Abstract
The molecular mechanisms of schizophrenia have been under investigation for decades; however, the exact causes of this debilitating neuropsychiatric disorder are still unknown. Previous studies have identified multiple affected neurotransmitter systems, brain regions, and cell types, each making a unique contribution to symptom presentation and pathophysiology. Numerous studies have identified gene and protein expression changes in schizophrenia, but the role of post-translational modifications, specifically N-glycosylation, has only recently become a target of investigation. N-glycosylation of molecules associated with glutamatergic neurotransmission is disrupted in schizophrenia, but it was unknown if these alterations are exclusive to the glutamatergic system or due to a more generalized deficit.
In normal human cortex, we found evidence for N-glycosylation of the α1, β1, and β2 γ-aminobutyric type A receptor (GABAAR) subunits using deglycosylation protein shift assays. This was confirmed with lectin affinity assays that revealed glycan attachment on the α1, α4, and β1-3 GABAAR subunits. Examining GABAAR subunit N-glycosylation in matched pairs of schizophrenia (N=14) and comparison (N=14) of superior temporal gyrus revealed a smaller molecular mass of immature N-glycans on the α1 subunit, more immature N-glycosylation of the 49-kDa β1 subunit isoform, and altered total N-glycosylation of the β2 GABAAR subunit in schizophrenia. Measures of altered N-glycosylation of the β1 and β2 subunits were confounded by an increased apparent molecular mass of all β1 and β2 subunit isoforms in schizophrenia. Although N-glycosylation of α1, β1, and β2 were all changed in schizophrenia, the concentrations of GABAAR subunits themselves were unchanged. These findings suggest that disruptions of N-glycosylation in schizophrenia are not exclusive to glutamate and may indicate a potential disruption of a central cell signaling process in this disorder.
Keywords: postmortem brain, superior temporal gyrus, post-translational modification, GABRA1, GABRB1, GABRB2
INTRODUCTION
The pathophysiology of schizophrenia has not been clearly elucidated, although it is generally thought that the disorder is likely due to dysregulation of one or more neurotransmitter systems. Previous research has focused on alterations of dopaminergic and glutamatergic receptors, abnormalities of which have been shown to be associated with the positive symptoms of the disorder. Disruptions in the GABA system have also been identified and are thought to contribute to the cognitive deficits seen in schizophrenia through disruptions in neural synchrony (Gonzalez-Burgos et al, 2010; Lewis et al, 2005; Sun et al, 2011; Uhlhaas and Singer, 2006). Research has long focused on identifying gene and protein expression changes in schizophrenia and ascertaining the functional effects of those alterations; more recently, the diverse mechanisms by which post-translational modifications can regulate function have become an additional target of investigation. Phosphorylation and N-glycosylation are the two most common experimentally identified post-translational modifications (Khoury et al, 2011). Although functional roles of protein phosphorylation have been widely studied in many disorders, the extent and mechanistic effects of disrupted N-glycosylation are less well characterized.
Glycosylation is the enzymatic attachment of a carbohydrate to a protein or lipid molecule; N-linked glycosylation is the specific attachment of a dolichol oligosaccharide precursor to the asparagine residue of a polypeptide. N-glycosylation can occur co- or post-translationally, and after initiation in the endoplasmic reticulum (ER) the glycan undergoes further modification as the glycoprotein is trafficked through the cell (Helenius and Aebi, 2004; Ohtsubo and Marth, 2006; Parodi, 2000; Varki, 2009). N-glycosylation has a role in many cell biological processes by facilitating protein folding and subunit assembly, serving as a quality-control signal in the ER and Golgi, regulating intracellular trafficking, and modulating protein–protein interactions, protein degradation, and the cell signaling pathways (Dennis et al, 2009; Helenius and Aebi, 2004; Ohtsubo and Marth, 2006; Parodi, 2000; Varki, 2009). Given this extensive functional diversity, altered N-glycosylation can phenotypically manifest in myriad ways to affect how individual neurons interact in the brain.
Recent work in our lab has focused on N-glycosylation abnormalities in the glutamatergic system in schizophrenia, where we have identified brain region-specific reductions in the N-glycosylation of transporter proteins EAAT1 and EAAT2 in the anterior cingulate cortex and dorsolateral prefrontal cortex, respectively (Bauer et al, 2010). We have also found decreased N-glycosylation of the AMPA receptor subunit, GluA2 (Tucholski et al, 2013a), and increased molecular mass of immature N-glycans attached to the GluR6 kainate receptor subunit in dorsolateral prefrontal cortex in schizophrenia (Tucholski et al, 2013b).
Although functional implications of the alterations found in the glutamate system are beginning to be examined, it is not yet known if these N-glycosylation abnormalities also extend to the GABAergic system. GABAARs are heteropentameric receptors of the cys-loop superfamily of ligand gated ion channels and mediate the majority of inhibitory neurotransmission; in mammalian brain, there are 19 subunits from eight different subunit classes expressed, though the most common receptor conformation consists of 2α, 2β, and 1γ subunit (Sieghart et al, 1999; Sieghart and Sperk, 2002). Several potential GABAAR subunit N-glycosylation sites have been identified by the presence of an N-glycosylation consensus sequence in an extracellular protein domain (Chen et al, 2012; Farriol-Mathis et al, 2004). N-glycosylation of GABAAR subunits has been confirmed in vivo using rodent models (Buchstaller et al, 1991a; Buchstaller et al, 1991b) and cell culture lines (Buller et al, 1994; Connolly et al, 1996; Lo et al, 2010; Tanaka et al, 2008); however, which subunits are N-glycosylated in human brain has not been characterized. This study aims to elucidate if alterations of N-glycosylation in schizophrenia are specific to the glutamatergic system or are more generalized, by characterizing the N-glycosylation pattern of GABAAR subunits in normal human cortex and identifying potential alterations in schizophrenia.
MATERIALS AND METHODS
Subjects and Tissue Acquisition
Samples of postmortem human cortex from two male and one female non-psychiatrically ill, non-antipsychotic treated subjects, with a mean age of 56.7±11.2 years, mean tissue pH of 6.1±0.1, and mean postmortem interval (PMI) of 22.1±1.1 h were obtained from the Alabama Brain Collection, as previously described (Bauer et al, 2009), and used exclusively for assay development and preliminary characterization of subunit glycosylation. Samples of gray matter from the full cortical thickness of the left superior temporal gyrus (STG, Brodmann area 22) of schizophrenia and comparison subjects were obtained from the Mount Sinai Medical Center brain collection (Table 1 and Supplementary Table S1), as previously described (Katsel et al, 2005; Rubio et al, 2013). Comparison and schizophrenia subjects were paired for age, sex, and tissue pH; our main priority in pairing was tissue pH, and as a result PMI was different between groups (t(13)=3.6, P<0.01). Patients with a diagnosis of schizophrenia by DSM-III-R criteria confirmed by at least two clinicians were recruited prospectively (Katsel et al, 2005). The patients had documented history of at least 10 years of hospitalization and demonstrated onset of psychotic symptoms before age 40. Multiple clinical assessments were used to evaluate the subjects, including NINDS-AIREN criteria for vascular dementia, NINCDS, DSM-IV, and CERAD guidelines for dementia, consensus criteria for a clinical diagnosis of probable or possible diffuse Lewy body disease, UPDRS for Parkinson's disease, and clinical assessment for frontotemporal dementia (Powchik et al, 1998; Purohit et al, 1998). In addition, subject medical history was assessed for psychiatric illnesses, history of drug or alcohol abuse, and tests of cognition, including the MMSE and CDR.
