Abstract
Background:
Buparvaquone (BPQ), a hydroxynaphthoquinone derivative, has been investigated for the treatment of many infections and is recommended as the gold standard for the treatment of theileriosis. Theileriosis, an intramacrophage infection is localized mainly in reticuloendotheileial system (RES) organs. The present study investigates development of solid lipid nanoparticles (SLN) of BPQ for targeted delivery to the RES.
Materials and Methods:
BPQ SLN was prepared using melt method by adding a molten mixture into aqueous Lutrol F68 solution (80°C). Larger batches were prepared up to 6 g of BPQ with GMS: BPQ, 2:1. SLN of designed size were obtained using ultraturrax and high pressure homogenizer. A freeze and thaw study was used to optimize type and concentration of cryoprotectant with Sf: Mean particle size, Si: Initial particle size <1.3. Differential scanning calorimetry (DSC), powder X-ray diffraction (XRD) and scanning electron microscope (SEM) study was performed on optimized formulation. Formulation was investigated for in vitro serum stability, hemolysis and cell uptake study. Pharmacokinetic and biodistribution study was performed in Holtzman rat.
Results:
Based on solubility in lipid; glyceryl monostearate (GMS) was selected for preparation of BPQ SLN. Batches of BPQ SLN were optimized for average particle size and entrapment efficiency at <100 mg solid content. A combination of Solutol HS-15 and Lutrol F68 at 2% w/v and greater enabled the desired Sf/Si < 1.3. Differential scanning calorimetry and powder X-ray diffraction revealed decrease in crystallinity of BPQ in BPQ SLN while, scanning electron microscope revealed spherical morphology. BPQ SLN revealed good stability at 4°C and 25°C. Low hemolytic potential (<8%) and in vitro serum stability up to 5 h was observed. Cytotoxicity of SLN to the U937 cell was low. The macrophage cell line revealed high (52%) uptake of BPQ SLN in 1 h suggesting the potential to RES uptake. SLN revealed longer circulation and biodistrbution study confirmed high RES uptake (75%) in RES organs like liver lung spleen etc.
Conclusion:
The high RES uptake suggests BPQ SLN as a promising approach for targeted and improved delivery in theileriosis.
KEY WORDS: Buparavaquone, solid lipid nanoparticles, targeting efficiency, theileriosis
Veterinary infections distress a large population animals and infection of livestock and companion animal is of serious concern, due to the propinquity of humans and transmission of disease to humans. Nanomedicines in targeted drug delivery to the cells in infectious diseases have been extensively investigated in the treatment of human disease. However, the application in the veterinary infection is still passive. The application of the nanomedicine in the therapy of veterinary infection could revolutionize animal health care especially in the developing countries where agriculture sector plays a major role in socioeconomic fabric of the country.
Buparvaquone (BPQ), a hydroxynaphthoquinone derivative, has been investigated for the treatment of many veterinary infections including leishmaniasis, pneumonia, cryptosporidiosis, equine piroplasmosis and is recommended as the gold standard for the treatment of theileriosis.[1,2,3,4] Mortality in untreated animals is very high. Moreover, almost all the recovered animals remain carriers for life, with growth and production potential never achieved. A cattle genetic improvement program focused on improving animal production; however, it suffered major setbacks as crossbreds are more vulnerable to the disease. A parasitic disease caused by the haemoprotozoan Theileria annulata and transmitted by ticks of the genus hyalomma, theileriosis is an intramacrophage infection of cattle that is localized mainly in the reticuloendotheleial organs namely lymph node, lymphocytes, spleen, lung and liver.[5] A strategy for targeted delivery of BPQ to the reticuloendotheileial system (RES) could therefore provide improved therapy in theileriosis.
In vitro cell uptake studies in monocytes and macrophage although limited, have shown considerable promise of high uptake of nanoparticles. Remarkable uptake of tacrolimus-loaded poly (lactic acid) nanoparticles in cultured human monocytes is reported,[6] whereas, poly-(lactic-co-glycolic acid) nanoparticles were readily taken up by peripheral blood mononuclear cells of a human immunodeficiency virus infected patient.[7] In another study, SMAD3 antisense oligonucleotides loaded solid lipid nanoparticles (SLN) were evaluated for uptake in activated murine macrophages for improved anti-inflammatory effect.[8] In vivo studies confirm high RES uptake of oridonin nanosuspension following intravenous (i.v) administration.[9] Our group has reported significant RES uptake of doxycycline hydrochloride loaded polymer lipid hybrid nanoparticles comprising gantrez as a polymer and various lipids following i.v administration.[10,11] Significant accumulation of microparticles of isoniazid and rifabutin in lung and liver has been demonstrated.[12] Ye et al. in their study observed significant accumulation of i.v injected actarit-loaded SLN in the lung, liver, spleen and kidney.[13] A biodistribution study of tetrandrine SLN following i.v administration revealed significant accumulation in the RES organs.[14] Furthermore, Shegokar et al. demonstrated higher accumulation and residence of stavudine entrapped lipid nanoparticles in the spleen and other lymphatic organs.[15]
Enhanced delivery to the RES organs has been reported even after oral and intrperitoneal administration of nanoparticles. Pandey et al. proclaimed that SLN of rifampicin, isoniazid and pyrazinamide accumulated in RES organ like lungs, liver and spleen up to 10 days following a single dose by oral administration.[16] Lamivudine and zidovudine loaded glyceryl monosterate (GMS)-P 188 SLN revealed higher uptake in the liver and spleen compared to the solution in mice following intraperitoneal administration.[17]
High RES uptake of SLN coupled with the relative safety of lipid based nanocarriers prompted us to investigate SLN of BPQ for targeted delivery to the RES in for improved efficacy in theileriosis.
