Abstract
Synaptic loss in the brain correlates well with disease severity in Alzheimer disease (AD). Deficits in brain-derived neurotrophic factor/tropomyosin-receptor-kinase B (TrkB) signaling contribute to the synaptic dysfunction of AD. We have recently identified 7,8-dihydroxyflavone (7,8-DHF) as a potent TrkB agonist that displays therapeutic efficacy toward various neurological diseases. Here we tested the effect of 7,8-DHF on synaptic function in an AD model both in vitro and in vivo. 7,8-DHF protected primary neurons from Aβ-induced toxicity and promoted dendrite branching and synaptogenesis. Chronic oral administration of 7,8-DHF activated TrkB signaling and prevented Aβ deposition in transgenic mice that coexpress five familial Alzheimer's disease mutations (5XFAD mice). Moreover, 7,8-DHF inhibited the loss of hippocampal synapses, restored synapse number and synaptic plasticity, and prevented memory deficits. These results suggest that 7,8-DHF represents a novel oral bioactive therapeutic agent for treating AD.
Keywords: 7,8-Dihydroxyflavone; Synapse; TrkB; Alzheimer's disease; neuroprotection
INTRODUCTION
The etiology and pathogenesis of Alzheimer disease (AD) have not been fully elucidated. Neuritic plaques and neurofibrillary tangles are the two major pathological hallmarks of AD. However, they are only weakly correlated with the degree of dementia in AD patients (Berg et al, 1998). It has been demonstrated that synaptic loss is the major correlate of cognitive impairment and acts as the pathological basis of cognitive alteration in AD brain (Sze et al, 1997; Terry et al, 1991).
Brain-derived neurotrophic factor (BDNF) is the most wildly distributed neurotrophin in the central nervous system. By binding to its specific cognate receptor tropomyosin-receptor-kinase B (TrkB), BDNF triggers the activation of Ras/Raf/MAKP, PLCγ, and PI3K/Akt signaling cascades, which have critical roles in neuronal plasticity, survival and neurogenesis (Diniz and Teixeira, 2011; Zuccato and Cattaneo, 2009). It has been documented that BDNF expression is reduced in the hippocampus, dentate gyrus, neocortex, and in the nucleus basalis of Meynert of AD patients (Murer et al, 2001; Narisawa-Saito et al, 1996; Phillips et al, 1991). Interestingly, neurons containing neurofibrillary tangles do not contain BDNF immunoreactivity, whereas intense BDNF-positive neurons are devoid of tangles (Murer et al, 1999). These studies suggest that BDNF may have a protective role against AD pathogenesis. In agreement with this hypothesis, BDNF gene delivery reversed synapse loss and cognitive deficits in both a rodent and a primate model of AD (Nagahara et al, 2009). However, the outcomes of several clinical trials using recombinant BDNF to treat amyotrophic lateral sclerosis have been disappointing (Beck et al, 2005; Ochs et al, 2000), presumably due to poor delivery and the short in vivo half-life of BDNF.
To search for a TrkB agonist with better bioavailability, blood–brain barrier penetration, and half-life than BDNF, we screened thousands of compounds from a chemical library. After extensive validation, we identified 7,8-dihydroxyflavone (7,8-DHF) as a selective small-molecular TrkB agonist that mimics the physiological actions of BDNF. Our pharmacokinetic experiments found that the oral bioavailablity of 7,8-DHF is about 5%, and its half-life is about 134 min in the plasma after oral gavage of 50 mg/kg. Furthermore, it can penetrate the blood–brain barrier (Liu et al, 2013). Remarkably, systemic administration of 7,8-DHF can activate TrkB receptors in the brain as evidenced by increases in phosphorylated TrkB in rodents (Andero et al, 2012; Andero et al, 2011; Choi et al, 2010; Jang et al, 2010; Liu et al, 2010). These findings provide a proof-of-concept demonstration that 7,8-DHF is a novel small molecule for the therapeutic intervention of AD.
It was recently reported that 7,8-DHF, when administered to the 5XFAD mouse model of AD at 12–15 months of age via daily intraperitoneal (i.p.) injection for 10 days, restored TrkB signaling, decreased β-secretase, reduced Aβ levels, and rescued Y-maze performance (Devi and Ohno, 2012). In the present study, we first examined the effect of 7,8-DHF on Aβ-induced neurotoxicity and synaptogenesis in vitro. Nest, we administered 7,8-DHF orally via drinking water to the 5XFAD transgenic mouse model of AD, which contains five familial Alzheimer's disease mutations (Swedish, Florida, and London human APP 695 mutations and M146L and L286V PS1 mutations). Treatment started at 2 months of age, before plaque deposition, and mice were assessed for AD-like neuropathology and cognitive performance at 6 months of age. We found that 7,8-DHF protected the primary cortical neurons and locus coeruleus (LC) neurons from Aβ-induced toxicity and promoted dendritic growth and synaptogenesis. In 5XFAD mice, chronic oral administration of 7,8-DHF prevented Aβ deposition, the loss of hippocampal synapses, synaptic dysfunction, and spatial memory deficits.
MATERIALS AND METHODS
Mice and Reagents
5XFAD mice on a C57BL/6J background were obtained from the Jackson laboratory (Bar Harbor, ME) and were bred in a pathogen-free environment in accordance with Emory Medical School guidelines. The mice received vehicle or 7,8-DHF in their drinking water. To dissolve 7,8-DHF in water, 1M NaOH was added drop wise to the water and stirred at room temperature overnight. The final concentration of 7,8-DHF was 22 mg/l (pH 7.6–7.8). Water (pH 7.6–7.8) was used as vehicle control. As the daily water intake of C57BL/6J mice is about 7 ml/30 g body weight (Bachmanov et al, 2002), the oral dose of 7,8-DHF is ∼5 mg/kg/day. The treatment started at 2 months of age, and mice were subjected to Morris water maze tests at 6 months of age. After behavior testing, the mice were killed for immunoblotting, immunohistochemistry, and ELISA experiments. Anti-TrkB antibody was purchased from Biovision (Milpitas, CA). Anti-phospho-TrkB Y816 antibody was raised against [H]-CKLQNLAKASPV-pY-LDILG-[OH] (a.a. 806–822)(EM437 and EM438) as rabbit polyclonal antibody. Anti-synaptotagmin, anti-MAP2, anti-Aβ, and anti-tubulin were purchased from Sigma-Aldrich (St Louis, MO). Anti-synapsin I, anti-PSD95, anti-spinophilin, anti-Akt, anti-p-Akt, anti-ERK, and anti-phospho-ERK1/2 antibodies were purchased from Cell Signaling (Boston, MA). Anti-vesicular GABA transporter (VGAT) was purchased from Thermo Scientific (Hudson, NH). Anti-bassoon was purchased from Stressgen (Kampenhout, Belgium). Anti-VGAT was purchased from Thermo Scientific. Anti-bassoon was purchased from Stressgen. Anti-tyrosine hydroxylase (TH) was from Calbiochem (Darmstadt, Germany). Synthetic Aβ (1–42) was purchased from rPeptide (Bogart, GA) and was dissolved in N2 medium at 0.5 mg/ml and incubated for 4 days at 37 °C to pre-aggregate the peptide. Histostain-SP and Aβ 1–42 ELISA kits were purchased from Invitrogen (Grand Island, NY). The In situ cell death detection kit was purchased from Roche (Indianapolis, IN). 7,8-DHF was purchased from TCI (Portland, OR). All chemicals not included above were purchased from Sigma-Aldrich.