Table 1. Subject Demographics.
| Comparison | Schizophrenia | |
|---|---|---|
| n | 14 | 14 |
| Age | 77.6±10.1 | 77.5±11.0 |
| Sex | 7M/7F | 7M/7F |
| Race | 11W/2B/1A/0H | 10W/2B/1A/1H |
| PMI (hours) | 8.1±6.6 | 15.6±6.4 |
| Tissue pH | 6.5±0.3 | 6.5±0.2 |
| On/Off Rx | 0/14 | 8/6 |
Abbreviations: A, Asian; B, Black; F, female; H, Hispanic; M, male; PMI, postmortem interval; Rx, antipsychotic medication; W, white.
Values are expressed as means±SD. Off Rx indicates patients that had not received antipsychotic medications for 6 weeks or more at time of death.
Systematic neuropathological examinations following the CERAD guidelines were used to macro- and microscopically evaluate the brain. Only subjects that did not exhibit neuropathology meeting the criteria for neurodegenerative disorders, including Alzheimer's disease, were used (Purohit et al, 1993). Comparison subjects were similarly evaluated and confirmed to be without neuropathological evidence of neurodegenerative disorders and had no documented history of psychiatric illness or substance abuse. All subjects with a history of substance abuse, death by suicide, or in a coma for more than 6 h before death were excluded. Consent to perform an autopsy on the body and brain for diagnostic and research purposes was obtained from the next-of-kin for each subject (Powchik et al, 1998; Purohit et al, 1998). Tissue samples were pulverized with small amounts of liquid nitrogen and stored at −80 °C until homogenization. Samples were reconstituted and homogenized on ice in 5 mM Tris-hydrochloride, pH 7.4, 320 mM sucrose, and a protease inhibitor tablet (Complete Mini; Roche Diagnostics, Manheim, Germany) with a Power Gen 125 homogenizer (Thermo Fisher Scientific, Waltham, MA). The homogenate was assayed for protein concentration with a BCA protein assay kit (Thermo Fisher Scientific) and stored at −80 °C until use.
Western Blot Analysis
The Novex Mini Cell NuPAGE system (Life Technologies, Grand Island, NY) with 4–12% Bis-Tris gradient polyacrylamide gels (Life Technologies) was used, and 10 μl of sample was added per lane. A molecular mass standard was run on each gel (Novex Sharp Pre-stained Protein Standard; Life Technologies). Gels were suspended in a bath of NuPAGE MES sodium dodecyl sulfate (SDS) running buffer (Life Technologies) with 500 μl NuPAGE antioxidant (Life Technologies) during electrophoresis.
Following electrophoresis, proteins were transferred onto 0.45 μm nitrocellulose membranes (Bio-Rad, Hercules, CA) using a semi-dry transfer apparatus (Bio-Rad) and then briefly rinsed with phosphate-buffered saline (PBS). The membrane was then blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE), 5% milk in PBS with 0.1% Tween (PBST), or 5% bovine serum albumin (BSA) in PBST for 1 h at room temperature, and then incubated overnight at 4 °C with primary antibody (Supplementary Table S2). After washing with PBST, blots were incubated with the appropriate secondary antibody and washed with PBST before being scanned on the Odyssey Infrared Imaging System (LI-COR Biosciences) at a resolution of 169 μm and intensity level of 5.
Deglycosylation Reactions
A concentration of 50 μg of each sample was denatured and deglycosylated with Denaturation Solution (New England Biolabs (NEB), Ipswich, MA) and either 10 × PNGase F Reaction Buffer (NEB) or 10 × EndoH Reaction Buffer (NEB), heated to 70 °C for 10 min, and then cooled to room temperature. For reactions with the enzyme peptide-N-glycosidase F (PNGase, NEB) only, 10% NP40 (NEB) was added. Deglycosylation enzymes, Endoglycosidase Hf (EndoH; NEB), and PNGase were added, and samples were incubated at 37 °C overnight. Loading buffer (0.5 M Tris-hydrochloride with 36% glycerol, 4.5% SDS, and 2% β-mercaptoethanol (BME)) was added to each sample and heated to 70 °C for 10 min. Non-enzyme-treated negative control samples were prepared identically to the enzyme-treated samples with the same buffers, except that water replaced the deglycosylating enzymes (Bauer et al, 2010; Tucholski et al, 2013a; Tucholski et al, 2013b).
Lectin Affinity Isolation
In a total volume of 1 ml, samples were incubated rotating with 1% Triton X-100 and 1% SDS at 4 °C overnight, and then centrifuged at 5000 r.p.m. for 10 min. The supernatant was reserved, assayed for protein concentration with a BCA protein assay kit (Thermo Fisher Scientific), and stored at −80 °C until use.
Tris-buffered saline with 0.1% Tween-20, 0.1% BSA, and 1 × Roche EDTA-free protease inhibitor Mini Complete (TBST+) was added to the supernatant to a final protein concentration of 0.8 μg/μl in 500 μl. To this volume, 20 μg of biotinylated lectin, including Concanavalin A (ConA; Vector Labs, Burlingame, CA), lycopersicon esulentum lectin (LEL; Vector Labs), narcissus pseudonarcissus lectin (NPL; Vector Labs), peanut agglutinin (PNA; Vector Labs), or aleuria aurantia lectin (AAL; Vector Labs), was added, and samples were incubated rotating at 4 °C overnight. Using a magnet, 250 μl of streptavidin-coupled Dynabeads (beads; Life Technologies) were washed with TBST+. The incubated lysate with lectin was added to the beads and incubated rotating at room temperature for 2 h. The supernatant was aspirated from the beads and reserved as a control. The beads were again washed with TBST+, ensuring that all liquid was removed after the final wash. Loading buffer (0.2 M Tris-hydrochloride with 12% glycerol, 1.5% SDS, and 0.7% BME) was added to the beads and then incubated on a heat block at 70 °C for 10 min. Using a magnet, the supernatant was then transferred to new tube and used immediately as the final prepared sample or stored at −20 °C (Tucholski et al, 2013a).
