy and contributed to 24-h memory persistence in the event arena. However, these procedural changes may not have further enhanced memory because a familiarized T-maze task alone is already sufficient to make spatial memory in the event arena last for 24 h. Similarly, Experiment 3 indicated that our protocol of novel object exploration could set up a memory of the initially novel objects that would last for at least 24 h but was insufficient to modulate spatial memory persistence in the event arena. That is, events other than novelty can modulate, and not all novel events are successful in doing so.
There are some caveats to this dual interpretation of our findings. First, in Experiment 1, the larger reward magnitude of three pellets relative to one pellet at encoding required that the animals make three visits from the start box to the single available sandwell at the time of encoding. Our further finding within that study that the availability of reward is actually not necessary for memory encoding of location raises the question of how the confounding between reward magnitude and number of visits should be interpreted. One possibility, consistent with Hebbian learning (see below), is that reward has minimal effect on memory encoding (O'Keefe and Nadel 1978), while multiple visits from the start box to the goal create the opportunity for more reliable Hebbian encoding that is more persistent over time. Second, in Experiment 3, novel object exploration was insufficient to modulate the persistence of previously encoded spatial memory, while walking across novel floor substrates was sufficient (Wang et al. 2010; Experiment 2 this study). In Experiment 3, the time spent exploring each of the novel objects during their exposure to the previously familiarized box (on average 37.8 sec per object, and 75.6 sec in total) was broadly comparable to the time taken (latency) to make either a sample or a correct choice in the baseline spatial memory task (∼30 sec). We know that a single visit to the encoding sandwell (one-pellet reward) is insufficient to create a lasting memory and thus the question arises, by analogy, whether a single trial of exposure to initially novel objects would be sufficient to produce an adequate memory. However, in novel object exploration, the animals will typically make multiple approaches to the individual objects. Moreover, the discrimination index data, calculated at 24 h, indicate that the period of 75.6 sec in a 5-min trial was sufficient to create a lasting novel object recognition memory. One possibility is that the threshold for novelty to modulate other memories may be higher than that for lasting novel object recognition memory itself. Another possibility is that there may be less arousal associated with novel object recognition. In the earlier report, the formation of flashbulb memories is described as a consequence of surprising or arousal events (Brown and Kulik 1977). The level of arousal or surprise may determine whether flashbulb memories are formed or whether trivial events surrounding these arousal events can last longer. Due to the lack of a physiological measurement that can objectively reflect the level of arousal unconfounded by other constructs such as novelty, reward, or stress, we are unable to support or rule out this interpretation.
A recent report indicates that exploration of different novel objects at distinct locations can be an effective modulating event to facilitate the persistence of memory in an inhibitory avoidance paradigm (Dong et al. 2012). However, we did not find that novel object exploration served as an effective memory modulatory event, despite resulting in good 24-h object recognition memory. This may reflect the impact of different forms of novelty, as the spatial version of novel object exploration might activate wider regions of the brain (including the hippocampus), which overlap with brain areas involved in the memory task being modulated, and/or provide greater neuromodulation in the key brain areas (see the section Neurobiological implications below). However, it seems more likely that it is due to the differential duration of habituation to the box that was used for object exploration. In our study, we used four or eight sessions (due to counterbalancing) of 10 min per session, while Dong et al (2012) used only one 15-min session of habituation, raising the possibility that the box itself remained novel and the effect observed was linked to general exploration of a still-novel box. Exposure to a novel box is sufficient to effectively modulate memory persistence of inhibitory avoidance (Moncada and Viola 2007; Lu et al. 2011), contextual fear conditioning and spatial object recognition (Ballarini et al. 2009), spatial memory in the event arena (Wang et al. 2010), spatial memory in the water maze (Almaguer-Melian et al. 2012), and extinction of contextual fear (de Carvalho Myskiw et al. 2013).
Third, in Experiment 2, the impact of the rewarded T-maze task is clear, but the novelty of a much larger amount of reward in the modulatory task 2 (four times the reward for each choice, resulting in a 50% increase in reward secured in the T-maze task) actually had little impact on memory persistence in task 1 beyond the mere doing of the T-maze task. Given that 24-h memory of task 1 was still observed after no change in reward magnitude (one pellet) or even a decrease rewarded choices in task 2, these point to “novelty” in the T-maze having minimal effect. The most parsimonious interpretation for the data of Experiment 2 is that rewarded performance of the T-maze task (not the consumption of reward alone) is sufficient to modulate the memory persistence in task 1.