Primary Neuron Culture
Primary rat cortical neurons and LC neurons were cultured as previously described (Chan et al, 2011). To measure the effect of 7,8-DHF on dendrite elongation, neurons cultured 3 days in vitro (DIV 3) were exposed to 500 nM 7,8-DHF or vehicle for 3 days, the neurons were then fixed in 4% formaldehyde, permeabilized, and immunostained with anti-MAP2 antibody. Pictures of the neurons were taken by fluorescence microscopy. Dendritic length and complexity were scored using computer software ImageJ (National Institute of Health, USA) as described (Chan et al, 2011). Data analysis were performed using Student's t-test. To assess the effect of 7,8-DHF on Aβ-induced toxicity, 7,8-DHF (500 nM) was added to the medium 20 min before Aβ treatment. Then cortical neurons and LC neurons were exposed to 20 μM pre-aggregated Aβ (1–42) and Aβ (25–35), respectively and incubated for 18 h. Neuronal apoptosis was detected with the in situ cell death detection kit. The apoptotic index was expressed as the percentage of TUNEL-positive neurons out of the total number of MAP2-positive neurons.
Immunofluorescence and Immunohistochemistry
For immunofluorescence and TUNEL staining, the sections were incubated overnight at 4 °C with anti-MAP2 antibody. After being washed with tris-buffered saline, the sections were incubated with Alexa Fluor 488-coupled secondary antibodies. The sections were then incubated with TUNEL reagent for 1 h at room temperature. After a phosphate-buffered saline (PBS) wash, images were acquired through an AxioCam camera on an Axiovert 200M microscope (Zeiss). For the analysis of synaptogenesis in primary cultured neurons, the neurons were costained with anti-VGAT and anti-bassoon antibody. The number and size of the synapses were analyzed with ImageJ software. Immunohistochemistry was performed according to the manufacturer's instructions (no. 956143 and no. 956543, Invitrogen). Briefly, tissue sections were deparaffinized and hydrated. After antigen-retrieval in boiling 10 mM sodium citrate (pH 6.0) for 20 min, the sections were incubated with primary antibodies (anti-trkb, anti-p-TrkB, or anti-Aβ) overnight at 4 °C. The signal was developed using Histostain-SP kit. The number of positive cells or plaques was analyzed using ImageJ software (National Institute of Health) and color deconvolution plugin as described previously (Josephs et al, 2008). First, color deconvolution was used to isolate AEC stain, which represents the positive area from the hematoxylin stain that represents the nuclei. Image was changed into 8-bit type (gray), and then processed into binary color image. Number of positive neurons or plaques was calculated using the ‘analyze particles' plugin of Imagine J.
Golgi Stain
Mice brains were fixed in 10% formalin for 24 h and then immersed in 3% potassium bichromate for 3 days in the dark. The solution was changed each day. Then the brains were transferred into 2% silver nitrate solution and incubated for 24 h in the dark. Vibratome sections were cut at 60 μm, air dried for 10 min, dehydrated through 95 and 100% ethanol, cleared in xylene, and coverslipped. For measurement of spine density, only spines that emerged perpendicular to the dendritic shaft were counted.
Western Blot Analysis
The mice brain tissue was lysed in lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, supplemented with protease inhibitors cocktail) and centrifuged for 15 min at 16 000 g. The supernatant was boiled in SDS loading buffer. After SDS-PAGE, the samples were transferred to a nitrocellulose membrane. Western blotting analysis was performed with a variety of antibodies.
Electron Microscopy
Synaptic density was determined by electron microscopy as described previously (Hongpaisan et al, 2011). After deep anesthesia, mice were perfused transcardially with 2% glutaraldehyde and 3% paraformaldehyde in PBS. Hippocampal slices were postfixed in cold 1% OsO4 for 1 h. Samples were prepared and examined using standard procedures. Ultrathin sections (90 nm) were stained with uranyl acetate and lead acetate and viewed at 100 kV in a JEOL 200CX electron microscope. Synapses were identified by the presence of synaptic vesicles and postsynaptic densities.
Electrophysiological Analysis
Electrophysiological analysis was carried out as previously described (Hong et al, 2012). Briefly, vehicle- and 7,8-DHF-treated 5XFAD mice were anaesthetized with isoflurane, decapitated, and the hippocampi were cut into 400-μm thick transverse slices with a vibratome. After incubation at room temperature in a-CSF for 60–90 min, slices were placed in a recording chamber on the stage of an upright microscope (Olympus CX-31) and perfused at a rate of 3 ml per min with a-CSF (containing 1 mM MgCl2) at 23–24 °C. A 0.1 MΩ tungsten monopolar electrode was used to stimulate the Schaffer collaterals. The field excitatory postsynaptic potentials (fEPSPs) were recorded in CA1 stratum radiatum by a glass microelectrode filled with a-CSF with resistance of 3–4 MΩ. The stimulation output (Master-8; AMPI, Jerusalem) was controlled by the trigger function of an EPC9 amplifier (HEKA Elektronik, Lambrecht, Germany). fEPSPs were recorded under current-clamp mode. Data were filtered at 3 kHz and digitized at sampling rates of 20 kHz using Pulse software (HEKA Elektronik). The stimulus intensity (0.1 ms duration, 2–4 V) was set to evoke 40% of the maximum fEPSP and the test pulse was applied at a rate of 0.033 Hz. Field potential input-output curves were constructed by measuring fEPSP slope responding to the stimulus intensity increased from 1 to 7 V, with an 0.5 V increment. LTP of fEPSPs was induced by 3 theta-burst-stimulation (TBS), it is 4 pulses at 100 Hz, repeated 3 times with a 200-ms interval). Paired-pulse facilitation (PPF) was examined by applying pairs of pulses, which were separated by 20–500 ms intervals. The magnitudes of LTP are expressed as the mean percentage of baseline fEPSP initial slope.
Aβ Plaque Staining
Amyloid plaques were stained with Thioflavin-S. The deparaffinized and hydrated sections were incubated in 0.25% potassium permanganate solution for 20 min, rinsed in distilled water, and incubated in bleaching solution containing 2% oxalic acid and 1% potassium metabisulfite for 2 min. After rinsing in distilled water, the sections were transferred to blocking solution containing 1% sodium hydroxide and 0.9% hydrogen peroxide for 20 min. The sections were incubated for 5 s in 0.25% acidic acid, then washed in distilled water and stained for 5 min with 0.0125% Thioflavin-S in 50% ethanol. The sections were washed with 50% ethanol and placed in distilled water. Then the sections were covered with a glass cover using mounting solution. Quantitative assessment of plaque areas was done using ImageJ software as described previously (Heneka et al, 2013). Briefly, the images were normalized and an automatic thresholding on the basis of the entropy of the histogram was used to identify the plaques. The pictures were converted to a binary, and then the plaque number and the plaque area were calculated using the ‘analyze particles' plugin of Imagine J.
Aβ42 ELISA
The mice brains were homogenized in 8X mass of 5 M guanidine HCl/50 mM Tris HCl (pH 8.0) and incubated at room temperature for 3 h. Then the samples were diluted with cold reaction buffer (PBS with 5% BSA and 0.03% Tween-20, supplemented with protease inhibitor cocktail), and centrifuged at 16 000 g for 20 min at 4 °C. The supernatant was analysed with a human Aβ42 ELISA kit according to the manufacturer's instructions (KHB3441, Invitrogen). The Aβ42 concentrations were determined by comparison with the standard curve.