Dephosphorylation Reactions
A concentration of 30 μg of each sample was dephosphorylated with Lambda Protein Phosphatase (NEB) in 1 × NEBuffer for Protein MetalloPhosphatases (NEB) supplemented with 1 mM MnCl2 (NEB) and incubated at 37 °C overnight. Loading buffer of (0.5 M Tris-hydrochloride with 36% glycerol, 4.5% SDS, and 2% BME) was added to each sample and heated to 70 °C for 10 min. Non-enzyme-treated samples were prepared identically to the enzyme-treated samples with the same buffers, except that they were incubated with water instead of phosphatase.
Data Analysis
For each protein band measured in western blots, the near-infrared fluorescence value and y-axis pixel position were collected using the Odyssey Infrared Imaging System. Near-infrared fluorescence was expressed as integrated intensity with right–left average intralane background subtraction using Odyssey V3.0 analytical software (LI-COR Biosciences). The y-axis pixel position used to calculate the molecular mass in kDa was expressed as the center-y pixel position value of a rectangle centered over the peak intensity of the protein band. Display adjustments were made using the Odyssey software. To calculate the molecular mass of the GABAAR subunit protein bands, we collected center-y values from a minimum of four molecular mass standards surrounding the region of interest and representing molecular masses of 110, 80, 60, 50, 40, or 30 kDa. The center-y value of the highest standard was subtracted from the center-y value of each standard and protein band of unknown molecular mass, giving the total pixels the protein moved relative to the position of the highest mw standard (Yadj). The Yadj values were then divided by the Yadj of the lowest molecular mass standard measured, to represent relative movement (Rm) of the protein in that region of the gel. For each set of standards, we plotted the Rm of each standard against the log of its molecular mass in kDa and used the resulting linear trendline to estimate the molecular mass of each protein band.
The protein expression of each GABAAR subunit was determined by measuring the integrated intensity of each subunit and normalizing to the integrated intensity of valosin-containing protein (VCP; ab11433; Abcam, Cambridge, MA); to analyze N-glycosylation of the α1 GABAAR subunit, which produces a characteristic protein shift when deglycosylated, we measured the molecular mass of the native subunit and calculated the molecular mass difference between enzyme-treated and non-enzyme-treated control samples. Both the β1 and β2 GABAAR subunits are expressed as multiple isoforms and, instead of producing a protein shift when treated with deglycosylating enzymes, produce additional immunoreactive bands. To analyze N-glycosylation of these subunits, we measured the molecular mass of each isoform, the band-separation distance (in kDa) between isoforms, and differences in band-separation distances between enzyme-treated and non-enzyme-treated controls. In addition, we assessed the relative contribution of each isoform to the total native subunit, by measuring the integrated intensity of each protein band divided by the sum of integrated intensities of all isoforms in the lane, and evaluated differences between enzyme-treated and non-enzyme-treated controls.
We used Statistica (StatSoft, Tulsa, Oklahoma) and Prism 5.0 (GraphPad Software, La Jolla, CA) for all statistical analyses. All dependent variables were found to be normally distributed. The main statistical tests used for planned comparisons were paired Student's t-tests, with α=0.05. Correlation analysis was performed to determine if there were any significant associations between any dependent variables and age, pH, or PMI. For dependent variables found to have significant associations, we used analysis of covariance (ANCOVA) with any significantly correlated variable as a covariate in addition to paired t-tests. In addition, we used post-hoc t-tests to assess dependent variables for effects of medication status in schizophrenia subjects. We defined subjects that had not received antipsychotic treatment for 6 weeks or more before death as ‘off medication' (n=6) and subjects who had received antipsychotic treatment within 6 weeks of death as ‘on medication' (n=8). To address the issue of multiple testing within the β1 and β2 GABAAR subunit data sets, we grouped subunit data by enzyme treatment and hypothesis family (molecular mass, molecular mass shift, integrated intensity, or integrated intensity change) and used the Benjamini–Hochberg method to control for the false discovery rate with q*=0.05 (Benjamini and Hochberg, 1995).
RESULTS
α1, α4, β1, β2, and β3 GABAA Receptor Subunits Are Glycosylated in Human Cortex
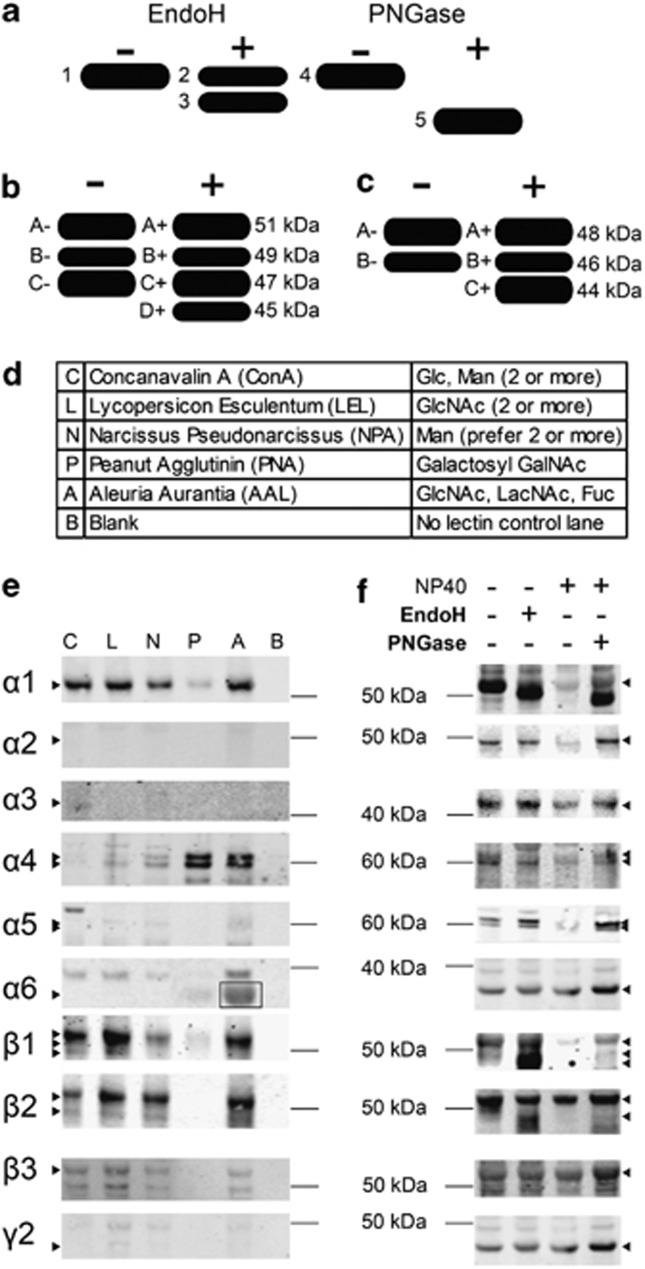
Postmortem human cortex, provided by the Alabama Brain Collection, was used to determine the glycosylation pattern of GABAAR subunits in normal brain. Cortical homogenates were treated with the deglycosylating enzymes EndoH or PNGase, and samples were then western blotted and probed with antibodies against 10 commonly expressed GABAAR subunits: α1–6, β1–3, and γ2 (Supplementary Table S2). Qualitative analysis of the immunoreactive protein bands showed that only the α1, β1, and β2 GABAAR subunits experienced an apparent molecular mass shift following cleavage of the attached N-glycans (Figure 1f). To confirm these findings, we performed a lectin affinity isolation to selectively retrieve lectin-bound molecules from cortical homogenates. The resulting glycoprotein enriched samples were western blotted and probed for the α1–6, β1–3, and γ2 GABAAR subunits. The presence of the α1, β1, and β2 GABAAR subunits confirmed the findings of the deglycosylation protein shift assays. We additionally identified the α4 and β3 GABAAR subunits as being glycosylated based on these lectin affinity isolations (Figure 1e), although molecular mass shifts after treatment with EndoH and PNGase were not evident for either the α4 or β3 GABAAR subunits in any subjects.