In parallel with our aim of examining whether other events can modulate memory persistence, it has recently been shown that the reconsolidation of contextual fear conditioning and place memory in the water maze can each help make an otherwise weak object-location memory last for 24 h (Cassini et al. 2013). These findings further expand the generality of the phenomenon. Many studies have suggested that when memory reactivation provides strengthening (Lee 2008), updating (Lee 2009), or mismatches from the training protocol (Pedreira et al. 2004; Morris et al. 2006), a protein-synthesis dependent reconsolidation process is engaged, especially in a hippocampus-dependent task (Wang and Morris 2010). It is important to recognize that memory reactivation, triggering reconsolidation, is procedurally different from memory encoding. Such procedural differences could contribute to the perception of novelty and this, in turn, could trigger a modulatory circuit. In terms of neurobiology, what encoding and reactivation can have in common is triggering plasticity protein synthesis, and it is precisely this that is essential for modulation of other closely timed memories.
Fourth, classical studies of “gradient of reinforcement” point to a very steep decay over time periods as short as a few seconds, whereas we see the impact of reward in task 2 given 30 min after task 1. Our interpretation of the difference is that reward and/or novelty here act as modulators of post-learning consolidation processes once memory encoding has occurred, whereas standard tasks use reward to mark one or another action as the correct things to do. Here the “correct thing to do” is simply to return to the same sandwell that has been dug in during trial 1, irrespective of whether reward was secured at that time. The classical gradient of reinforcement seems to be a gradient related exclusively to memory encoding and not relevant to memory modulation.
Fifth, our memory-modulating events in this study were mainly introduced after the memory-encoding event. This protocol is similar to post-training modulatory studies that examined memory-modulatory mechanisms related to the amygdala and to hormonal systems (McGaugh 2000; Diamond et al. 2007). It is suggested that memory undergoing post-learning consolidation in hippocampus can be modulated by neurotransmitters in amygdala (e.g., action at β-adrenergic receptors) and/or stress hormones (e.g., glucocorticoids) (Cahill and McGaugh 1998; McGaugh 2000).
Although this current study cannot distinguish between the memory-modulatory theory and synaptic tagging and capture (STC) theory, these ideas are not mutually exclusive. Combining our present data with our previous (Wang et al. 2010) and other studies (Moncada and Viola 2007; Ballarini et al. 2009; Moncada et al. 2011), the STC theory offers a flexible framework that covers both pre-learning and post-learning memory modulation. That is, the memory-enhancing effect can be also seen when the modulatory events are introduced before encoding as opposed to just after encoding (Moncada and Viola 2007; Wang et al. 2010). The STC theory explains this by suggesting that plasticity-related proteins (PRPs) are produced in the time window for which the tag is set by memory encoding. Additionally, based on the memory-modulatory theory, the post-training modulatory modulation is more effective “immediately” after encoding with its effectiveness decaying as the delay between the memory encoding and modulatory events (Cahill and McGaugh 1998; McGaugh 2000). Because the modulatory effects we observed are beyond such an immediate time window in both the current and previous studies (Moncada and Viola 2007; Ballarini et al. 2009; Wang et al. 2010; Moncada et al. 2011), STC theory offers a parsimonious explanation (Frey and Morris 1998).
Sixth, although we mainly use appetitive approaches that encourage exploration (e.g., novel box exploration) or involve rewards (e.g., the T-maze task), there can be other avenues for enhancing memory persistence. Diamond et al. (2007) reported different effects of a rat's exposure to a stressful stimulus (i.e., exposure to a cat) presented either immediately or 30 min before a radial arm water maze training. They found that acute stress exposure enhanced 24-h memory for radial maze only when the stress immediately preceded the training. In the same paper, they reported a second study in which they showed that prolonged stress (i.e., water immersion) impaired contextual, but not fear memory (Diamond et al. 2007). Although it seems that both stressful and novel/appetitive events can modulate memory persistence, there are some interesting differences. While stress seems to lose its effect on modulating memory as the gap between the two events is lengthened from 1 to 30 min (Diamond et al. 2007), there is a wider window for novel/appetitive events to modulate memory that comes 30–60 min later (Moncada and Viola 2007; Wang et al. 2010). In addition, when predator stress is introduced after learning, it impairs rather than improves a difficult spatial working memory task (Diamond et al. 1999). Moreover, in other scenarios in which post-training stress-released hormones can improve memory, there is also a tight window (immediate to 10-min delay rather than 30 to 120-min delay) for memory modulation (Gold and Van Buskirk 1975; Cahill and McGaugh 1998). In contrast, the work on behavioral tagging indicates there is a wider time window (15–60 min) for novel/appetitive events after learning to modulate memory (Moncada and Viola 2007; Wang et al. 2010). More research is required to delineate whether such differences reflect differences in the nature of the modulatory events (i.e., stressful/aversive or novel/appetitive) or different modulatory mechanisms in which plasticity-related protein may or may not be triggered by the modulatory events (Frey and Morris 1998).