Morris Water Maze
Female wild-type and 5XFAD mice maintained on standard drinking water or 7,8-DHF were trained in a round, water-filled tub (52 inch diameter) in an environment rich with extra maze cues. An invisible escape platform was located in a fixed spatial location 1 cm below the water surface independent of a subjects start position on a particular trial. In this manner, subjects needed to utilize extra maze cues to determine the platform's location. At the beginning of each trial, the mouse was placed in the water maze with their paws touching the wall from one of four different starting positions (N, S, E, and W). Each subject was given four trials/day for 5 consecutive days with a 15-min intertrial interval. The maximum trial length was 60 s and if subjects did not reach the platform in the allotted time, they were manually guided to it. Upon reaching the invisible escape platform, subjects were left on it for an additional 5 s to allow for survey of the spatial cues in the environment to guide future navigation to the platform. After each trial, subjects were dried and kept in a dry plastic holding cage filled with paper towels to allow the subjects to dry off. The temperature of the water was monitored every hour so that the mice were tested in water that was between 22 and 25 °C. Following the 5 days of task acquisition, a probe trial was presented during which time the platform was removed and the percentage of time spent in the quadrant that previously contained the escape platform during task acquisition was measured over 60 s. All trials were analysed for latency, swim path length, and swim speed by means of MazeScan (Clever Sys).
Statistical Analysis
All of the data were presented as mean±SEM. Statistical analysis were performed using either Student's t-test (two-group comparison) or one-way ANOVA followed by the Dunnett's post hoc multiple comparison test (more than two groups). The level of significance was set for P-value<0.05.
RESULTS
7,8-DHF Protects Primary Cortical Neurons and LC Neurons from Aβ-Induced Toxicity in a TrkB-Dependent Manner
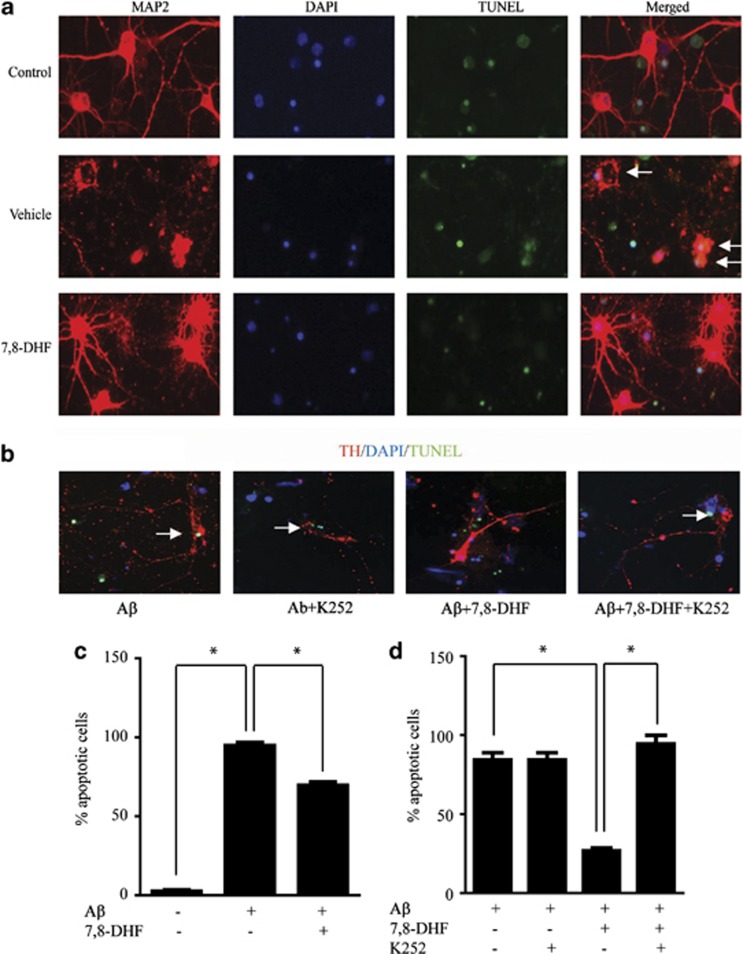
We previously reported that 7,8-DHF displays protective effect at 500 nM, and it induces TrkB tyrosine phosphorylation in hippocampal neurons (Jang et al, 2010; Liu et al, 2013). To examine whether 7,8-DHF has protective effects on neurons against Aβ-induced toxicity, we prepared rat primary cortical neurons and challenged them with pre-aggregated Aβ (1–42). The slides were immunostained with the neuronal marker MAP2 and then stained with TUNEL in situ cell death detection Kit. Aβ treatment provoked neuronal apoptosis as indicated by TUNEL assay. Neuronal apoptosis induced by Aβ was substantially blocked by 500 nM 7,8-DHF (Figures 1a and c). Some of the positive TUNEL signals were not overlapped with MAP2 (white arrow), indicating that other cell types but not neurons were dead. It has been demonstrated that AD patients present with an early and prominent loss of LC neurons (Chalermpalanupap et al, 2013; Matthews et al, 2002; Weinshenker, 2008). Accordingly, we also tested the effect of 7,8-DHF on Aβ-induced toxicity in LC neurons. 7,8-DHF significantly attenuated neuronal death induced by pre-aggregated Aβ. To determine whether the protective effect of 7,8-DHF is dependent on TrkB activity, we pretreated the neurons with K252, a Trk receptor inhibitor 30 min before 7,8-DHF introduction. K252 pretreatment completely blocked the protective effect of 7,8-DHF (Figures 1b and d), demonstrating that the protective effect of 7,8-DHF is dependent on TrkB activity.
Figure 1.
7,8-dihydroxyflavone (7,8-DHF) prevents Aβ-induced neurotoxicity in cultured cortical neurons and locus coeruleus (LC) neurons. (a) 7,8-DHF protected cortical neurons form Aβ toxicity. Cultured cortical neurons (DIV 12) were exposed to pre-aggregated Aβ (1–42, 20 μM) for 18 h in the presence or absence of 7,8-DHF (500 nM). Neurons were immunostained with neuronal marker MAP2. Neuronal apoptosis was detected by TUNEL staining. The neurons in apoptosis were indicated by white arrows. (b) 7,8-DHF protects LC neurons from Aβ toxicity. LC neurons were exposed to pre-aggregated Aβ (25–35, 20 μM) for 18 h in the presence or absence of 7,8-DHF (500 nM) and the Trk receptor inhibitor K252 (100 nM), and stained with tyrosine hydroxylase (TH) (red), DAPI (blue) and TUNEL (green). The percentage of apoptotic cells was determined by TUNEL staining. (c) Quantification of TUNEL-positive cells show that 7,8-DHF decreased the apoptotic rate of cortical neurons induced by Aβ. Data represent the mean±SEM from three independent experiments. *P<0.01. (d) Quantification of TUNEL-positive neurons shows that 7,8-DHF attenuated Aβ-induced apoptosis in LC neurons. The protective effect of 7,8-DHF was abolished by K252. Data represent the mean±SEM from three independent experiments. *P<0.01.
7,8-DHF Promotes Dendrite Branching and Synaptic Formation in vitro
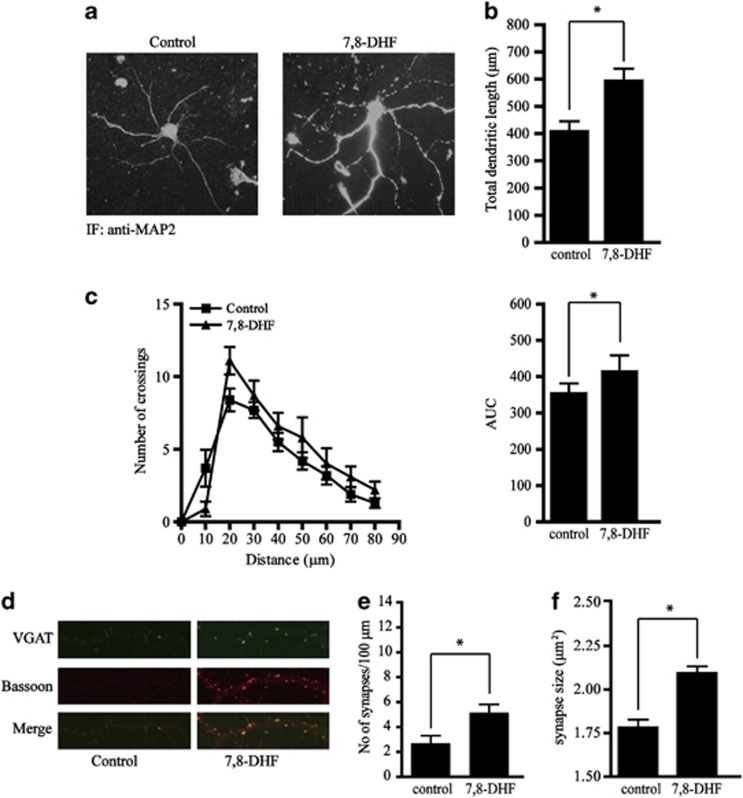
BDNF is required for normal dendritic morphology and synapse formation and maintenance (Gomes et al, 2006; Hiester et al, 2013). In order to test whether 7,8-DHF affects dendrite elongation, we assessed the dendritic length in the presence or absence of 7,8-DHF. We found that exposure to 500 nM 7,8-DHF for 3 days significantly increased the total dendritic length of primary cultured neurons (Figures 2a and b). We also tested the number of crossings between dendrites and calculated the area under the curve (AUC). The number of dendritic crossings was significantly increased by 7,8-DHF. These results indicate that 7,8-DHF promotes dendritic branching (Figure 2c).