Figure 1.
Characterization of GABAAR subunit glycosylation in normal human cortex. (a) Schematic of the expected assay results if a target protein is glycosylated with both immature and mature glycans. Bands 1 and 4 are non-enzyme-treated controls, representing the native glycoprotein; band 2 is the EndoH-insensitive protein that may have no glycans or only mature glycans attached; band 3 is the EndoH-sensitive protein with immature N-glycans cleaved; and band 5 is the PNGase-sensitive protein with both immature and mature N-glycans cleaved from the core glycoprotein. (b) Schematic representation of β1 isoforms present in control and deglycosylating enzyme-treated lanes. In addition to the 51, 49, and 47 kDa isoforms (bands A, B, and C, respectively) present in the control reaction, an additional band at 45 kDa (band D) is produced following deglycosylation. (c) Schematic representation of β2 isoforms present in control and enzyme-treated lanes. In addition to the 48 and 46 kDa isoforms (bands A and B, respectively) present in the control reaction, an additional band at 44 kDa (band C) is produced following deglycosylation. (d) Table of lectins used in the affinity assay and their corresponding sugar specificity. Fuc, fucose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; LacNAc, N-acetyl-D-lactosamine; Man, mannose. (e) Representative images from lectin affinity assays. Triton-solubilized, SDS-treated human brain homogenate samples were incubated with the biotinylated lectins ConA, LEL, NPA, PNA, and AAL, and then immunoisolated using streptavidin beads. The no-lectin control (blank) was treated identically to lectin-treated samples, except that the lectin was omitted. Western blots of the immunoisolation products were probed for the α1–6, β1–3, and γ2 GABAAR subunits. Representative images show the lectin affinity of the α1, α4, β1, β2, and β3 GABAAR subunits from postmortem human cortex. The α2, α3, α5, and γ2 GABAAR subunits were not detectable in the affinity isolation product. The α6 GABAAR subunit did not demonstrate lectin binding with ConA, LEL, NPL, or PNA; however, we are unable to determine if there is binding to AAL because of an artifact present in all AAL lectin affinity isolations at the same molecular mass as the α6 subunit (boxed region). Expected position of each subunit is indicated by an arrowhead. (f) Representative images from the protein deglycosylation shift assays. Postmortem human brain homogenates were treated with the N-deglycosylating enzymes EndoH or PNGase. Control (−) lanes were treated identically to the corresponding enzyme-treated (+) samples, except that the enzyme was omitted. Representative images showing the molecular mass shifts of α1, β1, and β2 GABAAR subunits after enzyme treatment; the α2–6, β3, and γ2 GABAAR subunits did not appear to have molecular mass shifts following these treatments. The expected position of each subunit before deglycosylation is indicated by an arrowhead.
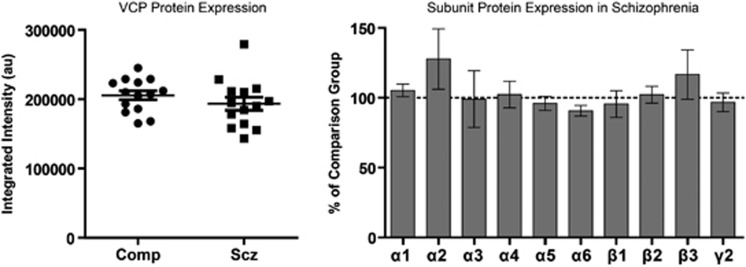
GABAAR Subunit Protein Expression is Unchanged in the STG of Schizophrenia Subjects
In STG samples not treated with deglycosylating enzymes, the relative subunit abundance of the GABAAR subunits α1–6, β1–3, and γ2 was determined, and results were normalized to intralane measures of VCP, a protein previously shown to be unchanged in schizophrenia brain (Bauer et al, 2009; Stan et al, 2006). None of the 10 subunits were significantly different between schizophrenia and comparison subjects (Figure 2).
Figure 2.
Total protein expression of GABAAR subunits is unchanged in schizophrenia. Western blot analysis of VCP and GABAAR subunits α1–6, β1–3, and γ2. Integrated intensity of the VCP immunoreactive protein band is unchanged in schizophrenia. VCP-normalized GABAAR subunit protein expression is unchanged in schizophrenia. Data are expressed as the mean±SEM integrated intensity of each GABAAR subunit in schizophrenia normalized to the comparison group.
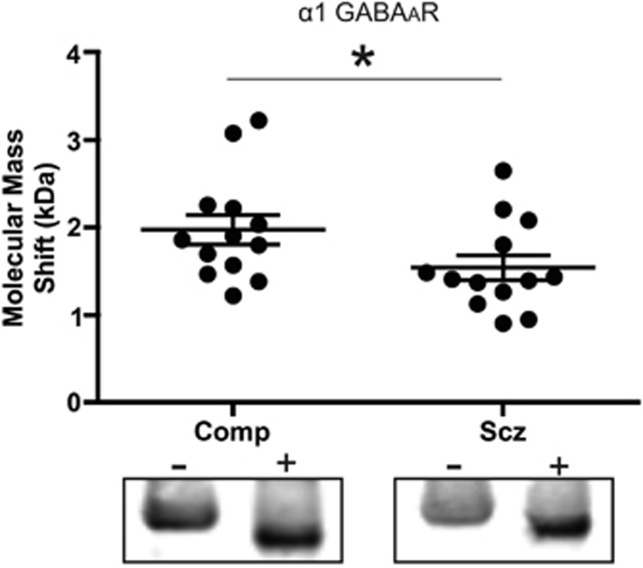
Decreased Molecular Mass Shift After EndoH Deglycosylation of the α1 GABAAR Subunit in Schizophrenia
Following treatment of STG samples with EndoH, but not PNGase, the molecular mass shift of the α1 subunit was less in schizophrenia than paired comparison subjects (t(12)=3.0, P=0.01; Figure 3). There was no difference in the native molecular mass of α1 GABAAR subunits between schizophrenia and comparison subjects.