There is a growing literature examining the effects of intervening tasks given after encoding of an unrelated task and there are studies reporting interference effects (Netto et al. 1991; Sandi et al. 2005; Diamond et al. 2006). These studies, however, typically use stressful stimuli as modulatory events (e.g., predator exposure, or an open field with flashing lights [OFL]). Netto et al. (1991) showed that exposure to an OFL for 2 h after encoding of a two-way active avoidance task impaired memory retrieval 24 h later. Impairment of memory in this type of protocol may be due to an excess of stress from the OFL. Indeed, the authors reported that suppression of the pituitary adrenocorticotropic hormone (ACTH) response to stress blocked the interference following OFL presentation, suggesting that this interference was partly due to the release of ACTH (Netto et al. 1991). Stimulation with flashing lights is typically used within chronic stress paradigms (Gamaro et al. 2003; Grippo et al. 2003), and has been shown to induce high blood pressure when paired with an intense sound (Florentino et al. 1988). As previously said, the events we used to modulate spatial memory did not include aversive stimuli.
We refer to our facilitation of memory persistence phenomenon as being, in certain respects, analogous to aspects of flashbulb memories. This type of memory has been studied in humans in relation to unpredictable and highly emotional events, such as the 9/11 attacks, but also to personal relevant events of emotional significance such as starting college (Talarico 2009). What is relevant in our work is that flashbulb memories are often surrounded by a halo of incidental memories (Stratton 1919), involving everyday life events, which could not be otherwise remembered. Events that carried an emotional value and surprise are able to induce long-lasting memory for normal everyday events occurring up to the preceding day and a few hours after the emotional event (Stratton 1919). However, in retrospective human studies, which usually investigate sporadic and unpredictable events, it is very difficult to manipulate the delay between the surprising event and other trivial ones in a systemic manner. This type of manipulation is possible in animal studies because prospective studies can be done. Rat studies reported that exploration of a novel box, or other novel events, could enhance memory in various hippocampus-dependent tasks when given 15 min (Almaguer-Melian et al. 2012), 30 min (Wang et al. 2010), or 1 h (Cassini et al. 2013; de Carvalho Myskiw et al. 2013) after encoding.
Neurobiological implications
Although no interventions of a neurobiological nature (lesions, drugs, genetic manipulations) were examined in this study, it is still appropriate to comment on the original intellectual context in which this work was conducted. Specifically, the “behavioral tagging” series of studies begun by Viola was inspired by the “synaptic tagging and capture” (STC) model of the persistence of synaptic potentiation (Frey and Morris 1997, 1998) and they also bear upon Lisman and Grace's model (Lisman and Grace 2005; Lisman et al. 2011) of how novelty activates a specific neural circuit in the brain to modulate memory.
In the STC model, two independent events interact to determine the persistence of change. One is the initial and relatively automatic encoding of memory traces by distributed patterns of synaptic potentiation and depression induced according to a Hebbian principle. For example, potentiation takes place when a specific pattern of glutamatergic presynaptic activity intersects with a specific pattern of postsynaptic activity. As has been noted a number of times, there is no necessity for “reward” in this process, the induced change being mediated exclusively by alterations in the strength of glutamatergic synapses (e.g., in hippocampus). Novel object encoding is one classic example of memory encoding in the absence of reward, as is spontaneous alternation, to which we can now add the act of finding a sandwell in which to dig irrespective of whether reward is actually available on that specific trial (it is, of course, available on most daily trials). Even when it is not available, the animal successfully encodes the location of the sandwell. The second event of importance in the STC model is the up-regulation of plasticity-related proteins (PRPs) within neurons that are then distributed through dendrites and can be captured at potentiated synapses by “tags” set at the time of synaptic potentiation. With respect to the present experiments, we can think of novelty exploration or changes in reward magnitude—either within task 1 or within task 2 some 30 min later—as events that are likely to up-regulate PRPs. An obstacle to a rigorous examination of this idea is that we still do not know the identity of the PRPs, although some controversial candidates have been proposed (Sacktor 2011).
Lisman and Grace's original 2005 model, guided by relevant electrophysiology, suggested that novelty was detected in the subiculum, and was then projected by a disinhibitory circuit that included the accumbens and ventral pallidum to activate the ventral tegmental area (VTA). The VTA in turn projects to the hippocampus where, they supposed, the release of dopamine would act on D1/D5 receptors to set in train intracellular events that triggered consolidation. The revised model (Lisman et al. 2011) establishes a link between these ideas and the STC model. Our findings are clearly compatible with these ideas, but the lack of modulatory effects after novel object exploration raises a question about access to this modulatory loop. One possibility is that the processing and retention of novel object memory traces is within perirhinal cortex and that no information is passed to the subiculum to trigger activity in the modulatory loop. As noted above, however, it may be that our protocol, while sufficient to produce 24-h memory for initially novel objects, was not strong enough. We cannot choose between these alternatives beyond noting that novelty is likely to be detected by comparators at different places and the insistence on the subiculum being the starting point of the modulatory loop may be too narrow.