Figure 2.
7,8-dihydroxyflavone (7,8-DHF) promotes synaptogenesis in primary cultured neurons. (a) Representative image of primary cortical neurons. The neurons were cultured in the presence or absence of 7,8-DHF (500 nM) for 3 days and immunostained with antibody to neuronal marker MAP2. (b) Quantification of total dendritic length. 7,8-DHF promoted dendritic elongation in primary neurons. (c) The number of crossings and area under the curve (AUC) following 7,8-DHF treatment.(d) The presynaptic structure of cultured neurons. Vehicle- or 7,8-DHF-treated neurons were double stained with the presynaptic markers VGAT (green) and bassoon (red). The number of synapses (e) and synapse size (f) was quantified. 7,8-DHF significantly promoted the number and size of neurons. Data represent the mean±SEM from three independent experiments. *P<0.01.
To further address the effect of 7,8-DHF on synaptic formation and maintenance in vitro, we stained vehicle- or 7,8-DHF-treated neurons with the presynaptic markers VGAT and bassoon. The density and structure of presynaptic structures were examined using immunofluorescent confocal microscopy. 7,8-DHF treatment increased the number of presynaptic structures expressing VGAT and bassoon. 7,8-DHF also increased synapse size (Figures 3d–f). These results suggest that 7,8-DHF promotes dendritic arborization and facilitates the formation and maintenance of synapses in vitro.
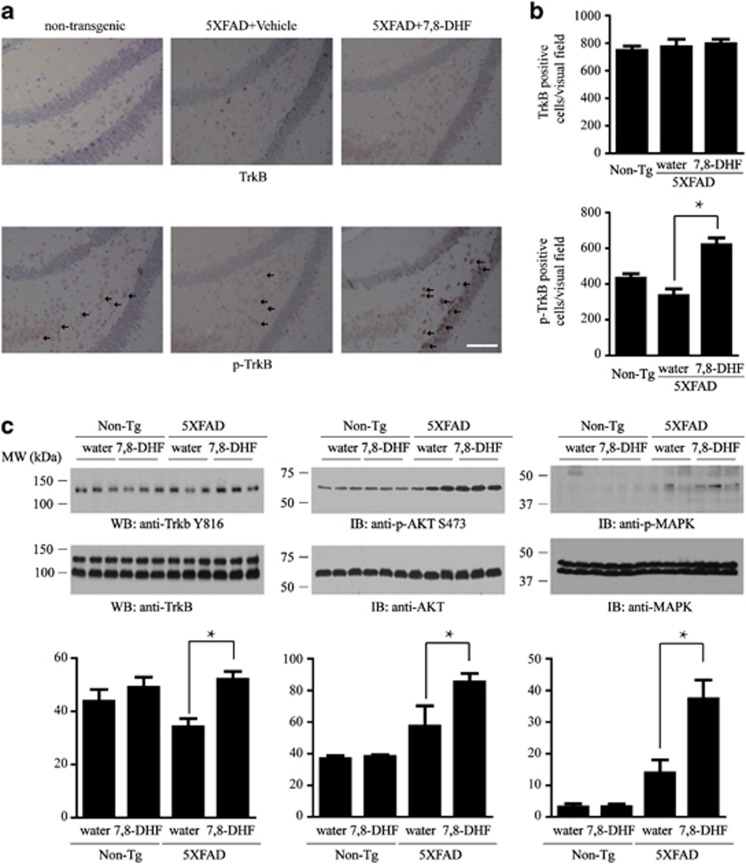
Figure 3.
7,8-dihydroxyflavone (7,8-DHF) elicits tropomyosin-receptor-kinase B (TrkB) and downstream signaling activation in 5XFAD mice. (a) Immunohistochemistry staining for TrkB and p-TrkB in 5XFAD brain sections. Two months old 5XFAD mice were treated with 7,8-DHF (5 mg/kg/day) or vehicle consecutively for 4 months. The phosphorylation of TrkB in dentate gyrus was detected by immunohistochemistry with anti-TrkB and anti-p-TrkB 816 antibody. Arrows indicate the p-TRKB-positive cells. Scale bar, 50 μm. (b) Quantification of p-TrkB-positive neurons in the dentate gyrus. Note that 7,8-DHF treatment elicited the phosphorylation of TrkB in 5XFAD mice. Data are shown as mean±SEM (n=n=3 mice per group). *P<0.01. (c) Immunoblotting analysis of the phophorylation of TrkB and its downstream signaling pathways Akt and ERK/MAPK. The level of p-TrkB, p-AKT and p-ERK/MAPK was increased by 7,8-DHF treatment, indicating that 7,8-DHF elicits TrkB and its downstream signaling pathways. n=3 mice per group. *P<0.05.
Chronic Oral Administration of 7,8-DHF Activates TrkB and Downstream Signaling Pathways in 5XFAD Mice
To explore whether oral administration of 7,8-DHF can activate TrkB in mouse brain, we supplied 7,8-DHF or vehicle in the drinking water of non-transgenic beginning at 2 months of age. After 4 months of drug treatment, we monitored TrkB activation in the brain by immunohistochemistry with anti-phosphorylated TrkB (p-TrkB) antibody. Quantitative analysis revealed that p-TrkB-positive neurons in 5XFAD mice appeared to be fewer compared with non-transgenic control, but the decrease was not statistically significant. However, 7,8-DHF treatment significantly elevated p-TrkB but not total TrkB levels in 5XFAD mice (Figures 3a and b). This result was confirmed by immunoblotting of the brain lysates. BDNF treatment elicits robust TrkB tyrosine phosphorylation and activates several downstream signaling pathways, such as PI3K/AKT and Ras/Raf/MAPK pathways. As expected, the TrkB receptor was more prominently phosphorylated in 5XFAD mice treated with 7,8-DHF than vehicle control, as were the downstream AKT and ERK/MAPK pathways (Figure 3c). Given the fact that 7,8-DHF can pass the blood–brain barrier (Liu et al, 2013), these results suggest that chronic oral administration of 7,8-DHF directly activates TrkB receptor and its downstream signaling pathways in the brain.