Figure 3.
Deglycosylation with EndoH of the α1 GABAAR subunit results in a smaller molecular mass shift in schizophrenia. Western blot analysis of the α1 GABAAR subunit from STG deglycosylated with EndoH in schizophrenia and comparison subjects. Representative images of control and EndoH-treated lanes from paired comparison and schizophrenia subjects. Controls (−) were treated identically to the corresponding enzyme-treated (+) samples, except that the enzyme was omitted. Data are expressed as the difference between the molecular mass in kDa of the native and enzyme-treated bands. *P<0.05.
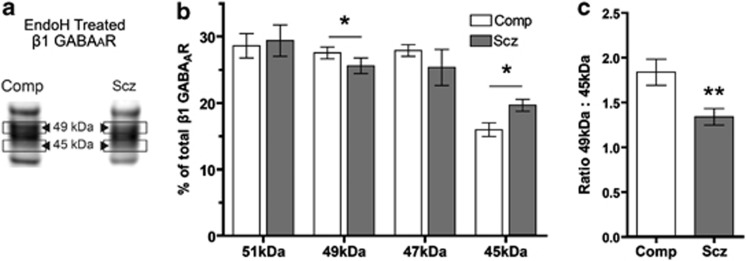
Ratio of β1 GABAA Receptor Subunit Isoforms is Altered in Schizophrenia
The β1 GABAAR subunit appears as three bands, with approximate molecular masses of 51, 49a, and 47 kDa. Following EndoH deglycosylation, an additional band with an approximate molecular mass of 45 kDa appears (Figure 4a). Correlational analysis of the amount of each β1 isoform in the enzyme-treated lane suggests that the formation of the 45 kDa isoform is primarily due to removal of N-glycans from the 49-kDa isoform (r(26)=−0.37, P=0.03). Although not significant, the weak correlations between the amount of 51 kDa (r(26)=−0.17) and 47 kDa (r(26)=−0.27) to the amount of 45 kDa isoform does not exclude the possibility that deglycosylation of the 47-kDa isoform may also contribute, to a lesser extent, to the formation of the N-deglycosylated 45-kDa β1 GABAAR subunit isoform.
Figure 4.
Deglycosylation with EndoH results in an altered ratio of 49 : 45 kDa β1 GABAAR subunit isoforms in schizophrenia. Western blot analysis of the β1 GABAAR subunit from STG deglycosylated with EndoH. (a) Representative images show the EndoH-treated lane from paired comparison and schizophrenia subjects. (b) Integrated intensity of each isoform band as a percentage of total β1-integrated intensity in the EndoH-treated lane. (c) Ratio of β1 GABAAR subunit 49 : 45 kDa isoform after treatment with EndoH. Data are expressed as means±SEM. *P<0.05 and **P<0.01.
Upon examination of the integrated intensity of each β1 isoform as a percentage of total β1 in the EndoH-treated sample, we identified a difference in the relative contribution of both the 49 and 45 kDa β1 isoforms to the total population of β1 subunits between schizophrenia and comparison subjects (Figure 4b). Schizophrenia subjects have less 49 kDa β1 isoform (t(13)=1.8, P=0.04, q<0.01) and more 45 kDa isoform (t(13)=2.4, P=0.02, q=0.02) than comparison subjects, and the ratio of 49 : 45 kDa isoforms is less in schizophrenia (t(13)=2.8, P=0.02, q<0.01; Figure 4c). PNGase control and treated samples display the same band pattern on western blots (Figure 1b), but no significant differences in molecular mass shifts, relative intensities, or ratio of each isoform to total β1 were identified between schizophrenia and comparison subjects.
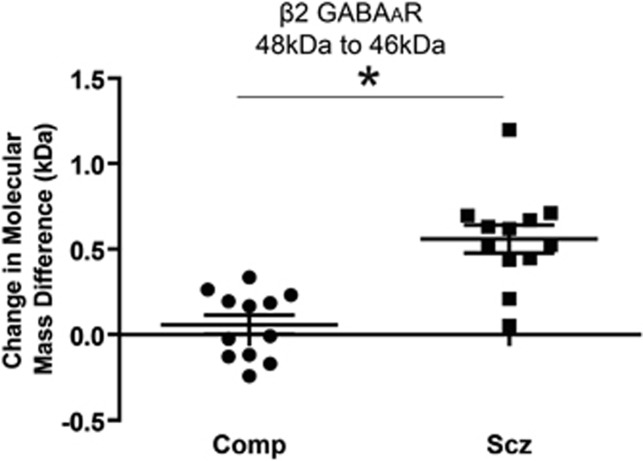
The Molecular Mass Difference of β2 GABAAR Subunit Isoforms is Greater in Schizophrenia Following PNGase Deglycosylation
The β2 GABAAR subunit appears as two bands, with approximate molecular masses of 48 and 46 kDa, with an additional antibody-reactive band with an approximate molecular mass of 44 kDa becoming apparent following deglycosylation (Figure 1c). The molecular mass difference between the two heaviest β2 subunit isoforms (48 and 46 kDa) was greater after deglycosylation with PNGase in schizophrenia subjects relative to comparison (t(8)=3.1, P=0.01, q=0.01). This molecular mass difference was correlated with the amount of total β2 protein expressed in schizophrenia subjects (r=0.67, P=0.03), but not in comparison subjects; ANCOVA revealed that the difference between schizophrenia and comparison subjects is independent of total β2 GABAAR subunit protein expression (F(1,17)=5.54, P=0.03). In addition, after PNGase treatment the 48 kDa β2 isoform demonstrates an apparent increase in molecular mass of approximately 0.3 kDa in schizophrenia subjects (t(11)=2.4, P=0.04, q=0.02, Figure 5). No significant differences in relative intensities or ratio of each isoform to total β2 were identified between schizophrenia and comparison subjects after EndoH or PNGase deglycosylation. No significant differences in molecular mass shifts were identified in EndoH-treated samples.
Figure 5.
Deglycosylation with PNGase results in a larger separation between the 48 and 46 kDa β2 GABAAR subunit isoforms in schizophrenia. Western blot analysis of the 48 and 46 kDa β2 GABAAR subunit isoforms from STG after deglycosylation with PNGase in schizophrenia and comparison subjects. Data are expressed as the mean difference between the isoform-separation distance in kDa of the native and enzyme-treated bands. *P<0.05.