This study focuses on the behavioral determinants of memory enhancement from a behavioral point of view. We do not yet have direct evidence to specify what molecular mechanisms are involved in our memory modulatory events. NMDA receptors are known to be essential for LTP induction and the subsequent formation of hippocampus-dependent memories. NMDA-dependent synaptic plasticity involves, as one step, an increase of intracellular calcium into postsynaptic cells (Morris 1989). Although, hippocampus-dependent memory is affected by stress through activation of NMDA receptors (Kim et al. 1996; Kim and Diamond 2002), we are not aware of evidence of enhanced LTP or memory following arousal via exclusive activation of these receptors. We therefore explain the enhanced consolidation according to STC theory. The modulatory events we used in our experiments were given to rats within the time window of persistence of tags that were set at the time of memory encoding, and so presumably of synaptic potentiation (Moncada and Viola 2007; Wang et al. 2010). Novelty and reward up-regulate immediately early genes leading to synthesis and distribution of PRPs. PRPs can be captured by the tags, enhancing LTP persistence. LTP, which would normally decay in time, can be sustained by PRPs, even when their availability is due to a different stimulus than the original one (Frey and Morris 1997, 1998). Several candidates and processes have been suggested to be part of the PRPs (Moncada et al. 2011; Redondo and Morris 2011).
To conclude, this behavioral study establishes that manipulations other than exploring an empty box with different floor surfaces have the potential to modulate the persistence of memory traces of a concurrent task. Animal models of the “flashbulb memory” phenomenon have the analytic advantage of being prospective (unlike retrospective human studies), but the successful use of a particular manipulation to date should not preclude thinking of other protocols for modulating the memory of inconsequential events.
Materials and Methods
Animals
Adult male Lister-hooded rats were used in these studies (n = 16, 200–225 g on arrival). Rats were group housed with four rats/cage. They had free access to food and water during acclimation to the animal room for 2–3 d and then were handled for 3 d. After this, they had free access to water but limited daily amount of food chow maintaining them at around 90% of free-feeding weight. This light food deprivation was introduced to keep the rats motivated to search for food in the event arena or in the T-maze. They were kept in a 12-h light–dark cycle (light 7 a.m. to 7 p.m.) and the experiment was conducted at the light phase of the cycle. Food chow (15–25 g per rat) was given at the end of daily training session separated by >1 h. All procedures followed the UK Home Office regulations of animal experimentation (Animals [Scientific Procedures] Act 1986) and were approved by qualified vets at the University of Edinburgh.
Apparatus
An event arena (L 160 × W 160 × H 40 cm) was used for the delayed matching-to-place training and memory test (task 1). There were 7 × 7 grid locations (20-cm spacing) that could be used for inserting sandwells. The arena was lined with ∼3 cm of sawdust with some locations uncovered for placing the sandwells. There were two intra-maze landmarks (a red metal pyramid and a plastic gray tower, ∼10 × 10 × 40 cm), which permanently sat at column 2, row 4 and column 6, row 4, respectively. Four start boxes (30 × 25 × 30 cm) were attached to the arena at the center of each wall (N, E, S, W) and had computer-controlled doors that could be remotely operated by the experimenter. Chocolate-flavored pellets (0.5 g, Bio-Serv) were used as rewards in the event arena. Plexiglas pots (6-cm diameter, 5-cm depth) were filled with sand for hiding rewards and hence were called sandwells. To mask the olfactory cues from the reward in the sandwell, the sand was mixed with ground pellets (at a ratio of 125-g pellets/2.5-kg sand) and all pots contained 10 pellets at the bottom of the pot that could not be accessed by the animal due to a separation by a mesh-wired metal disc installed at 1 cm from the bottom. The arena was placed at the center of a room that had clearly visible cues on the walls of the room to provide spatial information. Full details have been described in our previous publication (Wang et al. 2010).
The T-maze was composed of three sections: a left arm, a right arm (75 × 20 × 40 cm per arm), and a start box (30 × 25 × 30 cm), all made of Plexiglas. A shallow circular pot (6-cm diameter and 3-cm depth) was placed at the end of each arm. A food reward (0.5-g Bio-Serv pellet, Very Berry flavor) was placed in the pot and could not be seen or smelled by the rats from the start box. The floor was also made of Plexiglas but without sawdust to minimize its similarity with the event arena. The floor of the maze was wiped with diluted alcohol (30%) between animals to remove dust and potential traces left by previous rats.