Chronic Oral Administration of 7,8-DHF Prevents Synaptic Loss in 5XFAD Mice
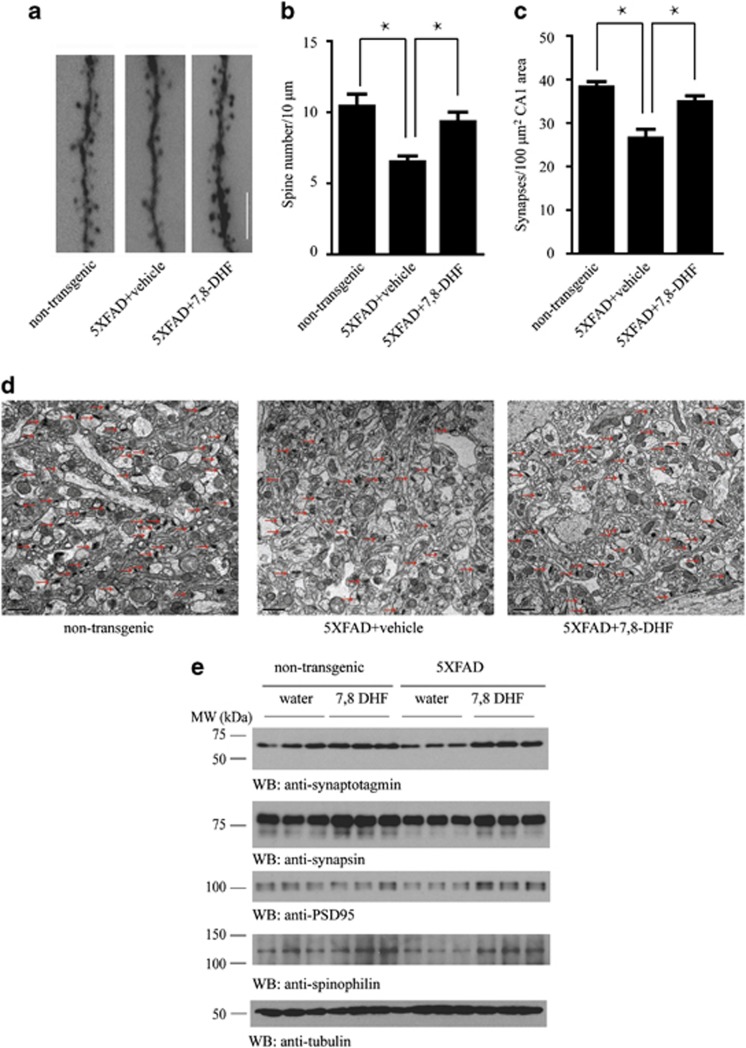
Synaptic loss is believed to be the basis of cognitive impairment in the early phase of AD (Scheff et al, 2011). In 5XFAD mice, significant synaptic loss and behavior deficits were detected at 5 months of age, in the absence of detectable neuronal loss (Hongpaisan et al, 2011). We first assessed the density of dendritic spines along individual dendrites of pyramidal neurons by Golgi stain. The density of dendritic spines was markedly decreased in the 5XFAD mouse model compared with the non-transgenic group, and this deficit was markedly rescued by 7,8-DHF treatment (Figures 4a and b). As each dendritic spine can form more than one synapse, we directly quantified the density of synapses in the CA1 area of 5XFAD mice brain by electron microscopy. 5XFAD mice showed a significant reduction in synaptic density compared with non-transgenic control mice. 7,8-DHF treatment notably reversed the loss of synaptic density (Figures 4c and d). We further confirmed these findings by immunoblotting using presynaptic markers (synaptotagmin and synapsin I) and postsynaptic markers (PSD95 and spinophilin). 5XFAD mice showed a significant decrease in these synaptic markers, indicating synaptic degeneration. 7,8-DHF treatment reversed the decrease of synaptic markers (Figure 4e). These results suggest that activation of TrkB receptors by 7,8-DHF inhibits the loss of synapse in 5XFAD mice.
Figure 4.
7,8-dihydroxyflavone (7,8-DHF) prevents the synaptic loss in hippocampal CA1 area of 5XFAD mice. (a) Golgi staining reveals the dendritic spines from apical dendritic layer of the CA1 region. Scale bar, 5 μm. (b) Quantitative analysis of the spine density. The decreased spine density in 5XFAD mice was reversed by 7,8-DHF. n=6 in each group, *P<0.01. (c) Quantitative analysis of the synaptic density in non-transgenic, vehicle- and 7,8-DHF-treated 5XFAD mice. 5XFAD mice show decreased synaptic density, which was reversed by 7,8-DHF. Data are shown as mean±SEM (n=3 mice per group). *P<0.01. (d) Representative electron microscopy of the synaptic structures. Arrows indicate the synapses. (e) Immunoblot analysis of synaptic markers in brain homogenates from mice treated with vehicle or 7,8-DHF. The expression of presynaptic markers (synaptotagmin and synapsin I) and post-synaptic markers (PSD95 and spinophilin) were reduced in 5XFAD mice, indicating synaptic degeneration. 7,8-DHF reversed the decrease of synaptic markers.
Chronic Oral Administration of 7,8-DHF Restores Synaptic Plasticity in 5XFAD Mice
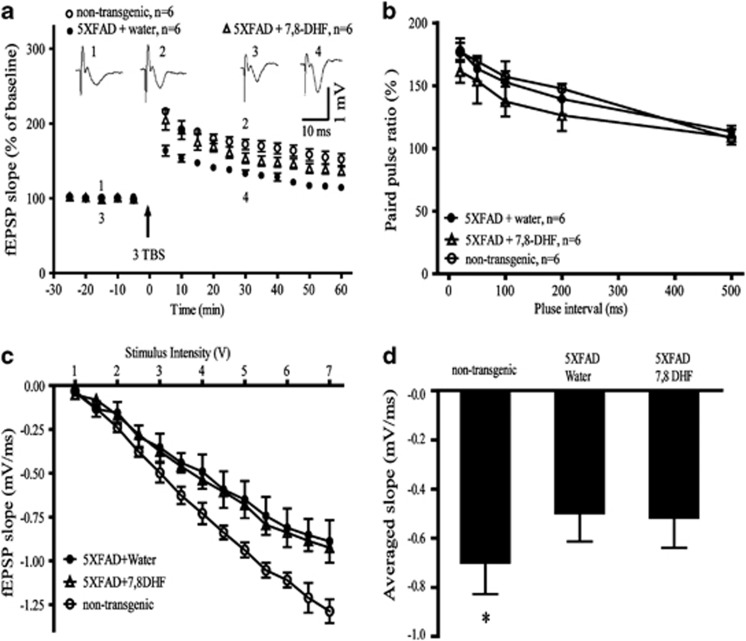
Long-term potentiation (LTP) is a measure of synaptic plasticity that underlies learning and memory. 5XFAD mice show significantly impaired LTP at the Schaffer collateral-CA1 pathways compared with non-transgenic mice (Kimura and Ohno, 2009). We examined whether chronic oral 7,8-DHF treatment can rescue LTP deficit observed in hippocampal slices from 5-month-old 5XFAD mice. As shown in Figure 5a, LTP magnitude was significantly decreased compared with the non-transgenic control mice, and the decreased LTP magnitude in 5XFAD mice was significantly reversed by 7,8-DHF treatment, suggesting a definite enhancement in synaptic plasticity by 7,8-DHF. We also tested PPF, which represents a short-term form of synaptic plasticity reflecting presynaptic function. Consistent with the previous report that PPF was not affected in 5XFAD mice (Kimura and Ohno, 2009), we found that the ratio of paired pulse was similar when comparing non-transgenic control, vehicle- and 7,8-DHF-treated 5XFAD mice (Figure 5b). Then we tested the basal synaptic transmission at CA1 synapses by measuring the input/output (I/O) curves. We found that the basal synaptic transmission was impaired in 5XFAD mice, and the I/O curve was not affected by 7,8-DHF treatment, indicating that 7,8-DHF did not rescue the basal synaptic transmission in 5XFAD mice (Figures 5c and d).
Figure 5.
