Abstract
Background:
The change in the reaction time of a tail or paw exposed to a thermal stimulus is a measure of nociceptive activity in laboratory animals. Tail-flick and plantar thermal sensitivity (Hargreaves) tests are non-invasive, minimize stress, and can be used to screen animals for phenotype and drug activity.
Objective:
Hargreaves testing has been widely used in rats. We investigated its use to measure the activity of opiate analgesia in mice.
Methods:
Mice were used in thermal stimulus studies at 1-5 hours and 1-5 days to test acute and extended release preparations of buprenorphine.
Results:
Hargreaves testing had limited value at 1-5 hours because mice can have an obtunded response to opiate therapy. Tail-flick studies with restrained mice are not affected by the initial locomotor stimulation.
Discussion:
The present report describes a simple restraint system for mice. The utility of the system is demonstrated by examining the efficacy of acute and extended release buprenorphine injections in Balb/c and Swiss mice.
Conclusion:
Standardized tail-flick testing provides a sensitive robust method to monitor opiate activity in mice.
KEY WORDS: Analgesia, buprenorphine, extended release, Hargreaves test, mouse, thermal latency test
Thermal latency tests are used to screen phenotypes, study drug development and investigate nerve conduction. One method consists of securing the animal, placing its tail in hot or cold water and recording the time (latency) it takes for the animal to “flick” or withdraw its tail from the thermal stress.[1] A second method places the unrestrained animal on a transparent plate. Radiant heat from a source is focused through the plate on the bottom surface of a foot pad. The time to move the foot in the control versus the test animal is the recorded as the thermal latency. The latter plantar thermal sensitivity tests are often referred to as the Hargreaves test based on their eponymous developer.[2]
Recent progress in Hargreaves protocols have been based on improved instrumentation. Vendors have developed equipment using laser heat sources, precise timing systems and chambers for mice and rats. We attempted to study the duration of action of a long-acting buprenorphine suspension in Balb/c mice using a Hargreaves system. Although these systems have been used to study sustained release analgesia in rats,[3] we found a challenge to their use in Balb/c mice. Buprenorphine analgesia produced an obtunded response. The mice stumbled in the test chamber from 4 to 6 h. The activity prevented short-term measurements of plantar thermal sensitivity. Tail-flick assays with restrained mice avoid this complication. However, the literature on tail-flick assays affords limited detail on the methods to restrain the mice. Free-hand restraint of mice with or without a towel is described in several reports. These methods are difficult to standardize because individual laboratory personnel will vary the amount of restraint and stress used to restrain the animal. Moreover, free-hand restraint may be contraindicated in surgically-treated animals. This complication is especially sensitive in our research on the spinal cord trauma.
Several investigators report the use of commercial tubes and cage-restraints. These products appear to work fine, but are tedious to clean and sterilize when multiple animals are screened. The present report describes the use of disposable tubes to restrain mice for measuring short and long-term opiate activity. Due to its simplicity, sensitivity and robust performance, the method may have general application in nociceptive research.
Materials and Methods
Animals
The JHU center is Assessment and Accreditation of Laboratory Animal Care accredited. The protocol was approved by the JHU Institutional Animal Care and Use Committee.
Male and female Balb/c mice (6-8 weeks old weighing 20-22 g) were obtained from Charles River Laboratories (Wilmington MA). Swiss mice SWR/J (6-8 weeks old weighing 15-17 g female; 24-26 g male) were obtained from The Jackson Laboratories (Bar Harbor ME). Mice were inspected for general health conditions before being housed at a density of 4 mice/cage in Smart Bio-Pak cages (Allentown NJ) and were allowed free access to Teklad Global Rodent Diet chow (Harlan, Madison WI) and Baltimore City water.
Drug solutions
United States Pharmacopeia grade buprenorphine HCl (Noramco, Wilmington DE), cholesterol, glycerol tristearate, (Sigma, St. Louis MO) and medium chain triglyceride (MCT) oil 812N (Sasol, Hamburg Germany) were used to prepare the drug and control subcutaneous (SC) injections. Solutions for osmotic pumps and acute injections of 0.05-0.10 mL were prepared by dissolving the drug in 0.1% lactic acid in sterile water. Pumps contained 0.75 mg/mL solutions of buprenorphine in 0.1% lactic acid.[4] Negative controls contained saline. Pump model 1002 (Durect Corporation, Cupertino CA) was used for Hargreaves tests. Long-acting injections were prepared from a drug powder supplied by Animalgesics Lab (Millersville MD). The drug powder prepared as described by Pontani and Misra contained 1.5% buprenorphine, 94.5% cholesterol and 4% triglyceride (w/w/w).[5] Injectable suspensions of 0.05 mL of the drug powder were prepared by suspending 80 mg of drug powder/1 mL of MCT oil. Drug-free, negative control powder was prepared by dry-tumble blending a mixture of cholesterol and glycerol tristearate (96/4, w/w) for 48 h at 5°C.