An opaque gray plastic box (80 × 60 × 50 cm) was used for novel object recognition. It was lined with ∼2 cm of sawdust. Two sets of objects, available in triplicates, were used. They differed in colors, textures, and shapes. One set of objects was a white square-shaped plastic bottle (7 × 7 × 18.5 cm) with a small blue cap (3 cm in diameter) on the top. It had a smooth texture at the bottle and ridged texture at the cap. The other set of objects was an orange plastic cup (5-cm diameter and 18 cm high) with a wider yellow top (8 cm in diameter). It had stud-like textures at the outer face and a concaved top. Neither set was known to have any ethological significance for the animals. Objects were placed near the corners of one wall of the box, 15 cm from each adjacent wall. The objects were secured to the floor of the box with Velcro strips so that the animals could not displace them.
Behavior procedures
Spatial memory task in the event arena
Training protocol
Daily training in the event arena consisted of a spatial memory encoding trial and a retrieval choice trial. At the beginning of the encoding trial the animals were given a single food pellet (0.5 g) in the start box to accustom them to eating in the start box. The experimenter then opened the door allowing the animal to enter the arena where a single sandwell was present and baited with three pellets. The rats were allowed to find the sandwell and dig in it in order to find the pellets. Animals collected the first pellet and returned to the start box to eat. They then returned to the sandwell to find the second pellet, and so on. In the daily training session, the rewarded location was changed across animals so that the rat could not find the pellet location based on some cryptic cues in the arena that were potentially left by the previous rat. Another advantage was that each quadrant of the arena would be used for reward locations on each day, allowing more reliable comparison across days. The start location was also varied among North, East, South, West start boxes across days. The retrieval trial was a choice trial, which occurred ∼30–40 min after encoding. During the retrieval trial the animal entered in the arena from the same start box as in the encoding trial. Five sandwells were present in the arena during this choice trial: four nonrewarded sandwells and one rewarded sandwell (which was located in same location as during the encoding phase). The animal would retrieve additional food in the “matching” location based on the information/memory from the encoding trial. The rewarded sandwell in the retrieval trial also contained three pellets that required the rats to collect in three visits. The trial ended when the animal collected all the pellets and consumed them in the start box. During the retrieval trial the number of errors was recorded (i.e., how many nonbaited sandwells the rats dug in before they dug in the correct, baited sandwell.)
Testing protocol
Testing days consisted of an encoding trial and a probe test. During the encoding trial, a single sandwell was baited with three, one, or no pellets depending on the objectives. The procedure was similar to the encoding trials in the training sessions except that in some cases (described below) there would be a different amount of pellets. During the probe test, five sandwells were present and none of the sandwells were rewarded. The animals were allowed to dig for 60 sec. Digging duration at each sandwell was recorded. Digging was defined as the direct contact of the sandwell with the forepaws. Sniffing or touching the well with the nose was not considered as digging. Sixty seconds later, the experimenter placed three pellets at the bottom of the correct well for the rats to collect in three visits. This was similar to the retrieval choice trial during training and was done to reduce the potential impact of extinction in the first 60 sec.
Testing conditions
The basic procedure was composed of an encoding trial in the event arena followed by, 30 min or 3 h later, a modulating event on Day 1. A test of the spatial memory in the event arena was done 24 h after the encoding on Day 2. The nature of the encoding trial and modulatory events changed in different conditions based on the scientific questions being addressed. These conditions were done in pairs with interleaving regular training in the event arena between them. They are described below in a chronological order.
We first asked whether spatial encoding with one-pellet reward and three-pellet reward could generate persistent memory at 24 h (Fig. 2). These two conditions were done in a counterbalanced order (i.e., half of the rats received the one-pellet condition and then the three-pellet condition, and vice versa for the other half of the rats). This half–half counterbalancing protocol also applied to all the following conditions.
After confirming that 24-h memory of one-pellet spatial encoding was at chance, we then asked whether procedural novelty by increasing reward magnitude in the T-maze (for details, see below), introduced at 30 min after the place encoding of one-pellet trial in the arena could facilitate this spatial memory persistence (Fig. 3). For comparison, the same T-maze procedure was delayed by 3 h. This was selected based on previous findings suggesting there is an effective modulatory time window (Moncada and Viola 2007; Wang et al. 2010) and 3 h is beyond this window and should not facilitate the persistence of other memories. These two conditions were done in a counterbalanced manner.
We examined whether a different type of procedural novelty by changing the reward regime in the T-maze (detail see below), introduced at 30 min after the place encoding of one-pellet trial, could facilitate this spatial memory persistence (Fig. 3). For comparison, a familiarized T-maze procedure with a consistent reward regime was conducted at 30 min after the place encoding in the event arena. These two conditions were done in a counterbalanced manner.