7,8-dihydroxyflavone (7,8-DHF) restores synaptic plasticity in 5XFAD mice. (a) Long-term potentiation (LTP) of field excitatory postsynaptic potentials (fEPSPs) was induced by 3xTBS (theta-burst-stimulation) (four pulses at 100 Hz, repeated three times with a 200-ms interval). Shown traces are representative fEPSPs recorded at the time point 1 and 2 (vehicle treated), 3 and 4 (7,8-DHF-treated mouse). The magnitude of LTP in 5XFAD mice is significantly lower than in non-transgenic control mice, and 7,8-DHF treatment reversed the LTP impairment. n=6 in each group. Data are presented as mean±SEM. *P<0.05 vs vehicle-treated mice. (b) The paired-pulse facilitation (PPF) of non-transgenic, vehicle-treated 5XFAD, and 7,8-DHF-treated 5XFAD mice. The ratio of paired pulse is similar between two groups. The inter-pulse interval for paired pulse is 20, 50, 100, 200, and 500 ms. n=6 in each group. (c) Synaptic transmission assessed by input/output (I/O) relation between stimuli intensity and fEPSP slope. I/O curves obtained in hippocampal slices prepared from non-transgenic, vehicle-, and 7,8-DHF-treated 5XFAD mice. (d) Averaged slope of I/O curves is significantly greater in non-transgenic mice (P<0.05, vs vehicle- or 7,8-DHF-treated 5XFAD mice). There is no difference between vehicle- and 7,8-DHF-treated 5XFAD mice. n=6 in each group. Data are presented as mean±SEM and analyzed with two-way ANOVA.
Chronic Oral Administration of 7,8-DHF Alleviates Aβ Deposition But not The Concentration of Total Aβ
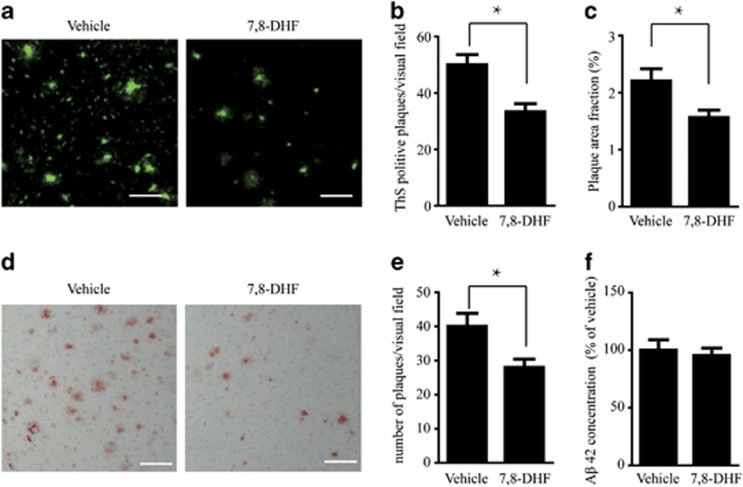
We further investigated the effect of 7,8-DHF treatment on senile plaque formation by Thioflavin-S staining. 5XFAD mice show significant plaque deposition at 6 months of age. Strikingly, the number of plaques in the hippocampus was significantly decreased in 7,8-DHF-treated mice as compared with vehicle control (Figures 6a and b). We further tested the deposition of Aβ by immunohistochemistry with anti-Aβ antibody. Aβ deposition was lower in 7,8-DHF-treated group when compared with mice given standard drinking water (Figures 6c and d). We also measured the concentrations of total Aβ42 by ELISA. Interestingly, total Aβ42 concentration in 5XFAD mice was not affected by 7,8-DHF treatment (Figure 6e). These results suggest that chronic oral 7,8-DHF may prevent Aβ deposition and plaque formation but not Aβ production.
Figure 6.
7,8-dihydroxyflavone (7,8-DHF) alleviates Aβ deposition but not the concentration of total Aβ. (a) Thioflavin-S staining of amyloid plaques in the hippocampus of 5XFAD mouse brain sections. Scale bar, 100 μm. (b and c) Quantitative analysis of amyloid plaques. The density of plaques (b) and the percentage of area occupied by Aβ deposits (c) in 5XFAD mouse brain were decreased by 7,8-DHF. n=3 mice per group, three sections per mice were analyzed. *P<0.01. (d) Immunohistochemistry of Aβ deposits in 5XFAD mice. Scale bar, 100 μm. (e) Quantitative analysis of amyloid plaques. Amyloid deposition in 5XFAD mice was significantly decreased by 7,8-DHF. n=3 mice per group, three sections per mice were analyzed. *P<0.01. (f) Aβ42 ELISA in vehicle- and 7,8-DHF-treated 5XFAD mice. n=4 in vehicle group, n=9 in 7,8-DHF group. 7,8-DHF did not change the concentration of total Aβ42.
Chronic Oral Administration of 7,8-DHF Rescues Memory Deficits in 5XFAD Mice
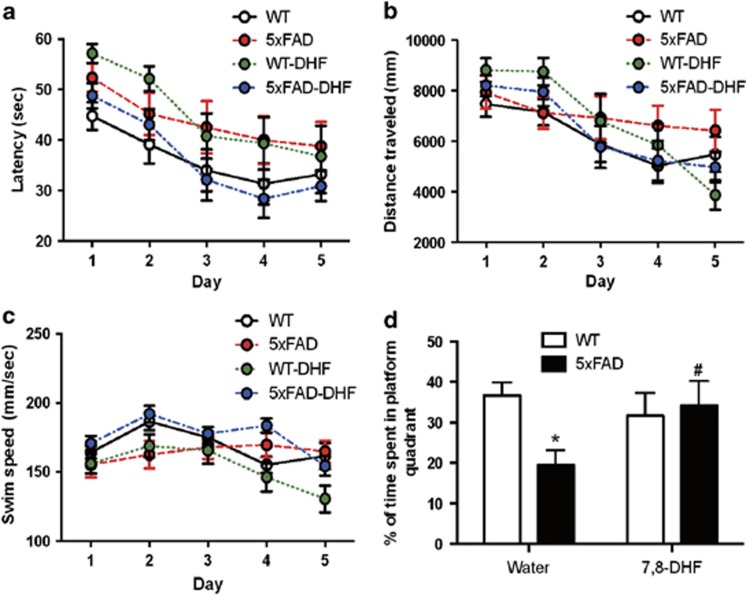
Hippocampal-dependent spatial memory of 5XFAD mice at 6 months of age was tested with the invisible platform task in the Morris water maze test. The average latency (Figure 7a), swim path length (Figure 7b), and swim speed (Figure 7c) for each of the 5 acquisition days were calculated and plotted. A two-way mixed analysis of variance (ANOVA) (Group × Training Day (repeated measure)) on latency revealed a main effect of training day (F (4, 156)=15.29, P<0.05) and group (F (3, 39)=3.88, P<0.05) but not interaction (F (12, 156)=0.4423, ns). A mixed two-way ANOVA on swim path distance revealed a significant main effect of training day (F (4, 156=14.81, P<0.05)) but no effect of group (F (3, 39)=0.84, ns) or interaction (F (12, 156)=1.16, ns). Separate one-way repeated measures ANOVAs on swim path distance revealed a significant effect of training day for water-treated wild-type (F (4, 40)=2.77, P<0.05), 7,8-DHF-treated wild-type (F (4, 28)=10.71, P<0.05), and 7,8-DHF-treated 5XFAD (F (4, 52)=6.48, P<0.05) mice, indicating significant learning over the 5 days of water maze training. Conversely, water-treated 5XFAD mice (F (4, 36)=0.58, ns) did not demonstrate this improvement. These results indicate that 5XFAD mice had impaired acquisition of the spatial learning task that was corrected by 7,8-DHF. A two-way mixed ANOVA on swim speed revealed a significant interaction between group and training day (F (12, 156)=2.387, P<0.05), and post hoc analysis revealed some small but inconsistent differences on various days. Analysis of the main effects of group on swim speed (F (3, 39)=3.412) revealed that, collapsed over the 5 training days, the only consistent effect of group on swim speed was that 7,8-DHF-treated wild-type mice swam significantly slower than 7,8-DHF-treated 5XFAD mice. Thus, it is highly unlikely that genotype or treatment effects on swim speed contributed to overall task performance, particularly as swim path distance is independent of swim speed.
