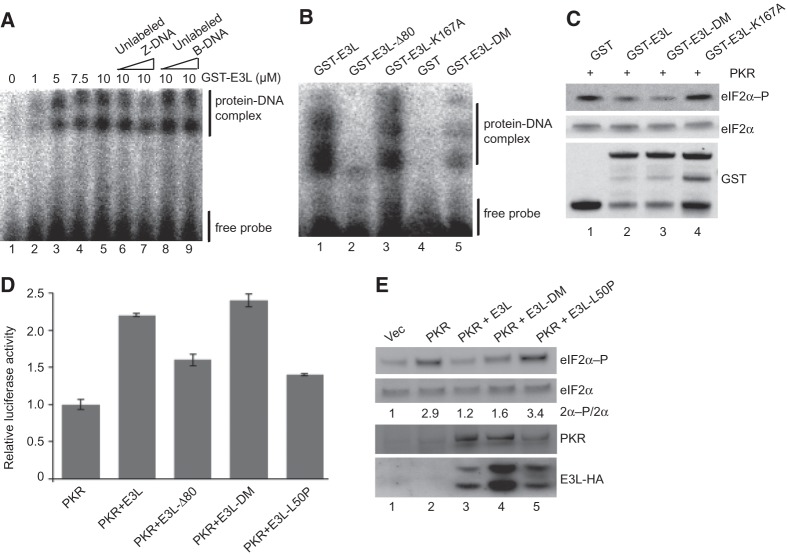
FIGURE 6.
E3L inhibition of PKR in vitro and in mammalian cells treated with dsRNA is independent of Z-DNA binding activity. (A) Electrophoretic mobility shift assay of GST-E3L binding to the Z-DNA substrate [32P]-labeled d(G:5BrC)20. Indicated concentrations of GST-E3L were incubated with labeled d(G:5BrC)20 in the presence of 30 nM or 300 nM unlabeled Z-DNA (lanes 6,7) or B-DNA (lanes 8,9). Positions of the GST-E3L–Z-DNA complex and free probe are indicated. (B) Z-DNA binding activity of E3L mutants. GST or the indicated GST-E3L derivative (5 µM) was incubated with the Z-DNA substrate and analyzed as described in A. (C) Z-DNA binding mutations do not impair E3L inhibition of PKR in vitro. The indicated GST or GST-E3L derivatives were incubated with purified Flag-His6-PKR and then mixed with His6-eIF2α1–200 and ATP. Reactions were resolved by SDS-PAGE and subjected to immunoblot analysis using antibodies specific for the Ser51-phosphorylated form of eIF2α (top panel) or using polyclonal antiserum against total yeast eIF2α (middle panel) or GST (bottom panel). (D) Human HeLa PKRkd cells were cotransfected with poly(I:C) (500 ng) and expression vectors for luciferase, knockdown-resistant human PKR (100 ng), and the indicated E3L-HA derivative (400 ng). After 40 h, cells were harvested, lysed, and extracts were assayed for luciferase activity. Luciferase activity was normalized to the transfections containing PKR but lacking E3L. Error bars indicate the standard deviation for three independent transfections. (E) Immunoblot analyses of WCEs from cells transfected as described in panel D. Blots were probed with antibodies specific for phosphorylated Ser51 in eIF2α (top panel), polyclonal antiserum against human eIF2α (second panel), monoclonal human PKR antibody (third panel), and monoclonal anti-HA antibody to detect HA-tagged E3L (bottom panel). The relative level of eIF2α-P to total eIF2α (2α-P/2α) was determined for three independent experiments using quantitative densitometry and Image J software (NIH) and normalized to the vector transformant.