Materials
BPQ (Intas Pharmaceutical, India), GMS (Gattefosse, India), trehalose (Hayashibara Co., Ltd., Japan), poloxamer 188 and Solutol HS 15 (BASF, India) were obtained as gift samples. Deionized water (Milli Q Plus system, USA) was used throughout the study. All other reagents/solvents used are either high-performance liquid chromatography (HPLC) grade or AR grade. Sodium acetate (Merk, India), methanol, acetonitrile and tetrahydrofuran (S.D Fine Chem, India) were purchased from their distributor.
Methods
BPQ solubility in different lipids
The lipids (200 mg) were maintained at 10°C above their melting point and a constant fraction of ~2 mg BPQ was added until no further drug dissolved. The lipids studied were GMS, glyceryl trimyristate (Dynsan 114), glyceryl tristearate (Dynsan 118), glyceryl behenate (Compritol) and glyceryl palmitostearate (Precirol).
Preparation of BPQ SLN
BPQ SLN was prepared by a reported melt method with some modifications.[18] Briefly, GMS (20-50 mg) was melted at 70-80°C and BPQ (10 mg) was added to the melt under stirring for 30 min. The melt was poured in to 1% or 2% w/v aqueous poloxamer 188 solutions at 80°C (30 mL). The resulting hot dispersion was subjected to probe sonication (DP120, Dakshin, India) for 10 min using a 10 s on/off cycle for 5 min. The dispersion was then cooled (5-10°C) in an ice bath with continuous stirring on a magnetic stirrer. The dispersion was centrifuged (Eltek 4100 D research centrifuge, India) at 10,000 rpm to separate free drug and surfactant. The nanoparticle pellet was dispersed in water using probe sonication. The ratios of GMS: BPQ evaluated were 2:1, 4:1 and 5:1.
Batches at larger scale (1, 2, 4 and 6 g BPQ) were prepared by maintaining GMS: BPQ ratio 2:1. The hot dispersion of BPQ SLN obtained as above was further subjected to a three step homogenization process. The first step involved homogenization using an ultra-turrax homogenizer (IKA T-18 basic, Germany) for 20 min at 3500 rpm followed by cooling for 20 min in an ice bath (5-10°C). In the second step, the chilled dispersion was subjected to high pressure homogenization (APV Gaulin, Germany). In the third step, 100 bar (10 cycle), followed by 200 bar (10 cycle) this fine dispersion was homogenized to obtain different batches at 500/1000/1200/1500 bar respectively. The homogenized dispersions were centrifuged at 10,000 rpm and the pellet separated. The supernatant obtained after centrifugation was suitably diluted with methanol and analyzed for BPQ by ultraviolet (UV) spectrophotometer (Shimadzu 1650 PC, Japan) at 252 nm. Percent entrapment efficiency (% EE) was calculated as follows;
% EE = ([BPQ] total−[BPQ] supernatant)/[BPQ] total × 100
The pellets were dispersed in filtered (0.22 μm) water by probe sonication.
In vitro Characterization
Drug loading
BPQ-SLN (freeze dried without cryoprotectant and stabilizer) equivalent to 2 mg of BPQ was dissolved in isopropyl alcohol (10 mL) by sonication. The solution was diluted suitably with the mobile phase (Refer stability study section) and filtered through a 0.45 μ filter (Millipore, Mumbai, India). The concentration of BPQ in BPQ SLN was determined by HPLC.
Particle size and zeta potential
The nanoparticles were centrifuged at 10,000 rpm for 30 min and the resultant pellet was dispersed in deionized water using a probe sonicator. The mean particle size (Sf) and zeta-potential of BPQ SLN were measured in triplicate at 25°C by dynamic light scattering (DLS) using Zetasizer Nano ZS (Malvern Instruments Ltd., UK). All the measurements were taken by scattering the He-Ne red laser light at 173°.
Freeze and thaw study
Freeze and thaw study was designed as a pre-test for optimization of type and concentration of cryoprotectant and/or stabilizers to be used during freeze drying.[19] An aliquot of 15 mL (~7 mg/mL BPQ) of nanoparticle dispersion was placed in amber color glass vials containing different type and concentrations of cryoprotectant and/or stabilizers. The dispersion was subjected to freezing at 40°C for a period of 6 h in a deep freezer (Eclipse 400 RS Biotech, UK) followed by thawing at 28°C. Samples were diluted suitably with filtered (0.22 μm) water and the particle size was measured. Ratio of Sf of the samples after freeze thawing to initial mean size (Si) was evaluated.
Freeze drying
The nanoparticles dispersion (~7 mg/mL BPQ) in filtered (0.22 μm) water was placed in a vial containing cryoprotectant and/or stabilizer. The dispersion was frozen at −40°C for 6 h and then subjected to drying using Labconco freeze dryer (FreeZone 4.5, USA). Drying was continued until 36 h and the condenser surface temperature was maintained at −54°C and vacuum pressure 54 × 10−3 bar.
Scanning electron microscopy study
The size and surface morphology of the BPQ SLN and blank SLN was analyzed using a SEM (JEOL JSM 6380) equipped with field emission. A small drop of BPQ SLN dispersion was deposited on an aluminum grid and allowed to air dry. The samples were sputtered with platinum using a coater (JEOL JSM 1600) and placed in the sample for examination.