Surgery
The surgical procedure used was based on the procedure used to implant mini-osmotic pumps in mice and rats. Access to approximately 11,000 publications and a video of the procedure, which is briefly described below, are available at the Alzet website.[6] Mice were given intraperitoneal anesthesia with a solution containing 25 mg/mL ketamine plus 2.5 mg/mL xylazine and 14.25% ethanol in saline. The dose of anesthesia was 0.15 mL/20 g mouse.
Following anesthesia, approximately 1 cm square of mid dorsal skin was shaved, washed with ethanol and then coated with Betadine. Mice were transferred to a procedural table that was cleaned with 70% ethanol solution and covered with a clean disposable towel. A sterile disposable no. 10 blade was used to make a 4-5 mm incision through the skin only. Bleeding, if any, was controlled with sterile gauze and light pressure. Sterile forceps were used to separate the skin and to create approximately a 2 cm × 4 cm SC pocket to accommodate the osmotic pump. The skin was then apposed and stapled. The same procedure was used as a sham surgery (no pump placement) in mice receiving long-acting drug injections. After the skin was stapled, mice were injected with drug or control suspension into the interscapular subcutis under the scruff of the dorsal neck.
Buprenorphine assay
Blood was collected by serial bleeds.[7] Plasma samples were examined by an enzyme-linked immunosorbent assay for buprenorphine.[8,9] Standards curves were prepared with solutions containing 0.0, 0.01, 0.05, 0.1, 0.5 and 1.0 ng/20 uL of buprenorphine. The absorbencies were recorded at 450 nm (reference wavelength: 650 nm) using a Perkin Elmer 1420 Vector3 micro plate reader with Wallac 1420 data manager software. Analyses of the standard curve and extrapolation of the sample absorbencies to the standard curve were made using Graph Pad Prism Software Version 5.04 (La Jolla CA).
Hargreaves tests
Thermal tests were conducted in a dedicated room in the Johns Hopkins Psychological Testing Core. Plantar Analgesia Meter Model 390G from IITC (Woodland Hills CA) equipped with a glass platform was used to test hind paw thermal latency. Mice were placed on a glass platform maintained at 32°C and acclimated to their environment for 2 days at 30 min/day and for 10-15 min prior to testing. A focused thermal heat stimulus was delivered from a fixed distance to the plantar surface of the hind paw for up to 10 s. The target paw was the right hind paw; however, the left hind paw was also used if the right hind paw could not be tested or was being repeat-tested too soon. A full leg raise specifically at the site the heat stimulus was directed at was considered a reaction to the thermal stimulus. Mice were tested once and data from each experimental group was averaged daily. Investigators were blinded to the treatment group.
Tail-flick measurements
Studies were conducted on an open bench. A 500 mL glass beaker filled with 450 mL of distilled water was warmed on a hot plate to the specified temperature as determined with a glass thermometer. Mice were allowed to acclimate to the laboratory environment for 1 h before testing. Mice were injected SC with drug or control solutions at time zero and returned to their cage. To restrain the mice for the test, disposable plastic 50 mL screw capped conical centrifuge were cut at the tip to create a 0.5 cm opening to allow the mouse to breath freely. A 0.5 cm square opening was cut into the caps to allow the tail access to the water bath, as shown in Figure 1. Warm water heat tests were performed at 20, 40 and 60 min intervals post dose.
Figure 1.
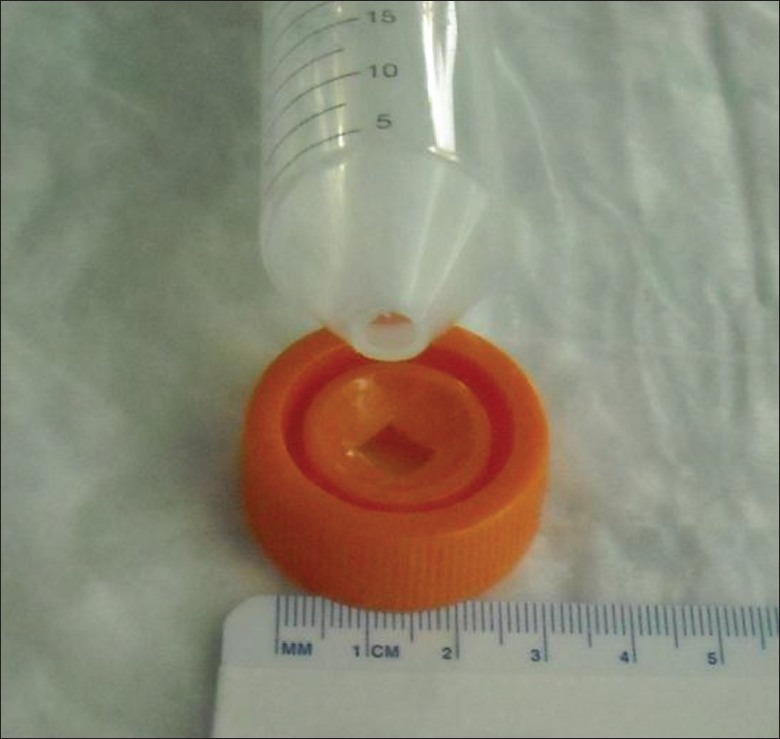
Warm water heat tests were performed at 20, 40, and 60 min intervals post dose
Approximately 5 min before the test, mice were removed from their cage and allowed to crawl into the precut 50 mL tubes. The tail was marked with ink at 3 cm from the distal tip. The tube holding the mouse was held in a horizontal position to allow the distal 3 cm of tail to be submerged in the water bath. The submersion time was limited to 30 s as measured on a digital laboratory timer. The movement of the tail out of the water was recorded as the tail-flick time. Investigators were blinded to the Balb/c mice treatment groups.
