Highlights
-
•
Primary invasive breast carcinoma can be found arising from within mammary-like glands in the vulva.
-
•
There is no standard management strategy for this rare disease; treatment recommendations should be similar to that for primary breast carcinoma.
-
•
The use of sentinel lymph node biopsy may offer another management option for this disease.
Keywords: Vulvar malignancy, Atypical mammary tissue
Introduction
While over 90% of vulvar malignancies are squamous cell carcinomas, other histologic types include melanomas, basal cell carcinomas, sarcomas, extramammary Paget's disease, and Bartholin gland adenocarcinomas. Although rare, breast tissue arising in the vulva can also have malignant potential. “Ectopic breast tissue” in the vulva was first described by Hartung in 1872, and since that time, several cases of both benign and malignant lesions arising in vulvar mammary tissue have been reported (van der Putte, 1994). The histology of the mammary-like tissue has multiple forms, ranging from simple, wide, slightly coiled tubular glands with a smooth outline, to more complicated forms in which the coiled structure has numerous branches and acini forming lobules. Similar to normal breast tissue, both epithelial and myoepithelial cells are present and the glands are subject to both the dysplastic and malignant changes that are seen in normal breast tissue (van der Putte, 1994). We report a case of a mammary-like invasive carcinoma presenting as an asymptomatic vulvar nodule.
Case
A 65 year old, multiparous, postmenopausal female status-post total hysterectomy with bilateral salpingo-oophorectomy 39 years prior for symptomatic myomatous uterus, presented for her annual well woman exam. She was without complaints and review of systems was negative; specifically, she denied any recent history of unintended weight loss or gain, fever, chills, nausea, melena or hematochezia, vaginal bleeding or discharge, abdominal pain or fullness. Her last mammogram was 1–2 years prior, after which she was diagnosed with right breast ductal papilloma which was excised. Colorectal screening was up to date.
In addition to her prior hysterectomy, gynecologic history was significant for estrogen replacement therapy for 20 years, which she stopped after developing what she reported as “vaginal cysts.” Two years prior to presentation, she had such a lesion removed from her right labia that had been present for one year and pathology was benign, per patient. On further discussion, she reported that the lesion had returned, was not painful, and was not associated with any further growth for the last six months. Clinical breast examination did not demonstrate evidence of palpable masses, retraction, skin changes, or axillary adenopathy. Genital examination revealed a mobile, soft, non-tender, 2.5 × 0.75 cm nodule at 11 o'clock on her right labia majora. Excisional biopsy was performed in clinic, which revealed ductal carcinoma in situ with positive margins. The patient then underwent a wide local excision of the area, which pathologically revealed invasive carcinoma with negative margins.
The specimen from the wide local excision revealed a surface which was predominantly dark brown and wrinkled with a smooth, white, flat lesion measuring 0.8 × 0.3 cm; the lesion was 1.2 cm from the 12 o'clock margin and 0.4 cm from the nearest (3 o'clock) margin. On serial sectioning, the lesion was firm and white, and extended to a depth of 0.3 cm.
Microscopic examination revealed invasive carcinoma, at least 1.6 mm in greatest dimension (Fig. 1), arising from within a background of mammary-like glands of the vulva demonstrating residual focal DCIS. The invasive lesion was noted to come within 1.3 mm of the margin, but all margins were negative for invasive disease. The histologic features were consistent with a primary carcinoma, not a metastatic lesion. Immunohistochemical (IHC) stains including smooth-muscle heavy chain (SMM-HC), CD 10, and p63 were performed and while there appeared to be some myoepithelial cells present along the smooth contoured edge by SMM-HC only, there were other areas without any definitive basal cell/myoepithelial cell staining (Fig. 2). The area of concern demonstrated an irregular growth pattern (no longer lobulocentric) and was not associated with the prior biopsy site changes. The residual mammary-like ducts (Fig. 3) exhibited strong immunoreactivity for all 3 IHC stains. The tissue was then tested for hormone receptor status, and was found to be estrogen receptor (ER) positive, but progesterone receptor (PR) and HER2/CEP17 negative.
Fig. 1.