In vitro drug release
In vitro release of BPQ SLN was determined by the dialysis bag method.[20] BPQ SLN dispersion in water (1 mL), equivalent to 5 mg of BPQ was filled in a dialysis bag (HIMEDIA®, Molecular weight cut off 12-14 kDa), which was placed in the basket of the USP Apparatus I (Electrolab, Mumbai, India). Release was commenced by submerging the basket (150 rpm) in 100 mL of 5% w/v aqueous SLS solution as dissolution medium at 37°C ± 0.2°C. An aliquot of 10 mL was withdrawn at predetermined time intervals up to 72 h and replaced with the same amount of medium. The aliquots were analyzed for BPQ by UV spectrophotometry at 278 nm. To study the release kinetics, data obtained from in vitro drug release studies were fitted into various kinetic models.
Differential scanning calorimetry (DSC) study
Thermal behavior of BPQ SLN and the SLN components was determined by DSC. Powdered samples were accurately weighed (5 mg) in aluminum pans, crimped and subjected to DSC under constant purging nitrogen at 20 mL/min using a Perkin Elmer Pyris 6 DSC (Perkin Elmer, Netherlands). Thermograms were recorded by heating samples from 35°C to 250°C at a heating rate of 10°C/min with empty aluminum pan as the reference.
Powder X-ray diffraction
X-ray diffraction spectra of BPQ SLN and SLN components were recorded at room temperature using PANalytical X’Pert PRO MPD θ/2 θ diffractometer (Almelo, Netherlands) with nickel filtered Cu Kα radiation operated at a voltage of 3 kV, 5 mA current. Scanning speed was adjusted at 4°/min and scanned at 7-70° (2 θ). Secondary side of the optical path consists of X’Celerator RTMS detector outfitted with beam monochromator.
Stability study of BPQ SLN
Freeze dried BPQ SLN was placed in glass vials stoppered with rubber cap and aluminum seal. The vials were stored at 4°C and 25°C ± 2°C to evaluate stability of the BPQ SLN. The SLN were monitored for particle size and drug content. BPQ in the SLN were quantified by dispersing the SLN equivalent to 2 mg of BPQ followed by isopropyl alcohol to adjust the volume and filtered through 0.45 μm filter (Millipore, India). The filtrate was diluted suitably with mobile phase and 100 μL was injected into HPLC (Jasco Instrument (PU-2080, Japan) attached with Waters spherisorb-C18 column and a UV-visible detector (UV-2075, Japan) at 252 nm. The mobile phase comprised of sodium acetate (0.05 M): Methanol acetonitrile: Tetrahydrofuran (30:20:20:30) and 0.1% v/v acetic acid at a flow rate of 1 mL/min. Data integration was performed using Borwin Chromatography software version 1.21 (JMBS developments, La Fontenil, France) and drug content was extrapolated from a linearity curve.
In vitro hemolysis study
An in vitro hemolytic study was carried out using previously reported method with slight modification.[21] Briefly, fresh rat blood (2 mL) was collected by puncturing the orbital sinus. Erythrocytes were separated by centrifugation at 3000 rpm for 15 min and washed thrice with phosphate buffer saline (PBS) to remove any protein remaining and other debris. Erythrocyte stock dispersion was prepared by making up the volume to 5 mL with PBS and allowed to equilibrate at 2-8°C for 24 h. Freeze dried BPQ SLN was dispersed in dextrose 5% w/v. BPQ SLN dispersion (1 mL) equivalent to 25 mg/mL BPQ was incubated at 37°C for 1 h with 100 μL of stock erythrocyte dispersion. The sample was centrifuged at 3000 rpm and 100 μL of supernatant was mixed with 2 mL of ethanol-HCl mixture (39 parts ethanol 99% v/v + l part HC1 37%, w/w). The absorbance of the mixture was recorded at 398 nm on a UV visible spectrophotometer against a blank sample. The blank sample was prepared using same procedure excluding the erythrocyte to eliminate the drug or excipient related interference. Triton X 5% v/v was used as a positive control and 0.9% w/v NaCl was used as a negative control. Hemolysis by the test sample was calculated using the following equation.
Hemolysis (%) = (Absorbance of sample) × 100/(Absorbance of Triton X 5% w/v)
In vitro serum stability study
The serum stability of BPQ SLN was evaluated with some modification in the method.[22] In brief, 0.5 mL (50 mg/mL) of BPQ SLN dispersion in water was incubated with 0.5 mL rat serum at 37°C ± 2°C. An aliquot (0.1 mL) was withdrawn at 0.25, 0.5, 0.75, 1, 2, 3, 4 and 5 h, diluted suitably with filtered water and evaluated for change in particle size by DLS.