We checked the behavior performance at 1-h memory test after spatial encoding with one-pellet reward and three-pellet reward (Fig. 2). These two conditions were done in a counterbalanced order.
After discovering that 24-h memory of one-pellet spatial encoding was good when the modulatory event was a familiarized task, we needed to confirm whether this was driven by simple consumption of reward pellets (Fig. 3). We gave animals the same amount of pellets that they typically obtained from the T-maze in their home cage as a modulatory event after the one-pellet encoding trial. In a separate condition, we investigated whether one pellet at the encoding trial in the event arena was required. We introduced a nonrewarded encoding trial followed by an exploration session in a novel box (Moncada and Viola 2007; Wang et al. 2010). During the encoding in the event arena, the animals would dig at the sandwell for several seconds, not find the reward, and voluntarily return to the start box (Fig. 2). At this point, the experimenter closed the door of the start box and returned the rats to their home cage. This nonrewarded digging was possible because the animals had been trained to dig in the single sandwell in all encoding trials so far. The above two conditions were run in a counterbalanced manner.
After finding that rewards in the encoding event are not always required for maintaining the spatial information, we replicated the same nonrewarded encoding and testing procedure in the event arena with modulatory events described in (2). A T-maze task with increased reward magnitude was introduced at 30 min or 3 h after the nonrewarded encoding trial (Fig. 3). These two conditions were done in a counterbalanced manner.
Finally, we examined whether a commonly used behavior paradigm of novelty, called novel object exploration, could be an effective modularity event (Fig. 4). The advantage of this paradigm is that we could then check whether the novel object exploration could produce a 24-h novel object recognition memory, which served as a good indicator of whether animals actually registered the novelty at the exploration phase (see below). Exposure to a habituated box that was used for novel object exploration was done in parallel for comparison. These two conditions were done in a counterbalanced manner.
T-maze rewarding task
The training in the T-maze was done in parallel with the training in the event arena. About 2–3 h after the daily encoding and retrieval training in the event arena, rats underwent a short T-maze training. On Day 1, they were trained under a win-shift regime, with each arm baited with one pellet at the beginning. Rats collected one pellet in the right (or left) arm in trial 1, returned and ate it in the start box, collected the other pellet in the left (or right) arm in trial 2, and returned and ate it in the start box. For the following trials, the one-pellet reward was baited in alternative arms so the rats had to shift to win the reward. Training session on Day 1 stopped after rats collected three pellets from the right arm and three pellets from the left arm. This took on average 15 min. The same win-shift regime was repeated for Days 2–5, with sessions stopped after the rats collected five pellets from each arm. The session length gradually reduced from ∼10 min to 5 min. The percentage of choices that were rewarded was calculated as the behavior index. Two heterogeneous groups were identified. One group of rats (n = 8 of the total 16 rats) preferentially revisited the arm that was previously baited and as a result showed lower percentage of rewarded choices (65.3 ± 1.4%), while the other group (the other eight of the 16 rats) preferentially switched across arms and had higher percentage of rewarded choices (73.3 ± 0.8%).
On Days 6–8, the first group was trained under the win-stay regime, with the reward baited in the same arm, since they were naturally more likely to revisit the same arm based on Days 1–5 performance (n = 4 baited at the right arm, n = 4 baited at the left arm). The second group was trained under the same win-shift regime as on Day 1–5. After these three days of training, we found the eight rats in the win-stay group still visited the same arm to collect rewards and resulted in a high percentage of rewarded visits (77 ± 1.8%). Four rats in the win-shift group maintained their preference to alternate between arms to collect rewards (77.2 ± 2.1% of rewarded visits). Four rats in the win-shift group, however, changed their alternating pattern and preferentially revisited the same arm to try to collect rewards and resulted in a reduced rewarded probability per visit (63.3 ± 1.4%). These four rats were then trained with win-stay regime for the rest of the study. The aim was to train the rats based on their natural tendency so the procedural changes to be introduced later on as modulating events in various testing conditions would exert greater effects on generating procedural novelty.
On Days 9–12, 12 rats were trained under the win-stay regime (n = 6 rewarded at the right arm, n = 6 rewarded at the left arm) and four rats were trained under the win-shift regime. The percentage of rewarded choices remained very high and stable throughout these sessions. The win-stay group remained rewarded with a win-stay regime and the win-shift group remained rewarded with a win-shift regime for the rest of the study. The T-maze task, with or without procedural novelty, was then used as modulatory events for various testing conditions described above. One regular T-maze training session was interleaved between those testing conditions that involved the T-maze as a modulatory event.