Figure 7.
Effects of 7,8-dihydroxyflavone (7,8-DHF) on spatial learning and memory of wild-type (WT) and 5XFAD mice. Female WT and 5XFAD mice (n=10–14/group) given standard drinking water or 7,8-DHF dissolved in their drinking water were trained in the water maze over 5 days. Shown are mean±SEM latency to mount the escape platform (a), swim path length (b), and swim speed (c). A probe trial was performed on day 6 during which the platform was removed. Shown is the mean±SEM percentage of time spent in the target quadrant (d). *P<0.05 compared with vehicle treated wild-type mice, #P<0.05 compared with vehicle treated 5XFAD mice.
Assessment of memory recall for the platform location on the probe trial revealed impaired memory in water-treated 5XFAD mice and its rescue by 7,8-DHF (Figure 7d). A two-way ANOVA (Genotype × Treatment) performed on the percentage of time spent in the target quadrant during the 60 s probe trial indicated a significant interaction (F (1, 41)=2.293, P<0.05). Sidak's multiple comparisons revealed that, when compared with water-treated wild-type mice, water-treated 5XFAD mice spent a significantly lower percentage of their time in the quadrant that formerly contained the hidden platform. Comparison of the percentage of time spent in the target quadrant between water- and 7,8-DHF-treated 5XFAD mice revealed a significant difference between these groups (t (1,22)=2.293, P<0.05), demonstrating rescue of spatial memory recall by 7,8-DHF.
DISCUSSION
Our results in the present study demonstrate that 7,8-DHF mimics BDNF and displays neurotrophic actions by protecting cortical neurons and LC neurons from Aβ-induced toxicity, promotes dendritic arborization, and synaptogenesis in vitro. In the 5XFAD mouse model, chronic oral administration of 7,8-DHF activated TrkB signaling pathways in the brain, attenuated synaptic loss, reversed synaptic plasticity, Aβ deposition, and rescued spatial memory deficits. These results suggest that 7,8-DHF simulates the physiological actions of BDNF and prevents the synaptic dysfunction and cognitive deficits in a rodent model of AD.
Impairment of the BDNF-TrkB pathway has long been believed to have an essential role in AD pathogenesis. Accumulating evidence supports that BDNF provides a novel therapeutic strategy for AD treatment (Arancibia et al, 2008; Nagahara et al, 2009). Nevertheless, BDNF is a 25 KDa protein, and as such, possesses the intrinsic drawbacks of any polypeptide as a pharmacological agent, including in vivo instability and poor pharmacokinetics. To circumvent these issues, we screened thousands of compounds and successfully identified a small molecule 7,8-DHF, which selectively provokes TrkB but not TrkA activation in mouse brain upon i.p. or oral administration (Andero et al, 2012; Andero et al, 2011; Jang et al, 2010; Liu et al, 2010). We also found many flavone derivatives, such as chrysin (5,7-DHF) fails to active TrkB receptor, indicating that the 8-position hydroxy group is essential for flavone derivatives to bind TrkB receptor (Jang et al, 2010). Here we show that 7,8-DHF promotes both cortical and LC neuron survival in the face of Aβ-induced neurotoxicity. LC neurons are one of the earliest populations of neurons affected in AD and Parkinson disease, probably because that they are more vulnerable than other neuronal populations. Nevertheless, our in vitro result suggests that 7,8-DHF may be used to protect these neurons in the early phase of AD.
5XFAD mice have been shown to develop cerebral amyloid plaques at 2 months of age and show memory impairment at 4–5 months of age (Oakley et al, 2006). It has also been shown that the level of mature BDNF is dramatically reduced in 5XFAD mice, beginning at 3 months of age (Devi and Ohno, 2012). Given the key roles that BDNF-TrkB signaling has in learning and memory, we propose that our TrkB agonist 7,8-DHF may protect memory decline in 5XFAD mice. Here we show that TrkB receptors in the dentate gyrus were activated by 7,8-DHF treatment. The activation of TrkB downstream Akt and MAPK pathways were coupled to TrkB phosphorylation. Therefore, chronic oral administration of 7,8-DHF activates BDNF-TrkB signaling in the brain of 5XFAD mice. Finally, our results are consistent with the previous report that systematic administration of 7,8-DHF triggers TrkB activation in a transgenic mouse model of AD and in cognitively impaired aged rats by activating the TrkB signaling pathway (Devi and Ohno, 2012; Zeng et al, 2012). Interestingly, we noticed that 5XFAD mice did not show significant impairment of basal TrkB activity. Presumably, the decreased BDNF level is compensated by TrkB receptor hypersensitivity. However, we observed that 7,8-DHF triggered TrkB activation and exerted the protective effect. It is worth noting that in our experiment the increase of p-TrkB signaling is not as robust as in the Devi and Ohno's study. This may be due to the different basal TrkB activity. They used 12- to 15-month-old mice, which demonstrate significant impairment of TrkB signaling. We previously reported that 7,8-DHF binds to TrkB and quickly induces TrkB dimerization, phosphorylation, and the activation of downstream signaling pathways (Andero et al, 2011; Jang et al, 2010). Many different labs have independently reported that 7,8-DHF activates the TrkB signaling pathway in cortical neurons, motor neurons, retinal ganglion cells, dopaminergic neurons, and so on (Apawu et al, 2013; Gupta et al, 2013; Mantilla and Ermilov, 2012). All of these data suggest that 7,8-DHF exert its neurotrophic activity through TrkB. To confirm a small molecule indeed acts as a receptor agonist, the best evidence might be co-crystallization. However, the crystal structure of TrkB extracellular domain (ECD) has not been fully solved yet, we had not yet directly observed the co-crystal structure of the 7,8-DHF and TrkB complex.
Synaptic dysfunction is a major pathophysiological hallmark in AD and other neurodegenerative diseases. Substantial evidence indicates that there is a decrease in the number of synapses in AD patients and in patients with amnestic mild cognitive impairment (aMCI), a prodromal stage of AD (Scheff et al, 2011; Selkoe, 2002). Hence, synaptic dysfunction is an early event in AD pathogenesis. Furthermore, synaptic loss appears to be the best pathologic correlate of dementia in AD (Sze et al, 1997; Terry et al, 1991). It has been suggested that ‘synaptoprotective' therapy will probably be more clinical relevance than neuroprotective therapy (Coleman et al, 2004). BDNF/TrkB signaling has a direct role in the formation and maintenance of synapses (Gomes et al, 2006; Hiester et al, 2013). Like BDNF, 7,8-DHF can significantly promote dendritic elongation and arborization in primary cultured cortical neurons (Figure 2). Dendrites constitute >90% of the neuronal surface available for synaptic contact (Coleman et al, 2004). We also show that 7,8-DHF promotes synaptogenesis. Conceivably, our in vitro results indicate that 7,8-DHF may provide protective effects on synapses in AD. Activation of TrkB is required for multiple aspects of neuronal functions, including neuronal survival, morphological change of neurons, and synaptic plasticity (Bekinschtein et al, 2008; Diniz and Teixeira, 2011; Lu et al, 2013; Zuccato and Cattaneo, 2009). TrkB signaling promotes the formation of dendritic spines (Zeng et al, 2011). We observed a decrease in dendritic spine density in the hippocampus of 5XFAD mice, and that TrkB agonist 7,8-DHF increased the spine density in apical dendrites of CA1 neurons of hippocampus. In agreement with this observation, 7,8-DHF also exerted a beneficial effect on the number of synapses in the CA1 area of 5XFAD mice. Furthermore, the expression of synaptic markers was also increased by 7,8-DHF treatment, indicating that 7,8-DHF possesses the profound protective effects on synapses in vivo.