Cytotoxicity and cell uptake study
Human macrophage cell line U-937 was purchased from the National Center for Cell Science, Pune, India. Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium with gentamycin and 10% v/v bovine fetal calf serum at 37°C and 5% CO2. Cell viability was evaluated by trypan blue dye exclusion technique. Briefly, cells (100 μL) from each well were mixed with Trypan Blue solution (100 μL; 0.16% w/v,) and cells were counted in a Neubauer hemocytometer. The cytotoxicity of BPQ SLN and BPQ was evaluated using the reported methylthiazoletetrazolium (MTT) assay.[23] Briefly, U-937 cells were plated at 2 cells/mL × 106 cells/mL density in 96-well plates. BPQ SLN was dispersed in filtered (0.22 μm) water and BPQ was dissolved in methanol water mixture (8:2). Calculated amount of stock solution was transferred to the cell suspension to obtain BPQ concentrations of 0.5, 1, 2, 5, 10, 20, 25, 50 and 100 μg/mL. The solutions were incubated in 5% CO2 at 1 and 24 h of incubation at 37°C, 5% with CO2, the medium was replaced with 100 μL of MTT solution and incubated for 4 h followed by addition of 100 μL of dimethyl sulfoxide to each well. The cell viability was determined by measuring the absorbance at 570 nm using an ELISA reader. Quantitative cell uptake was determined by incubating BPQ-SLN/BPQ (equivalent BPQ 2 μg/mL) with U-937 cell line (Density 2 cells/mL × 106 cells/mL) containing 10% fetal calf serum in RPMI media, at 37°C in presence of 5% CO2. At the end of 1 h the cells were separated from the non-internalized nanoparticles by centrifugation at 1000 rpm for 10 min. The cell pellet was lysed using aqueous solution of sodium dodecyl sulfate. The supernatant/lysed cell suspension was mixed with methanol: Acetonitrile (1:1) 200 μL to precipitate the proteins. The suspension was centrifuged and the supernatant analyzed by HPLC to determine BPQ in the supernatant and cell lysate to ensure mass balance. Percent BPQ uptake by cells was calculated.
In vivo Evaluation
Animals and husbandry conditions
The protocol was duly approved by Institute Animal Ethics Committee of the National Institute for Research in Reproductive Health, Mumbai, India (NIRRH) and the studies were performed according to the guidelines of committee for the purpose of control and supervision of experiments on animals, India. The rats were bred in the premises of NIRRH and were housed in polypropylene cages furnished with autoclaved corn cob bedding. Healthy male and female Holtzman rats (male/female) with average body weight (BW) 280 ± 20 g were used for the study. The ambience was controlled at 22 ± 1°C and humidity of 55-70%, in a 14 h light/10 h dark cycle was maintained throughout the study. The animals were provided with pelleted feed and filtered drinking water, ad libitum.
Pharmacokinetic study
Rats were separated in to two group based on sex. Freeze dried BPQ SLN were dispersed in water for injection (1 mg/mL equivalent to BPQ) and administered through tail vein (BPQ 1 mg/kg BW). Blood (250-500 μL) was collected in BD Microtainer® tube coated with dipotassium ethylenediaminetetraacetic acid at the end of 0.25, 0.5, 1, 3, 6, 12, 24 and 72 h by puncturing the retro orbital plexus. Blood was centrifuged at 3000 rpm for 15 min and plasma was stored at −70°C until HPLC analysis.
Biodistribution study
Freeze dried BPQ SLN were dispersed in water for injection (1 mg/mL equivalent to BPQ) and administered through tail vein. Six rats were randomly selected and sacrificed at the end of 0.25, 6, 12 and 24 h by cervical dislocation. Internal organs (lung, liver, spleen, heart, brain, uterus/testis and kidneys) were isolated. All the organs were pressed gently to remove the blood and blotted on the absorbent paper. The tissues were preserved at −70°C until complete analysis was performed. Organs were homogenized using a tissue homogenizer (Polytron PT 1300D, Switzerland) using PBS as a dispersing medium and BPQ content was analyzed by HPLC.
Overall targeting efficiency (TE) of BPQ loaded SLNs was calculated using following formula:[24]

The numerator refers to BPQ exposure to target organ and denominator refers to the sum total of BPQ exposure to all the tissues, including the target tissue (AUC0–∞)i
HPLC analysis
BPQ in various plasma/organ homogenates (100 μL) was spiked with internal standard parvaquone (100 μL) and BPQ was separated using methanol: Acetonitrile (1:1; 200 μL). The mixture was centrifuged at 18,000 rpm (10 min) and supernatant was analyzed by validated HPLC method. HPLC (Jasco Instrument (PU-2080, Japan) attached with Waters spherisorb ODS2 column (Pore size-80Å, Particle size-5 μ and width × length-4.6 mm × 250 mm) and a UV-visible detector (UV-2075, Japan) at 252 nm was used for the analysis. The mobile phase comprised of sodium acetate (0.05 M): Methanol: Acetonitrile: Tetrahydrofuran (30:20:20:30) and 0.1% v/v acetic acid at a flow rate of 1 mL/min. Data integration was done using Borwin Chromatography software version 1.21 and drug content was extrapolated from a linearity equation.
Statistical analysis
All the data are expressed as mean ± standard deviation. Vehicle control group were compared with the treatment group unless otherwise specified. Data was treated with one way analysis of variance followed by Dunnetts multiple comparison tests using GraphPad Prism 5 (La Jolla, CA 92037 USA), USA software. Difference was considered to be significant at P < 0.05.
Results and Discussion
Intracellular infections are onerous to eliminate using conventional drug delivery strategies especially when the parasite is localized intracellularly. Nanoparticles, by enabling high cellular uptake, are an important tool for effective therapy of intracellular infection. Conventional nanocarriers are rapidly cleared from circulation after i.v injection (~90%) by RES organs, mainly fixed macrophages in the liver, spleen and lung after opsonization by proteins present in the blood stream.[25] The clearance is significantly influenced by nanoparticle properties, with lipophilic particles exhibiting more rapid clearance.