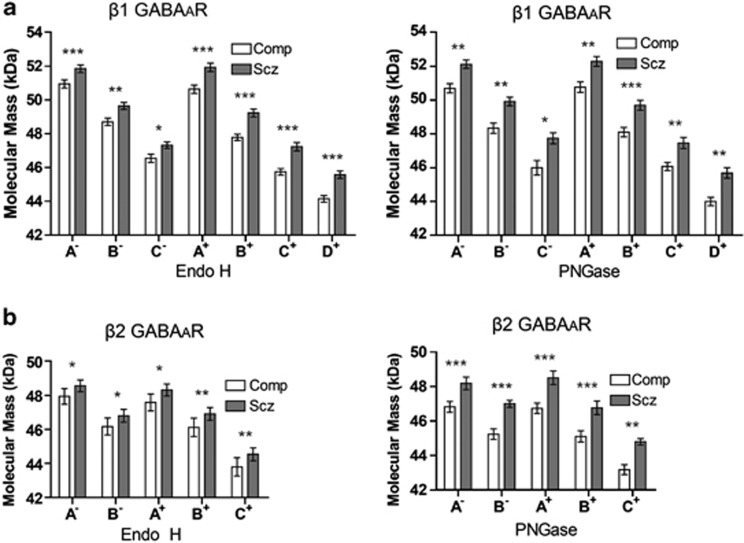
Molecular Mass Increase of β1 and β2 GABAAR Subunit Isoforms in Schizophrenia is not due to additional N-Glycosylation
N-glycosylation alterations of the β1 and β2 GABAAR subunits in schizophrenia are confounded by the finding that all isoforms of these subunits, in both control and deglycosylating enzyme-treated lanes, demonstrate an increased molecular mass relative to comparison subjects (Figure 6, Supplementary Table S3). Although the molecular mass of some isoforms was found to correlate with PMI, with the exception of the β2 48 kDa isoform in the PNGase control lane (band A−), the molecular mass increase of these isoforms remained significant in secondary ANCOVAs with PMI held as the covariate (Supplementary Table S3). In addition, dephosphorylation of homogenate samples did not produce a corresponding reduction in molecular mass of the β2 subunit isoforms in schizophrenia subjects, suggesting that this increased molecular mass in schizophrenia is not due to differences in phosphorylation.
Figure 6.
Molecular mass of the β1 and β2 GABAAR subunits is increased in schizophrenia. Western blot analysis of the β1 (a) and β2 (b) GABAAR subunits. The mean apparent molecular mass of each band associated with the β1 subunit is 0.8–1.7 kDa heavier, and each β2-associated band is 0.6–1.6 kDa heavier in schizophrenia than in comparison subjects. (a) β1 is expressed as three isoforms; bands A, B, and C in non-enzyme-treated control reactions (−), with an additional band D produced following deglycosylation (+). (b) β2 is expressed as two isoforms; bands A and B in non-enzyme-treated control reactions (−), with an additional band C produced following deglycosylation (+). Data are expressed as means±SEM. *P<0.05, **P<0.01, and ***P<0.001.
DISCUSSION
This study demonstrates altered patterns of N-glycosylation of GABAAR subunits in the STG in schizophrenia. Although N-glycosylation abnormalities of glutamatergic signaling molecules have been previously reported in the anterior cingulate and dorsolateral prefrontal cortices (Bauer et al, 2010; Tucholski et al, 2013a; Tucholski et al, 2013b), this study establishes that N-glycosylation deficits may be more extensive in schizophrenia, affecting multiple neurotransmitter systems and including multiple cortical regions known to be relevant in schizophrenia pathophysiology. The STG is of particular interest, having been shown to have increased density of GABAARs (Deng and Huang, 2006), as well as decreased volume in schizophrenia (Barta et al, 1990; Sun et al, 2009). Functionally, the left STG has been shown to have a role in the development of auditory hallucinations (Barta et al, 1990; Nenadic et al, 2010; Silbersweig et al, 1995) and has been associated with disrupted thought processes (Rajarethinam et al, 2000) and impaired working memory in schizophrenia (Zierhut et al, 2013).
Previous studies have shown that altered N-glycosylation of some GABAAR subunits in model systems affects the processing, assembly, receptor stability, and cell surface expression of mature GABAARs, as well as affecting channel gating properties and receptor function (Buller et al, 1994; Gurba et al, 2012; Lo et al, 2010; Tanaka et al, 2008). N-glycosylation has a functional role regulating forward trafficking of proteins along the secretory pathway from the ER through the Golgi before vesicular transport to the plasma membrane. This is accomplished by enzymatically attaching or pruning the proper sequence of glycans in a specific, step-wise manner. N-glycosylation is initiated in the ER by the attachment of a high-mannose oligosaccharide at the Asn–Xaa–Ser/Thr consensus sequence, where Xaa is any amino acid except proline. These high-mannose glycans in the ER are considered immature until enzymatic processing proceeds, sequentially cleaving the terminal glucose and mannose residues until the protein moves forward into the Golgi for mature N-glycan processing (Helenius and Aebi, 2004; Ohtsubo and Marth, 2006; Parodi, 2000; Varki, 2009).
Relatively little information has been published on the glycosylation patterns of the GABAAR subunits in normal human brain. Before studying N-glycosylation of GABAAR subunits in schizophrenia, we first identified which of the subunits are N-glycosylated in normal human cortex. The Swiss-prot database (www.uniprot.org) provides manually curated information about the proteome, including amino acid sequence annotation of potential, probable, and experimentally verified post-translational modifications, and lists at least two potential N-glycosylation sites on each of the human GABAAR subunits under investigation (α1–6, β1–3, and γ2) (Farriol-Mathis et al, 2004; Khoury et al, 2011). Western blot analysis of normal human cortex treated with the deglycosylating enzymes EndoH and PNGase demonstrated apparent molecular mass changes of the α1, β1, and β2 GABAAR subunits, indicating the presence of N-linked glycans, and the presence of glycans attached to these subunits was confirmed by lectin affinity assays. The lectin affinity assay enabled specific isolation of glycoproteins and demonstrated that, in addition to the α1, β1, and β2 GABAAR subunits, the α4 and β3 subunits exhibit lectin-binding affinity, confirming that at least five GABAAR subunits are glycosylated. It is possible that deglycosylation of the α4 and β3 subunits did not produce a visible molecular mass shift because the molecular mass change was too small to detect via western blot, or these subunits may instead have attached O-linked glycans, which would not have been cleaved by either of the deglycosylating enzymes used in the assay.