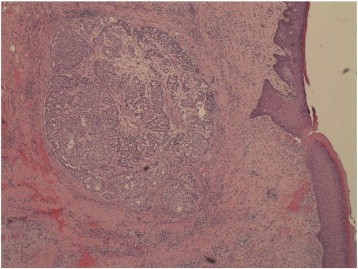
Invasive carcinoma in greatest dimension.
Fig. 2.
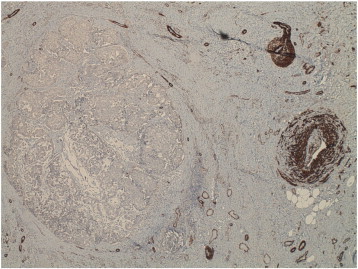
Myosin (SMM-HC) stain — This image demonstrates loss of the myoepithelial cell (MEC) layer, which is considered the gold standard for the diagnosis of invasive cancer. An intact MEC layer is seen in both benign and in situ lesions.
Fig. 3.
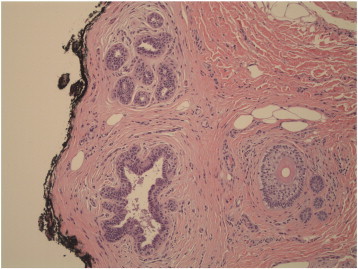
Focal mammary-like duct near the margins, without atypia.
Secondary to narrow margins, re-excision was performed as well as an ipsilateral groin sentinel lymph node dissection, both of which were negative for DCIS or malignancy. After referral to a medical oncologist, she was also administered letrozole, an aromatase inhibitor, as adjuvant therapy for the ER positive cancer. 18 months after excision, she is without evidence of recurrent disease.
Comment
A relative paucity of reports has documented complications of ectopic breast tissue in the vulva, beginning with Hartung in 1872. Mammary ridges were theorized to have developed in a line from the axilla to the groin, and while the large majority of these ridges would normally regress, it was possible for rudimentary tissue to persist and develop into supernumerary mammary tissue (van der Putte, 1994). These mammary-like glands have an unknown function, but were distinguished from eccrine and apocrine glands. The glands are simple tubular structures and are sparsely concentrated; however, in some women they are more numerous and assume more complicated forms that resemble mammary tissue (van der Putte, 1994). The more complicated forms contain the basic coiled structure, but are obscured by numerous branches and acini which form lobules. They are distinctly different from eccrine and apocrine glands by multiple factors including: 1) the type of epithelium, 2) the formation of diverticula, branches, and lobuli, 3) the shedding of clusters of epithelium, and 4) the expression of receptors for estrogen and progesterone.
Additionally, mammary-like glands are also noted to be different than normal mammary tissue derived from the mammary ridges for multiple reasons as well: 1) their simpler configuration, 2) the higher number of glands than would be expected in rudimentary elements from mammary ridges, and 3) the direct relationship to eccrine glands. Furthermore, based on human embryologic studies, the mammary ridges could not involve the anogenital area, as at the height of the formation of the mammary ridges in 9 mm and 10 mm embryos, the labia majora are still far from their first appearance (van der Putte, 1994). However, ectopic breast tissue has been described in the vulva, as well as in any other area of the body. Both ectopic breast tissue and mammary-like glands are susceptible to the physiologic, dysplastic, and malignant changes seen in normal breast parenchyma (Castro and Deavers, 2001), as there have been reported cases of invasive ductal carcinoma, ductal carcinoma in situ (DCIS), lobular carcinoma, mucinous adenocarcinoma, phyllodes tumors, and fibroadenomas in accessory breast tissue in the vulva (Lopes et al., 2006).
Although ectopic mammary tissue in the vulva is believed to be present in two to six percent of women, few cases of invasive ductal carcinoma have been reported in the literature (Kazakov et al., 2011). The most common clinical presentation is a painless, solitary nodule, arising most often in the labia majora. Patient age at presentation has been between 45 and 84 years, with most being 60 or older (Kazakov et al., 2011). Most carcinoma cases are histologically consistent with invasive ductal carcinoma, which is also the most common histology in orthotopic breast tissue (Kazakov et al., 2011). This still leaves a differential diagnosis of primary mammary carcinoma, carcinoma of a skin appendage, or metastatic mammary carcinoma. Recognition of an in situ component is the best clue to establishing the primary origin in the skin, as was seen in our case, rather than a metastatic process (Kazakov et al., 2011).