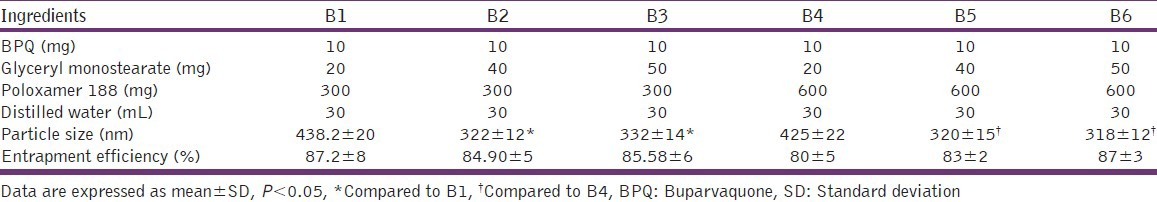
SLN have revolutionized nano-drug delivery systems, as their application has been extended from the delivery of simple hydrophobic molecules to the delivery of complex biomolecules like DNA/Si RNA.[26,27] Solid lipids are biocompatible, biodegradable and many are classified under generally recognized as safe (GRAS) category. SLN are ideally suited for hydrophobic drug such like BPQ, which has high log P - 5.3. Moreover, being lipophilic they present the possibility of high RES uptake hence an improved localization of drug at the infection site. Among the lipids studied the solubility of BPQ was found to be in range of 2-3% however maximum solubilization was found in GMS (3% w/w) hence GMS was selected for SLN preparation. An increase in the GMS concentration resulted in a significant reduction in particle size. An increase in the pluronic concentration also resulted in decrease in particle size. The decrease in size with surfactant is well reported[28] and attributed to lower surface tension, which enables smaller droplet formation. The reduction in size with GMS could be related to hydrophilic nature of GMS. The EE was however high and >80% in all the batches [Table 1].
Table 1.
Particle size and entrapment efficiency of BPQ solid lipid nanoparticles with different batches
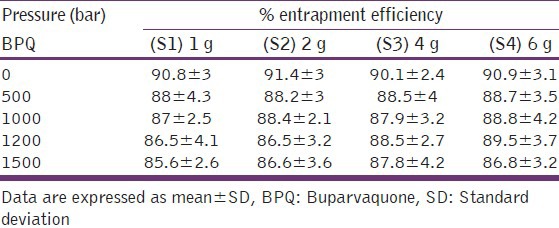
As the scale increased from 1 to 6 g BPQ, micron size particles were obtained [Table 2]. Further, a continuous increase in particle size was observed [Table 2]. Homogenization enabled decrease in particle size to the nano range. An optimal homogenization cycle was important. A decrease in particle size was seen up to a pressure of 1000/1200 bar, while at 1500 bar a considerable increase was observed [Table 2]. Homogenization, a high pressure operation, results in generation of heat during the process with the temperature directly related to pressure. At high homogenization pressure of 1500 bar it is possible that the temperature was high enough to cause agglomeration of the lipid nanoparticles. 1000 bar was considered as the optimal pressure for BPQ SLN and 10 cycles at this pressure were required. It is pertinent to note that at all scales high EE of >85% was observed [Table 3].
Table 2.
Effect of pressure on the average particle size of BPQ solid lipid nanoparticles

Table 3.
Effect of pressure on the entrapment of BPQ
The nanoparticle dispersions following centrifugation were subjected to freeze drying. Freeze drying is generally carried out in two stages namely freezing stage followed by drying. It is suggested that if nanoparticles are adequately protected during freezing stage the final freeze dried product would have a size comparable with initial size. The scientific basis of the freeze and thaw study is the underlying assumption that a cryoprotectant, which protects during the freezing stage, could be considered as effective for subsequent freeze drying.[19] Hence an initial freeze and thaw study was carried out using trehalose. Trehalose up to 4% w/v did not reveal adequate cryoprotection [Figure 1] on the basis of Sf/Si ratios. Hence, a combination of trehalose with number of other stabilizers was evaluated. The Sf/Si ratios are shown in Figure 2. Among the various stabilizers evaluated, the electrolytes sodium citrate, sodium phosphate and the poly anionic gantrez were unable to adequately protect the nanoparticles. Surfactants and long chain polymers are known to adsorb on the surface of particles and provide stabilization due to the surface-active property on rehydration or due to dehydration of surfactant during the freezing process forcing the surfactant to the particle surface, providing cryoprotective effect.[29]
Figure 1.

Effect of trehalose concentration on mean particle size/initial particle size ratio. Data are expressed as mean ± standard deviation
Figure 2.

Effect of cryoprotectant type and concentration on particle size of nanoparticles after freeze thaw cycling (mmean ± standard deviation [SD])* with 1% w/v trehalose. Data are expressed as mean ± SD
Among the surfactants AOT, Solutol HS-15 and combination of Solutol HS-15 with Lutrol F68 exhibited comparable Sf/Si ratio. A combination of Solutol HS-15 and Lutrol F68 at 2% w/v and greater enabled the desired Sf/Si < 1.3. Freeze drying of nanoparticles using a combination of trehalose 1% w/v Lutrol F68 + Solutol HS-15, 2% w/v (1:1) revealed Sf/Si 1.2, considered acceptable.[29] Our study again confirms the role of freeze and thaw study as an important screening test to limit the number of freeze drying experiments. Freeze dried BPQ SLN revealed a high drug loading of 30% ± 2.16% and spherical morphology confirmed by SEM [Figure 3]. The average particle size of freeze dried sample by DLS was 580.8 ± 12 nm. The negative zeta-potential-16.2 ± 0.38 mV suggested good colloidal stability.[30]
Figure 3.