In schizophrenia, we found a smaller molecular mass shift after removal of the immature N-glycans from the α1 GABAAR subunit, and altered immature N-glycosylation of β1 subunit isoforms. These alterations suggest possible abnormalities in glycoprotein processing and quality-control mechanisms in the ER. A smaller molecular mass change after EndoH deglycosylation, as we found for the α1 GABAAR subunit, indicates that the immature N-glycans removed have a smaller molecular mass in schizophrenia, likely due to impaired glycoprotein processing in the ER. Proper immature N-glycan processing is necessary for accurate protein folding via the calnexin–calreticulin cycle, which allows the cell to recognize when glycoproteins have achieved the proper tertiary or quaternary structure before export to the Golgi. The role of ER N-glycan processing in protein folding is a well-characterized process that has been reviewed extensively (Helenius and Aebi, 2004; Parodi, 2000; Varki, 2009). As total protein expression of the GABAAR subunits was not altered in schizophrenia, a smaller molecular mass immature N-glycan may indicate that the subunit has undergone some N-glycan processing, but remains relatively retained in the ER rather than being localized at the plasma membrane. This may be because of glycoproteins being folded inefficiently and held in the calnexin–calreticulin cycle, impaired targeting of terminally misfolded proteins for ER-associated degradation, or failure of the (UDP)-glucose : glycoprotein glucosyltransferase conformation sensor to recognize the correct protein tertiary structure. Alternatively, the reduced molecular mass shift after immature N-glycan cleavage may be due to an altered conformation of the α1 subunit, causing the protein to migrate differently in the gel; this alternative might also suggest aberrant protein folding in the ER.
Following deglycosylation, the relative amount of the β1 GABAAR subunit 49 kDa isoform is reduced and a new 45 kDa isoform appears. Correlation analysis supports the interpretation that this new 45 kDa β1 isoform is produced by deglycosylation of the 49 kDa β1 isoform. In schizophrenia, there is a greater reduction in the relative amount of the 49 kDa isoform and greater expression of the 45 kDa isoform after deglycosylation by EndoH. This suggests that more of the 49-kDa isoform is immaturely N-glycosylated in schizophrenia, supporting the hypothesis that there is a disruption in the processing of N-glycosylated proteins in the ER. The alterations seen with the β1 GABAAR subunit are suggestive of either an additional N-glycosylation consensus sequence in the 49 kDa β1 isoform or unmasking of a consensus sequence that facilitates additional N-glycan attachment in schizophrenia.
Aberrant N-glycosylation of the α1 and β1 GABAAR subunits is only apparent after EndoH deglycosylation and is consistent with ER-related abnormalities of immature N-glycan processing. N-glycosylation differences in schizophrenia seen after deglycosylation with PNGase, which cleaves both immature and mature N-glycans, are only apparent with the β2 GABAAR subunit. When both immature and mature N-glycans are cleaved, the highest molecular mass isoform of the β2 GABAAR subunit in schizophrenia undergoes a larger molecular mass shift than in comparison subjects. This may suggest the presence of higher molecular mass N-glycans attached to the core protein in schizophrenia, which may be attributed to more extensive complex oligosaccharide branching of mature N-glycans, or differences in the specific sugar composition of attached N-glycans. Similar to the α1 GABAAR subunit, an alternate possibility may be that the conformation of the core subunit is altered in schizophrenia, producing an aberrant migration pattern in the gel. Although many measures of N-glycosylation of the β1 and β2 GABAAR subunits are significantly changed in schizophrenia, these findings are complicated by the apparent increased molecular mass of all β1 and β2 isoforms in schizophrenia. Because all of the β1 and β2 subunit isoforms demonstrate an increased molecular mass in schizophrenia subjects, independent of N-glycosylation status, these alterations cannot solely be attributed to increased N-glycosylation of these GABAAR subunits in schizophrenia.
It has been shown in cell culture that the less glycosylated isoforms of the α1, β2, and γ2L subunits are preferentially incorporated into intact, functional receptors (Connolly et al, 1996), and in cells expressing α1 subunits with mutated N-glycosylation consensus sequences, GABAAR ligand binding can be significantly reduced or completely eliminated (Buller et al, 1994). Mutations of consensus sequences on the β2 subunit inhibiting N-glycosylation have been shown to decrease peak current amplitude and mean channel open time of GABAARs and, in an N-glycosylation consensus sequence-specific manner, have variable effects on subunit stability, assembly of receptors in the ER, and expression of N-glycosylation-deficient β2 subunit-containing receptors in the plasma membrane (Lo et al, 2010). It has been postulated that aberrant glycosylation of specific GABAAR subunits may lead to altered protein folding and tertiary structure, causing the assembly of correctly N-glycosylated subunits to be more energetically favorable and altering GABAAR subunit stoichiometry in the plasma membrane (Gurba et al, 2012). Alternatively, it has been suggested that incorrect N-glycosylation may impair receptor trafficking to the plasma membrane, reducing GABA-evoked currents and decreasing the efficacy of GABAergic signaling (Tanaka et al, 2008). These findings suggest that the alterations of GABAAR subunit N-glycosylation that we have found have meaningful effects on the functional properties of intact GABAARs in schizophrenia.
Although we view it as less likely, there is a possibility that the glycosylation alterations for α1 and β1, which are only immature, ER-associated glycosylation changes, are compensated for within the cell, and intact receptors incorporating these subunits are appropriately N-glycosylated when inserted into the plasma membrane, thus producing no functional alterations. On the other hand, the β2 GABAAR changes we found reflect aberrant total N-glycosylation and most likely involve changes in mature patterns of glycosylation, which are likely to be associated with functional consequences. Future studies might explore whether there are alterations in the trafficking and intracellular localization of α1-, β1-, and β2-containing GABAARs and if these subunits are abnormally expressed at the cell surface in schizophrenia, as our data would suggest.
Dysfunctional GABAergic neurotransmission is central to the disinhibition hypothesis of schizophrenia, often put forward to explain cognitive deficits in schizophrenia. This hypothesis suggests that NMDA receptor hypofunction in GABAergic interneurons disinhibits excitatory output to pyramidal cells in the prefrontal cortex, leading to overactivation of glutamatergic neurons, which contributes to altered firing in other downstream neurotransmitter systems; this theory explains many of the converging lines of evidence and elucidates how functional alterations in multiple neurotransmitter systems and brain regions contribute to schizophrenia pathophysiology (Gonzalez-Burgos et al, 2010; Lewis et al, 2005; Lewis and Moghaddam, 2006; Nakazawa et al, 2012; Sun et al, 2011; Uhlhaas and Singer, 2006). Evidence of reduced production of GABA across multiple brain regions (Thompson et al, 2009) and increased GABAAR density (Deng and Huang, 2006) provide further indications that GABAergic dysfunction in the STG may have a role in the generation of psychotic behaviors and auditory hallucinations that are characteristic of the disorder.