The first type of modulating event was a regular T-maze training session but with procedural novelty caused by increasing the reward magnitude. This involved an ∼5 min T-maze task with increased reward in the baited arm (2 × double-sized reward, i.e., four times the amount). The 5 min was chosen specifically to be as similar as the length of novel exploration in a box that was typically used as an effective modulatory event (Moncada and Viola 2007; Ballarini et al. 2009; Wang et al. 2010; Moncada et al. 2011). In order to minimize the disruption of the animals’ behavior, the session stopped when the rats collected the pellet between 4.5 min and 5 min and naturally returned to the start box, the door of which was then closed. The same logic applied to all T-maze sessions as a modulatory event. This procedure of increasing reward greatly increased the reward efficacy per choice (see Results). This T-maze with increased reward was introduced at either 30 min or 3 h after encoding with one pellet or with no pellet in the event arena (link to testing conditions [2] and [6] described above).
The second type of modulating event was a regular T-maze training setup but with procedural novelty caused by a changed rewarding regime. The win-stay group would be rewarded by a win-shift regime and the win-shift group would be rewarded by a win-stay regime in an ∼5-min session (link to testing condition [3] described above). This effectively reduced the reward efficacy per choice (see Results).
The third type of modulating event was an ∼5 min regular T-maze training without explicit introduction of procedural novelty. This was done in counterbalance with the previous condition of changed rewarding regime (linked to testing condition [3] described above).
Finally, a similar amount of pellets (10 pellets per rat) that the rat would typically collect in a regular T-maze training session was given in their home cage. This was to check whether consumption of pellets alone without engaging a learning/memory process could be an effective modulatory event. It took rats ∼4–5 min to consume all the pellets.
Novel object recognition
On Days 1–4, animals (n = 12, excluding one cage of four rats in which one rat died naturally and one rat developed a weak limb) were habituated to the box without objects for 10-min daily before exposure to the objects. On Day 1, the animals remained in their home cage and the cage was put in the gray box that would later be used for object exploration. On Days 3 and 4, animals were placed individually in the gray box for 10 min. Habituation took place ∼1–2 h after training in the event arena. On Day 5, animals were placed in the familiarized box with or without objects for 5 min at 30 min after the spatial encoding trial with one-pellet reward in the event arena. The test phase was conducted 24–26 h later and at ∼2 h after the memory probe test in the event arena.
Animals were placed in the gray box facing the wall opposite to the objects and allowed 5-min exploration. In the object exploration condition two identical objects were presented in the box. Time spent exploring each object was recorded. Exploratory behavior was defined as directing the nose toward the object within 2 cm and actively exploring them. Sitting on the objects or rearing near them without signs of active exploration was not considered as exploratory behavior (Ennaceur and Delacour 1988; Ennaceur et al. 2005). After 5 min the animal was removed from the box from the same side from which it entered and brought back to the home cage.
The next day rats were reintroduced in the box for a test phase in order to assess memory for the previously encountered objects. During this test phase the box contained two different objects, one copy of the previously seen object and a novel one, placed in the same locations used in the initial exposure. Animals were allowed to spend 5 min in the box. Time spent exploring the objects was recorded as before. The type of objects used as familiar or novel, and the position of the novel object in the test phase, were counterbalanced between subjects. An overhead camera was used to monitor the movements of the animal in the box.
The procedure, i.e., event arena and objects exploration, was repeated twice. After the first test animals were rehabituated to the box without stimuli for 2 d, and received 3 d of training in the event arena. On Day 4 the spatial memory encoding and the box exploration (with and without objects) was performed as before. All animals explored both the familiarized empty gray box and the box with novel objects in a counterbalanced order.
Data collection and statistical analysis
Overhead cameras were used to monitor the movements of the animal in the event area, T-maze, and box. In the training in the event arena, the number of errors (0–4) that the rats made by digging in wrong sandwells at the retrieval-choice trial was recorded and used to calculate the performance index (100—(error/4) × 20). In the probe trial of memory test, digging time in each well was recorded by using a multitimer software developed in-house (LabView by P. Spooner). In the T-maze task, the choices made (left or right) and whether it was rewarded were recorded at each trial to calculate the percentage of rewarded choices per training session. In the novel object exploration, measurement of the exploration time was performed manually by using the same multitimer software. The design of the study was mainly within-subjects and hence paired t-tests (Figs. 2–4) and repeated measures ANOVAs (Figs. 1–4) were used in this study. One exception was that the rewarding regime in the T-maze was between-subjects. Two-tailed tests were used and Type One error was at 0.05.
Acknowledgments
This study was designed by S.-H.W and conducted by S.-H.W. and B.S. It was supported by the Caledonian Research Foundation and the Royal Society of Edinburgh (S.-H.W.) and by a European Research Council Advanced Investigator Grant (R.G.M.M.). We thank Patrick Spooner for technical assistance with the apparatus, Jane Tulloch for lab management, and Richard Watson for animal care.