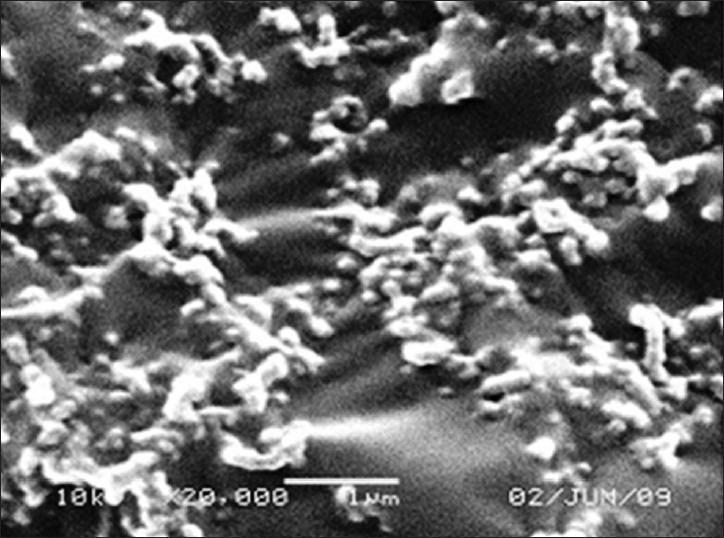
Scanning electron microscope photomicrograph of buparvaquone solid lipid nanoparticles
Sustained drug release was obtained for up to 72 h, which was best explained by first order or Higuchi model based on coefficient of correlation [Figure 4 and Table 4]. The SLN comprise of a lipid with a BPQ dispersed in the lipid matrix. Drug release from SLN would occur by diffusion of the drug through channels created within the insoluble matrix would hence be related to tortuosity and porosity of the nanoparticles explaining the t/2 kinetics.
Figure 4.

In vitro release profile of buparvaquone (BPQ) and BPQ solid lipid nanoparticles. Data are expressed as mean ± standard deviation
Table 4.
Drug release kinetic models

The DSC study revealed diffuse melting endotherm of BPQ SLN suggested reduced crystallinity of BPQ/partial amorphization of the drug in the lipid matrix [Figure 5]. X-ray diffraction spectra confirmed these observations [Figure 6]. BPQ SLN exhibited good stability with drug content 99.98 ± 1.4 and average size was 619 ± 15 nm even at the end of 12 months at 25°C/60% RH [Table 5].
Figure 5.

Differential scanning calorimetry thermogram of (1) Buparvaquone (BPQ) (2) Glyceryl monostearate and (3) BPQ solid lipid nanoparticle
Figure 6.

X-ray diffraction diffractogram of (1) Buparvaquone (BPQ) (2) Glyceryl monostearate and (3) BPQ solid lipid nanoparticle
Table 5.
Scanning electron microscope photomicrograph of buparvaquone solid lipid nanoparticles

Extensive hemolysis in vivo can lead to a severe anemic condition; hence the hemolytic potential of all i.v administered formulations must be evaluated especially when the formulation contains surfactants. Further the small size and unique physicochemical properties of nanoparticles may cause their augmented interactions with erythrocytes and hence determination of hemolytic properties is one of the most common tests in studies of nanoparticle interaction with blood components. According to several studies in vitro, percent hemolysis is rated as “no concern’ when it varies from 5% to 25% respectively.[31,32,33,34] BPQ SLN and SLN components showed <8% hemolysis suggesting their safety for i.v administration. BPQ SLN revealed no significant (P < 0.05) change in particle size in presence of serum up to 120 min was observed. The changes in SLN size after 120 min could be due to adsorption of serum proteins on the nanoparticles. Macrophage uptake is an important requisite for possible uptake by the RES in vivo. The human macrophage cell line U-937 represents a macrophage like cell line and hence was used to confirm the uptake of BPQ SLN by macrophages, in vitro.[35] The low cytotoxicity of BPQ SLN as observed from the MTT assay could be attributed to the GRAS status of the lipid [Figure 7].
Figure 7.

Percent cell viability of buparvaquone (BPQ) and BPQ solid lipid nanoparticles at 1 and 24 h using the methylthiazoletetrazolium assay. Data are expressed as mean ± standard deviation
Quantification of BPQ in U-937 cells revealed up to 52% uptake at 1 h. The HPLC method developed for detection of BPQ from plasma and organ homogenates was found to be specific and precise and could resolve the BPQ peak from other analyte peaks. Since a large number of steps is involved in the processing of biological samples, a close analogue of BPQ namely parvaquone was selected as internal standard. The limit of detection was 40 ng/mL and linearity of developed method was 50-5000 ng/mL. Average extraction efficiency was found to be >90%.
Pharmacokinetics and biodistribution of drugs can be altered by loading them into particulate carriers. Loading of BPQ in particulate carriers could reduce off target effect and hence toxicity related to BPQ, whereas simultaneously increasing the drug concentration at the target site, thereby improving therapeutic efficacy. The pharmacokinetic study revealed a biphasic curve with rapid drop in the plasma concentration suggesting BPQ SLN follows a two compartment models due to rapid uptake in RES organs [Figure 8]. Half-life of BPQ SLN was found to be more than 20 h, suggesting longer circulation of BPQ SLN. Plain BPQ was not evaluated as it was difficult to solubilize for i.v. administration. BPQ SLN revealed total overall TE of >75 in the RES organs spleen, liver and lung [Figure 9].