The reduced immature N-glycosylation of the α1 GABAAR subunit may reduce ligand-binding efficacy of the receptor or alter subunit stoichiometry. Increased N-glycosylation of the 49 kDa isoform of the β1 GABAAR subunit may also contribute to disinhibition by altering channel gating and reducing the mean open time of the receptor, limiting chloride ion entry and reducing inhibition of post-synaptic, glutamatergic neurons. In conjunction with reduced activation of GABAergic interneurons by NMDA receptor hypofunction and decreased GABA release, functional alterations of GABAARs containing aberrantly N-glycosylated subunits may contribute to overactivation of downstream glutamatergic and dopaminergic neurons.
There are several limitations to this work. Although we were able to match subjects for tissue pH, there is a significant difference between groups for PMI. The schizophrenia subjects had longer PMIs in the face of identical tissue pH, which if anything would suggest that we would be less likely to find the changes that we report here. We have performed a series of post-hoc statistical tests with PMI as a covariate, and this resulted in identical findings. These considerations suggest that PMI is not likely the explanation for our findings, but is a caveat to the study. The effects of antipsychotic medication on N-glycosylation in brain tissue have not been previously reported. However, olanzapine treatment has been shown to affect the abundance of some N-linked glycoforms in both serum and cerebrospinal fluid in early-stage, antipsychotic naive patients (Telford et al, 2012). We performed post-hoc analyses to assess possible medication effects on our results, finding no indication that antipsychotic treatment affected the outcomes of these experiments. A caveat is that this is a relatively underpowered secondary analysis and should be interpreted with caution. Our subjects are all elderly, and these findings may not generalize to younger patients at earlier stages of this illness.
In summary, the results of this study demonstrate that the α1, α4, β1, β2, and β3 GABAAR subunits are glycosylated in normal human cortex, with the specific attachment of N-linked glycans on the α1, β1, and β2 GABAAR subunits. We also demonstrate that aberrant N-glycosylation in schizophrenia occurs in the GABAergic system and is not exclusive to the processing of glutamatergic molecules as we have previously reported (Bauer et al, 2010; Tucholski et al, 2013a; Tucholski et al, 2013b). In addition, the N-glycosylation alterations of the GABAergic system that we observed could functionally contribute to the pathophysiology of schizophrenia. Although alterations of immature N-glycosylation are distinctly evident in schizophrenia, the possibility of more extensive N-glycosylation deficits in multiple cellular compartments is not excluded. Taken together, these observations are indicative of a more generalized mechanism of pathophysiology in schizophrenia that affects the intracellular processing of multiple neurotransmitter receptors and transporters, and is perhaps associated with the wide variation of patient symptom presentation, severity of phenotype, and therapeutic or pharmaceutical efficacy among the patient population.
FUNDING AND DISCLOSURE
This work is supported by the National Institutes of Health Grant MH53327 (JHM-W), MH064673 (VH), and MH066392 (VH). Tissue for characterization of normal cortical GABAAR glycosylation was generously provided by Dr Rosalinda C. Roberts and the Alabama Brain Collection. The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Buchstaller A, Adamiker D, Fuchs K, Sieghart W. N-deglycosylation and immunological identification indicates the existence of beta-subunit isoforms of the rat GABAA receptor. FEBS Lett. 1991;287:27–30. doi: 10.1016/0014-5793(91)80008-q. [DOI] [PubMed] [Google Scholar]
- Buchstaller A, Fuchs K, Sieghart W. Identification of alpha 1-, alpha 2- and alpha 3-subunit isoforms of the GABAA-benzodiazepine receptor in the rat brain. Neurosci Lett. 1991;129:237–241. doi: 10.1016/0304-3940(91)90470-e. [DOI] [PubMed] [Google Scholar]
- Buller AL, Hastings GA, Kirkness EF, Fraser CM. Site-directed mutagenesis of N-linked glycosylation sites on the gamma-aminobutyric acid type A receptor alpha 1 subunit. Mol Pharmacol. 1994;46:858–865. [PubMed] [Google Scholar]
- Chen ZW, Fuchs K, Sieghart W, Townsend RR, Evers AS. Deep amino acid sequencing of native brain GABAA receptors using high-resolution mass spectrometry. MCP. 2012;11:M111 011445. doi: 10.1074/mcp.M111.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Deng C, Huang XF. Increased density of GABAA receptors in the superior temporal gyrus in schizophrenia. Exp Brain Res. 2006;168:587–590. doi: 10.1007/s00221-005-0290-9. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- Farriol-Mathis N, Garavelli JS, Boeckmann B, Duvaud S, Gasteiger E, Gateau A, et al. Annotation of post-translational modifications in the Swiss-Prot knowledge base. Proteomics. 2004;4:1537–1550. doi: 10.1002/pmic.200300764. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurba KN, Hernandez CC, Hu N, Macdonald RL. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters alpha1beta3gamma2L gamma-aminobutyric acid type A (GABAA) receptor expression and channel gating. J Biol Chem. 2012;287:12083–12097. doi: 10.1074/jbc.M111.332528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophrenia research. 2005;77:241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. 2011;1:pii: srep00090. doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lo WY, Lagrange AH, Hernandez CC, Harrison R, Dell A, Haslam SM, et al. Glycosylation of {beta}2 subunits regulates GABAA receptor biogenesis and channel gating. J Biol Chem. 2010;285:31348–31361. doi: 10.1074/jbc.M110.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Smesny S, Schlosser RG, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. Br J Psychiatry. 2010;196:412–413. doi: 10.1192/bjp.bp.109.070441. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Parodi AJ. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem J. 2000;348 (Pt 1:1–13. [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, et al. Postmortem studies in schizophrenia. Schizophrenia Bull. 1998;24:325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, et al. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993;33:255–260. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia: a postmortem neuropathologic study of 100 cases. Arch Gen Psychiatry. 1998;55:205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41:303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910–1920. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, et al. Structure and subunit composition of GABA(A) receptors. Neurochemistry international. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter. Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61:14–32. doi: 10.1016/j.brainresrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, et al. Gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Olsen RW, Medina MT, Schwartz E, Alonso ME, Duron RM, et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am J Hum Genet. 2008;82:1249–1261. doi: 10.1016/j.ajhg.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford JE, Bones J, McManus C, Saldova R, Manning G, Doherty M, et al. Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. J Proteome Res. 2012;11:3743–3752. doi: 10.1021/pr300218h. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr Res. 2013;146:177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013;24:688–691. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Varki A.2009Essentials of Glycobiology2nd edn.Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY; xxix784. [PubMed] [Google Scholar]
- Zierhut KC, Schulte-Kemna A, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Distinct structural alterations independently contributing to working memory deficits and symptomatology in paranoid schizophrenia. Cortex. 2013;49:1063–1072. doi: 10.1016/j.cortex.2012.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.