Abstract
Measurements of adenosine 3′:5′-cyclic monophosphate (cAMP) concentrations have been made in Escherichia coli under various conditions. Different strains of E. coli accumulate different extracellular concentrations of cAMP (0.2-4 μm) at stationary phase. Mutation at the RNA control locus does not affect the accumulation pattern. Growth of the bacteria in minimalsalts medium leads to a greater accumulation of cAMP than growth in nutrient broth. Partition studies show that essentially all of the cAMP that is accumulated is found in the medium rather than in the cells. Kinetic studies show that most of the cAMP is formed coincidentally with exhaustion of glucose from the medium. Growth on high concentrations of glucose leads to inhibition of cAMP formation. Other carbon sources cannot substitute for glucose in this inhibitory effect. Measurements of enzyme activities indicate that glucose suppression of cAMP formation cannot be accounted for by a decreased activity of adenylate cyclase or an increased activity of cAMP phosphodiesterase (EC 3.1.3.7).
Keywords: growth, glucose ultilization, cAMP production, extracellular cAMP
Full text
PDF
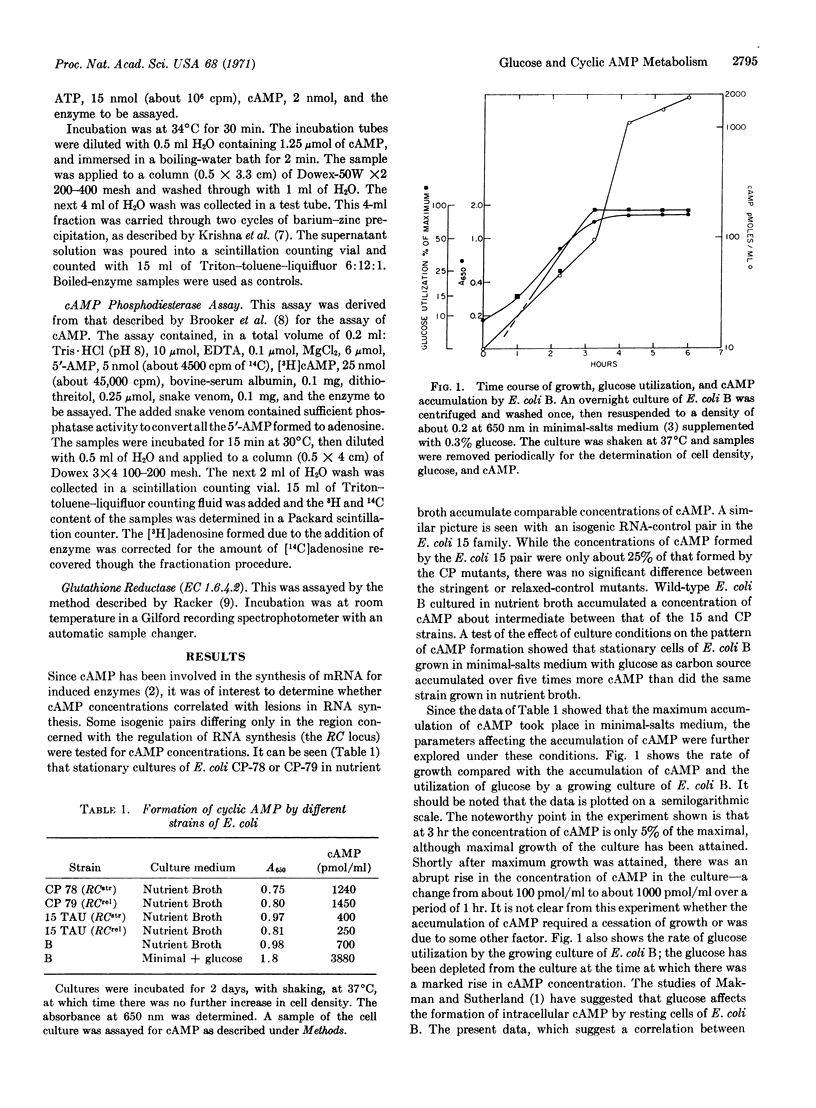

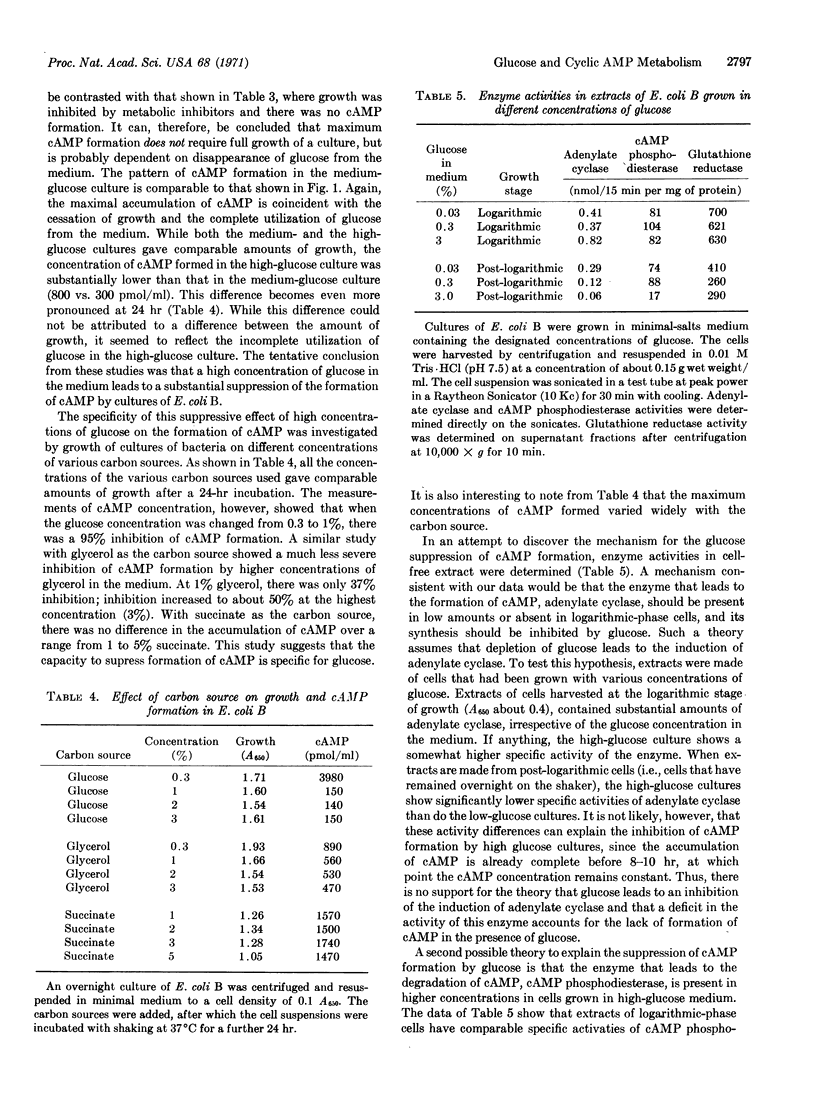

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y. Cyclic 3',5'-adenosine monophosphate phosphodiesterase produced by the slime mold Dictyostelium discoideum. Science. 1968 Jul 5;161(3836):57–59. doi: 10.1126/science.161.3836.57. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn T. M., Van De Meene J. G., Bonner J. T., Barkley D. S. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Tao M., Huberman A. Some properties of Escherichia coli adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):236–240. doi: 10.1016/0003-9861(70)90127-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