Figure 8.

Pharmacokinetic profile of buparvaquone solid lipid nanoparticles. Data are expressed as mean ± standard deviation
Figure 9.

Overall targeting efficiency of buparvaquone solid lipid nanoparticles following intravenous administration in rat. Data are expressed as mean ± standard deviation
The noteworthy finding of the study is substantially high TE (~35%) was observed at the end of 24 h in the spleen suggesting therapeutic benefit in theileriosis.[36] Similar observations have been reported by Yang et al. and Manjunath and Venkateswarlu[36,37,38] for campothecin SLN and clozapine SLN (average size ~ 200 nm) respectively, which demonstrated the substantial accumulation in liver and spleen. As the major load of the theilerial parasite is localized in the lymphatic organs[39] and in the organs of the RES namely liver and spleen, accumulation of BPQ SLN in these locations, could provide major therapeutic advantages. Moreover, the spleen being a lymphoreticular organ, splenic delivery of BPQ SLN essentially provides dual targeting, namely lymphatic as well RES targeting.[39,40] This could provide enhanced therapeutic advantage in diseases like Theileria.[41]
Conclusion
SLN represent an attractive nanocarrier system for the hydrophobic drug BPQ. High accumulation of BPQ SLN in the RES organs liver, spleen and lungs, suggests the possibility of improved therapy in theileriosis.
Acknowledgement
The authors are thankful to Department of Biotechnology, Ministry of Science and Technology, Government of India for funding the project (Project sanction no. [BT/PR/9908/NNT/28/66/2007]).
Footnotes
Source of Support: Department of Biotechnology, Ministry of Science and Technolgy, Government of India for funding the project (Project sanction no. (BT/PR/9908/NNT/28/66/2007)
Conflict of Interest: None declared.
References
- 1.Reimão JQ, Colombo FA, Pereira-Chioccola VL, Tempone AG. Effectiveness of liposomal buparvaquone in an experimental hamster model of Leishmania (L.) infantum chagasi. Exp Parasitol. 2012;130:195–9. doi: 10.1016/j.exppara.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Kaneshiro ES, Sul D, Basselin M, Kayser O. Pneumocystis carinii synthesizes four ubiquinone homologs: Inhibition by atovaquone and bupravaquone but not by stigmatellin. J Eukaryot Microbiol. 2001;(Suppl):172S–3. doi: 10.1111/j.1550-7408.2001.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 3.Zaugg JL. Buparvaquone in the treatment of equine piroplasmosis (Babesia equi) of European origin. Equine Pract. 1993;15:19–22. [Google Scholar]
- 4.Alidadi N, Dezfouli M, Rahbari S, Ali AM, Nouri M, Bahanar A, et al. Buparvaquone on Cryptosporidium parvum oocysts shedding in calf. Asian J Animal Vet Adv. 2008;3:275–7. [Google Scholar]
- 5.Manual on meat inspection for developing countries. Manual on meat inspection for developing countries 1994, version 119. 1994. [Accessed on 2013 May 15]. ISBN no. 9251033048. Available from: http://www.fao.org/docrep/003/t0756e/t0756e04.htm .
- 6.Tammam S, Mathur S, Afifi N. Preparation and biopharmaceutical evaluation of tacrolimus loaded biodegradable nanoparticles for liver targeting. J Biomed Nanotechnol. 2012;8:439–49. doi: 10.1166/jbn.2012.1403. [DOI] [PubMed] [Google Scholar]
- 7.Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin SE, Kim CK. Long-term stable cationic solid lipid nanoparticles for the enhanced intracellular delivery of SMAD3 antisense oligonucleotides in activated murine macrophages. J Pharm Pharm Sci. 2012;15:467–82. doi: 10.18433/j3z312. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Zhang D, Chen M, Duan C, Dai W, Jia L, et al. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int J Pharm. 2008;355:321–7. doi: 10.1016/j.ijpharm.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Patil RR, Gaikwad RV, Samad A, Devarajan PV. Role of lipids in enhancing splenic uptake of polymer-lipid (LIPOMER) nanoparticles. J Biomed Nanotechnol. 2008;4:359–66. [Google Scholar]
- 11.Devarajan PV, Jindal AB, Patil RR, Mulla F, Gaikwad RV, Samad A. Particle shape: A new design parameter for passive targeting in splenotropic drug delivery. J Pharm Sci. 2010;99:2576–81. doi: 10.1002/jps.22052. [DOI] [PubMed] [Google Scholar]
- 12.Verma RK, Kaur J, Kumar K, Yadav AB, Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob Agents Chemother. 2008;52:3195–201. doi: 10.1128/AAC.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, Wang Q, Zhou X, Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm. 2008;352:273–9. doi: 10.1016/j.ijpharm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Ji Z, Zou M, Nie X, Shi Y, Cheng G. Preparation, characterization, pharmacokinetics and tissue distribution of solid lipid nanoparticles loaded with tetrandrine. AAPS Pharm Sci Tech. 2011;12:1011–8. doi: 10.1208/s12249-011-9665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shegokar R, Singh KK, Müller RH. Production and stability of stavudine solid lipid nanoparticles – From lab to industrial scale. Int J Pharm. 2011;416:461–70. doi: 10.1016/j.ijpharm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Pandey R, Sharma S, Khuller GK. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis (Edinb) 2005;85:415–20. doi: 10.1016/j.tube.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Sankar V, Nareshkumar PN, Ajitkumar GN, Penmetsa SD, Hariharan S. Comparative studies of lamivudine-zidovudine nanoparticles for the selective uptake by macrophages. Curr Drug Deliv. 2012;9:506–14. doi: 10.2174/156720112802650707. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz C, Mehnert W, Lucks JS, Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release. 1994;30:83–96. [Google Scholar]