Secondary to the rarity of these lesions, there is no widely accepted recommended management strategy. A recent review only identified approximately 25 cases reported in the literature (Benito et al., 2013). Management strategies have ranged from wide local excision for DCIS to radical vulvectomy with bilateral inguinofemoral nodal dissection for invasive carcinomas (Irvin et al., 1999). Adjuvant therapies have included radiation, anthracycline-based chemotherapy, and hormonal therapy, in an attempt to decrease recurrence and improve survival. Patients that did not receive adjuvant therapy had a median survival of 4 months (Lopes et al., 2006). Only 1 patient out of 8 who received adjuvant therapy had died at the time of the previous publications, and the median survival was greater than 22 month (Lopes et al., 2006, Irvin et al., 1999). Not surprisingly, there have been no clinical trials to evaluate the effectiveness of chemotherapy and radiation in such a small number of patients, so information on treatment has been, and should continue to be, extrapolated from treatment of cancers in the breast — i.e. excisional procedure with or without lymph node dissection, followed by other adjuvant therapies as needed.
There is little to no data reporting on the utility of sentinel lymph node biopsy in this clinical scenario. Breast carcinoma is almost exclusively managed using sentinel lymph node mapping (Krag et al., 2010), while promising data support the use of this technique for vulvar cancer (Levenback et al., 2012). Vulvar cancer is ideally suited for sentinel lymph node mapping secondary to the predictable lymphatic drainage of the vulva, the low false negative rate, and the substantially reduced morbidity (e.g. lymphedema) compared to a full groin lymph node dissection (Levenback et al., 2012). We demonstrate this to be a potentially feasible technique for primary breast adenocarcinomas arising within the vulva in mammary-like glands, and in the context of such a rare histology, may be included in the management options of this disease.
Conflict of interest statement
None of the authors have any financial disclosures.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Presented at the Armed Forces District Annual Meeting of Obstetrics and Gynecology, San Diego, California, October 2011.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of San Antonio Military Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense or the U.S. Government.
References
- Benito V., Arribas S., Martinez D., Medina N., Lubrano A., Arencibia O. Metastatic adenocarcinoma of mammary-like glands of the vulva successfully treated with surgery and hormonal therapy. J. Obstet. Gynaecol. Res. 2013;39(1):450–454. doi: 10.1111/j.1447-0756.2012.01937.x. (Jan) [DOI] [PubMed] [Google Scholar]
- Castro C.Y., Deavers M. Ductal carcinoma in-situ arising in mammary-like glands of the vulva. Int. J. Gynecol. Pathol. 2001;20(3):277–283. doi: 10.1097/00004347-200107000-00012. (Jul) [DOI] [PubMed] [Google Scholar]
- Irvin W.P., Cathro H.P., Grosh W.W., Rice L.W., Andersen W.A. Primary breast carcinoma of the vulva: a case report and literature review. Gynecol. Oncol. 1999;73(1):155–159. doi: 10.1006/gyno.1998.5269. (Apr) [DOI] [PubMed] [Google Scholar]
- Kazakov D.V., Spagnolo D.V., Kacerovska D., Michal M. Lesions of anogenital mammary-like glands: an update. Adv. Anat. Pathol. 2011;18(1):1–28. doi: 10.1097/PAP.0b013e318202eba5. (Jan) [DOI] [PubMed] [Google Scholar]
- Krag D.N., Anderson S.J., Julian T.B., Brown A.M., Harlow S.P., Costantino J.P. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenback C.F., Ali S., Coleman R.L., Gold M.A., Fowler J.M., Judson P.L. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2012;30(31):3786–3791. doi: 10.1200/JCO.2011.41.2528. (Nov) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes G., DeCesare T., Ghurani G., Vincek V., Jorda M., Gluck S. Primary ectopic breast cancer presenting as a vulvar mass. Clin. Breast Cancer. 2006;7(3):278–279. doi: 10.3816/CBC.2006.n.041. (Aug) [DOI] [PubMed] [Google Scholar]
- van der Putte S.C. Mammary-like glands of the vulva and their disorders. Int. J. Gynecol. Pathol. 1994;13(2):150–160. doi: 10.1097/00004347-199404000-00009. (Apr) [DOI] [PubMed] [Google Scholar]