Several transgenic mouse models of AD show age-dependent deficits in hippocampal LTP (Chapman et al, 1999; Oddo et al, 2003), which correlate with the impairment in hippocampal-dependent memory. LTP is regarded as cellular mechanism for learning and memory. In the adult brain, the main functions of BDNF are to enhance synaptic transmission, facilitate synaptic plasticity, and promote synaptic growth. BDNF is also critical for LTP (Lu et al, 2013). It has been reported that BDNF promotes persistence of long-term memory storage through activation of ERK signaling (Bekinschtein et al, 2008). Like BDNF, 7,8-DHF can also promote the activation of ERK, a likely mechanism by which 7,8-DHF rescues LTP impairments in 5XFAD mice. In the water maze test, 7,8-DHF improved not only the acquisition of the spatial learning task but also the spatial memory recall. This is consistent with previous reports which demonstrated that 7,8-DHF improves spatial memory in APP transgenic mice(Devi and Ohno, 2012) and cognitively impaired aged rats (Zeng et al, 2012). We previously found that the brain 7,8-DHF peaked at 10 min with a concentration of 50 ng/g of the brain (about 200 nM) after oral administration of 50 mg/kg dosage (Liu et al, 2013). Here the mice received an approximate dose of 5 mg/kg/day. We estimate that the brain concentration in the present study is lower than the in vitro beneficial concentration of 500 nM. These results suggest that chronic exposure of the brain to lower concentration of 7,8-DHF is sufficient to exert the protective effect in vivo.
Polymerization of Aβ is believed to have a key role in the pathogenesis of AD. We tested whether 7,8-DHF interferes with Aβ aggregation. We found that 7,8-DHF reduced the formation of plaques as demonstrated by Thioflavin-S staining and Aβ immunohistochemistry. However, 7,8-DHF treatment did not affect total cerebral Aβ42 concentrations. Considering that numerous flavonoids exhibit inhibitory activity against Aβ42 aggregation (Sato et al, 2013). We cannot exclude the possibility that 7,8-DHF may also directly inhibit the aggregation of Aβ as well. Recently, it was reported that 10 days of i.p. 7,8-DHF administration decreased cerebral Aβ concentration and BACE1 expression in 5XFAD mice at 12–15 months old (Devi and Ohno, 2012). One of the possible reasons for this discrepancy is the different route of drug administration. Our oral administration pharmacokinetics data show that 7,8-DHF's oral bioavailability is around 5% and i.p. injection may display much higher levels of bioavailability (Liu et al, 2013). This difference might explain why p-TrkB signals in 7,8-DHF i.p. injected mouse brains are stronger than what we have shown in the brain lysates when 7,8-DHF was orally administrated. Moreover, 10 days of i.p. treatment may lead to higher drug levels, which may then suppress BACE1 expression, resulting in blockade of Aβ production, although 7,8-DHF does not inhibit BACE1 activity directly. On the other hand, the age of the mice may also contribute to this discrepancy. The 12–15 months old 5XFAD mice in the Devi and Ohno's study represent an advanced age of AD, whereas in our study the 5XFAD mice begun to receive 7,8-DHF at 2 months of age, which represent an earlier stage of disease progression. The expression of BACE1 in the brains of older 5XFAD mice is much higher than that in younger mice, which may make it easier to demonstrate the inhibitory effect of drugs (Zhang et al, 2009). Together, the difference in mice age and different routes of drug administration may result in different drug exposure in the brain, contributing to the different effects of 7,8-DHF on BACE1 expression in the two studies. Except for these differences, the present study differs from the Devi and Ohno's work in several ways. First, we focused on the effect of 7,8-DHF on synaptic dysfunction, because synaptic loss is the physical basis of cognitive alteration in AD. Demonstration of the ‘synaptoprotective' effect of 7,8-DHF will provide more clinically relevant information. In addition, we measured the effect of 7,8-DHF using the water maze test, because it is the most widely used test to measure hippocampal-dependent spatial-based learning and memory, which is early and prominent memory loss in AD patients, whereas the Devi and Ohno's study used Y-maze to test the general cognitive function.
In summary, this study demonstrates that chronic oral administration of 7,8-DHF exerts therapeutic effect in 5XFAD mice. This effect can largely be attributed to the protective effect of 7,8-DHF on synapses. Our study identifies 7,8-DHF as a novel ‘synaptoprotective' strategy for the treatment of AD and other neurodegenerative diseases.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by a grant from the National Institute of Health (RO1, DC010204) to K Ye, the NIH/NIAP50 ADRC center grant to both K Ye and D Weinshenker, and a grant from the National Natural Science Foundation of China (No. 81100958) to Z Zhang.
References
- Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22:399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apawu AK, Maina FK, Taylor JR, Mathews TA. Probing the ability of presynaptic tyrosine kinase receptors to regulate striatal dopamine dynamics. ACS Chem Neurosci. 2013;4:895–904. doi: 10.1021/cn4000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, et al. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, et al. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther. 2013;5:21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Liu X, Pradoldej S, Hao C, An J, Yepes M, et al. Phosphoinositide 3-kinase enhancer regulates neuronal dendritogenesis and survival in neocortex. J Neurosci. 2011;31:8083–8092. doi: 10.1523/JNEUROSCI.1129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci USA. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL. Brain-derived neurotrophic factor and Alzheimer's disease: physiopathology and beyond. Neuromolecular Med. 2011;13:217–222. doi: 10.1007/s12017-011-8154-x. [DOI] [PubMed] [Google Scholar]
- Gomes RA, Hampton C, El-Sabeawy F, Sabo SL, McAllister AK. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. J Neurosci. 2006;26:11487–11500. doi: 10.1523/JNEUROSCI.2364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013;49:96–104. doi: 10.1007/s12031-012-9899-x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiester BG, Galati DF, Salinas PC, Jones KR. Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci. 2013;56C:115–127. doi: 10.1016/j.mcn.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Chan CB, Kwon IS, Li X, Song M, Lee HP, et al. SRPK2 phosphorylates tau and mediates the cognitive defects in Alzheimer's disease. J Neurosci. 2012;32:17262–17272. doi: 10.1523/JNEUROSCI.3300-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongpaisan J, Sun MK, Alkon DL. PKC epsilon activation prevents synaptic loss, Abeta elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, et al. A Synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J Med Chem. 2010;53:8274–8286. doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qi Q, Xiao G, Li J, Luo HR, Ye K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology. 2013;91:185–200. doi: 10.1159/000346920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KL, Chen CP, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, et al. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Wakabayashi K, Tsuji S, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer's disease. Neuroreport. 1996;7:2925–2928. doi: 10.1097/00001756-199611250-00024. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Sato M, Murakami K, Uno M, Nakagawa Y, Katayama S, Akagi KI, et al. Site-specific inhibitory mechanism for amyloid-beta42 aggregation by catechol-type flavonoids targeting the Lys residues. J Biol Chem. 2013;188:23212–23224. doi: 10.1074/jbc.M113.464222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011;24:547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer's disease. Curr Alzheimer Res. 2008;5:342–345. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q. 7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J Neurochem. 2012;122:800–811. doi: 10.1111/j.1471-4159.2012.07830.x. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, et al. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang XM, Cai Y, Xiong K, Cai H, Luo XG, Feng JC, et al. Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2009;30:2271–2283. doi: 10.1111/j.1460-9568.2009.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]