- 19.Date PV, Samad A, Devarajan PV. Freeze thaw: A simple approach for prediction of optimal cryoprotectant for freeze drying. AAPS Pharm Sci Tech. 2010;11:304–13. doi: 10.1208/s12249-010-9382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guhagarkar SA, Gaikwad RV, Samad A, Malshe VC, Devarajan PV. Polyethylene sebacate-doxorubicin nanoparticles for hepatic targeting. Int J Pharm. 2010;401:113–22. doi: 10.1016/j.ijpharm.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Bock TK, Müller BW. A novel assay to determine the hemolytic activity of drugs incorporated in colloidal carrier systems. Pharm Res. 1994;11:589–91. doi: 10.1023/a:1018987120738. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–34. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Lecároz C, Blanco-Prieto MJ, Burrell MA, Gamazo C. Intracellular killing of Brucella melitensis in human macrophages with microsphere-encapsulated gentamicin. J Antimicrob Chemother. 2006;58:549–56. doi: 10.1093/jac/dkl257. [DOI] [PubMed] [Google Scholar]
- 24.Wang JX, Sun X, Zhang ZR. Enhanced brain targeting by synthesis of 3’,5’- dioctanoyl-5-fluoro-2’- deoxyuridine and incorporation into solid lipid nanoparticles. Eur J Pharm Biopharm. 2002;54:285–90. doi: 10.1016/s0939-6411(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 25.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 26.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Bondì ML, Craparo EF. Solid lipid nanoparticles for applications in gene therapy: A review of the state of the art. Expert Opin Drug Deliv. 2010;7:7–18. doi: 10.1517/17425240903362410. [DOI] [PubMed] [Google Scholar]
- 28.Quintanar-Guerrero D, Ganem-Quintanar A, Allémann E, Fessi H, Doelker E. Influence of the stabilizer coating layer on the purification and freeze-drying of poly (D, L-lactic acid) nanoparticles prepared by an emulsion-diffusion technique. J Microencapsul. 1998;15:107–19. doi: 10.3109/02652049809006840. [DOI] [PubMed] [Google Scholar]
- 29.Saez A, Guzmán M, Molpeceres J, Aberturas MR. Freeze-drying of polycaprolactone and poly (D, L-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur J Pharm Biopharm. 2000;50:379–87. doi: 10.1016/s0939-6411(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 30.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin K, Dannenfelser RM. In vitro hemolysis: Guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173–6. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- 32.Krzyzaniak JF, Raymond DM, Yalkowsky SH. Lysis of human red blood cells 1: Effect of contact time on water induced hemolysis. PDA J Pharm Sci Technol. 1996;50:223–6. [PubMed] [Google Scholar]
- 33.Krzyzaniak JF, Alvarez Núñez FA, Raymond DM, Yalkowsky SH. Lysis of human red blood cells. 4. Comparison of in vitro and in vivo hemolysis data. J Pharm Sci. 1997;86:1215–7. doi: 10.1021/js970184o. [DOI] [PubMed] [Google Scholar]
- 34.Krzyzaniak JF, Yalkowsky SH. Lysis of human red blood cells. 3: Effect of contact time on surfactant-induced hemolysis. PDA J Pharm Sci Technol. 1998;52:66–9. [PubMed] [Google Scholar]
- 35.De Jaeghere F, Allemann E, Feijen J, Kissel T, Doelker E, Gurny R. Cellular uptake of PEO surface-modified nanoparticles: Evaluation of nanoparticles made of PLA: PEO diblock and triblock copolymers. J Drug Target. 2000;8:143–53. doi: 10.3109/10611860008996860. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Zhu J, Lu Y, Liang B, Yang C. Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm Res. 1999;16:751–7. doi: 10.1023/a:1018888927852. [DOI] [PubMed] [Google Scholar]
- 37.Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107:215–28. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Bansal GC. Bovine theileriosis in India: An overview. Proc Natl Acad Sci India. 2005;75:134–43. [Google Scholar]
- 39.Dhar S, Malhotra DV, Bhushan C, Gautam OP. Chemoimmunoprophylaxis with buparvaquone against theileriosis in calves. Vet Rec. 1987;120:375. doi: 10.1136/vr.120.15.375-c. [DOI] [PubMed] [Google Scholar]
- 40.Dingler A, Blum RP, Niehus H, Müller RH, Gohla S. Solid lipid nanoparticles (SLN/Lipopearls) – A pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J Microencapsul. 1999;16:751–67. doi: 10.1080/026520499288690. [DOI] [PubMed] [Google Scholar]
- 41.Anandaraja R, Ahmed NM, Hafeez M. 01. S-2. India: Achrya N.G. Ranga Agricultural University; 2001. Theileriosis in crossbred cattle - A report on autopsy examination. Paper Read at the Twelfth National Congress of Veterinary Parasitology; p. 45. [Google Scholar]