References
- Ainge JA, van der Meer MA, Langston RF, Wood ER 2007. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus 17: 988–1002 [DOI] [PubMed] [Google Scholar]
- Almaguer-Melian W, Bergado-Rosado J, Pavón-Fuentes N, Alberti-Amador E, Mercerón-Martínez D, Frey JU 2012. Novelty exposure overcomes foot shock-induced spatial-memory impairment by processes of synaptic-tagging in rats. Proc Natl Acad Sci 109: 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H 2009. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci 106: 14599–14604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett D, Wood ER, Dudchenko PA 2012. The postsubiculum is necessary for spatial alternation but not for homing by path integration. Behav Neurosci 126: 237–248 [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J 1977. Flashbulb memories. Cognition 5: 73–99 [Google Scholar]
- Cahill L, McGaugh JL 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci 21: 294–299 [DOI] [PubMed] [Google Scholar]
- Cassini LF, Sierra RO, Haubrich J, Crestani AP, Santana F, de Oliveira Alvares L, Quillfeldt JA 2013. Memory reconsolidation allows the consolidation of a concomitant weak learning through a synaptic tagging and capture mechanism. Hippocampus 23: 931–941 [DOI] [PubMed] [Google Scholar]
- de Carvalho Myskiw J, Benetti F, Izquierdo I 2013. Behavioral tagging of extinction learning. Proc Natl Acad Sci 110: 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM 1999. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9: 542–552 [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF 2006. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus 16: 571–576 [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR 2007. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plast 2007: 60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT 2012. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J Neurosci 32: 11980–11990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S 2005. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res 159: 247–266 [DOI] [PubMed] [Google Scholar]
- Florentino A, Castro A, Fuentes JA 1988. High blood pressure induced by audiovisual stimulation in young rats: Effect of antihypertensive agents. Clin Exp Hypertens A 10: 873–885 [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM 1997. Synaptic tagging and long-term potentiation. Nature 385: 533–536 [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM 1998. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci 21: 181–188 [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C 2003. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int 42: 107–114 [DOI] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB 1975. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13: 145–153 [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK 2003. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav 78: 703–710 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM 2002. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3: 453–462 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF 1996. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci 93: 4750–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL 2008. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci 11: 1264–1266 [DOI] [PubMed] [Google Scholar]
- Lee JL 2009. Reconsolidation: Maintaining memory relevance. Trends Neurosci 32: 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA 2005. The hippocampal–VTA loop: Controlling the entry of information into long-term memory. Neuron 46: 703–713 [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E 2011. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci 34: 536–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ji Y, Ganesan S, Schloesser R, Martinowich K, Sun M, Mei F, Chao MV, Lu B 2011. TrkB as a potential synaptic and behavioral tag. J Neurosci 31: 11762–11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL 2000. Memory—a century of consolidation. Science 287: 248–251 [DOI] [PubMed] [Google Scholar]
- Moncada D, Viola H 2007. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J Neurosci 27: 7476–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H 2011. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci 108: 12931–12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG 1989. Synaptic plasticity and learning: Selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci 9: 3040–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA 2006. Memory reconsolidation: Sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50: 479–489 [DOI] [PubMed] [Google Scholar]
- Netto CA, Valente JT, Borges-Sobrinho JB, Walz R, Tomaz CA 1991. Posttraining presentation of a flashing light alters retrieval of a two-way active avoidance task in rats. Physiol Behav 49: 33–39 [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L 1978. The hippocampus as a cognitive map. Oxford University Press, Oxford,, UK [Google Scholar]
- Pedreira ME, Perez-Cuesta LM, Maldonado H 2004. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem 11: 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RG 2011. Making memories last: The synaptic tagging and capture hypothesis. Nat Rev Neurosci 12: 17–30 [DOI] [PubMed] [Google Scholar]
- Sacktor TC 2011. How does PKMζ maintain long-term memory? Nat Rev Neurosci 12: 9–15 [DOI] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM 2005. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry 57: 856–864 [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RGM 1999. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9: 118–136 [DOI] [PubMed] [Google Scholar]
- Stratton GM 1919. Retroactive hyperamnesia and other emotional effects on memory. Psychol Rev 26: 474–486 [Google Scholar]
- Talarico JM 2009. Freshman flashbulbs: Memories of unique and first-time events in starting college. Memory 17: 256–265 [DOI] [PubMed] [Google Scholar]
- Wang SH, Morris RG 2010. Hippocampal–neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61: 49–79, C41–C44 [DOI] [PubMed] [Google Scholar]
- Wang SH, Redondo RL, Morris RG 2010. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci 107: 19537–19542 [DOI] [PMC free article] [PubMed] [Google Scholar]