Statistical analyses
Microsoft Excel 2007 (Microsoft Excel, Microsoft office 2007) was used to generate average (avg) and standard deviation (Stdev) data. For post-hoc analysis of significance, a two-tailed equal variance t-test was used to compare raw values between groups. The threshold for statistical significance was set at P ≤ 0.05.
Results
Efforts to measure opiate efficacy through plantar thermal sensitivity in male and female Balb/c mice dosed with 0.5 and 2.0 mg/kg buprenorphine were confounded for at least 4 h by locomotor activity in the drug-treated animals. The level and duration of the activity were the same at both dose levels. Mice wandered the perimeter of the Plexiglas containment chamber at intervals of 1-12 rotations/min. Efforts to test mice in smaller chambers generally stimulated the activity. A second challenge was in blinding the observations to the treatment group. Mice injected with saline control solution could be immediately identified by the number of fecal pellets they produced in the test chamber. Opiated-treated mice produced only 1-2 pellets/test cycle.
Although the locomotor activity largely resolved by 6 h, there was no significant difference in thermal latency scores in male or female mice in control and acutely dosed groups at 6 h. Plasma levels of the drug were measured in separate groups of 4 female mice injected with the 0.5 and 2.0 mg/kg dose. Blood was collected at 6 h. The buprenorphine plasma levels in the 0.5 and 2.0 mg/kg dose group were 0.4 ± 0.3 and 22 ± 3 ng/mL (avg ± Stdev), respectively.
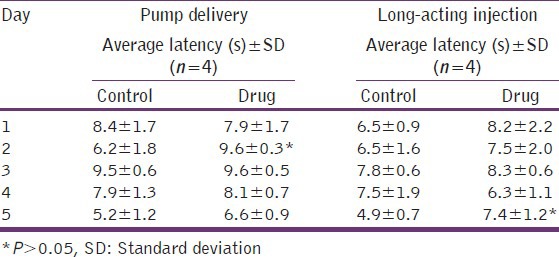
To investigate the sensitivity of Hargreaves testing in mice after the initial locomotor activity had resolved, Balb/c mice were treated to two extended-release buprenorphine delivery systems and examined daily for 5 days. The results are shown in Table 1. The applied daily dose of buprenorphine delivered by the osmotic pump was 0.25 mg/kg. The applied dose of the long acting suspension was 3.2 mg/kg. Previous studies with the long-acting suspension demonstrate average plasma levels of drug ranged from approximately 50 ng/mL in the mice injected with the long acting buprenorphine suspension on day-1-1 ng/mL on day-4. Plasma levels in the mice dosed by osmotic pumps were not measured. Female mice treated with continuous-release analgesia supplied by the two delivery systems demonstrated an average increase of approximately 1 s or 15% thermal latency increase in the drug-treated groups. However, as shown in Table 1, the differences generally failed to meet levels of statistical significance.
Table 1.
Hargreaves tests of female Balb/c mice treated with buprenorphine from osmotic pumps and long-acting suspension
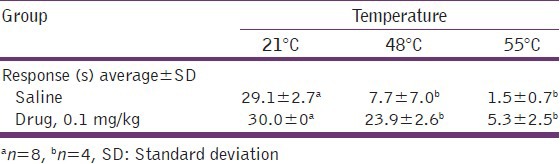
Tail-flick tests with restrained mice readily detected opiate activity. The data in Table 2 illustrate base line responses of an assay in Balb/c female mice. At room temperature, saline-injected mice show remarkably stable baseline values within the 30 s test window. At increased temperatures, the response in control mice increased to an average time of 7.7 s at 48°C to 1.5 s at 55°C. Similar results were shown by male mice. The restraining tube does not appear to condition responses. Tail-flick response was examined in groups of 8 male and female Balb/c mice at 48° after mice were injected with 0.05 mL of saline. The repeat response time from the same mouse tested at 20, 40 and 60 min post-injection was virtually identical (data not shown). The temperature sensitivity at 48° with the control mice was examined daily for 3 days. Again, virtually identical test scores for the 3 days revealed no evidence that the mice became conditioned to, or averse to the restraining tubes.
Table 2.
Tail-flick response in female Balb/c mice
Dose response data from female Balb/c mice injected SC with increasing single doses of buprenorphine and measured 60 min post-injection at 55° are shown in Table 3. Male mice provide essentially the same response (data not shown).
Table 3.
Dose sensitivity of tail-flick response in female Balb/c mice at 55°C

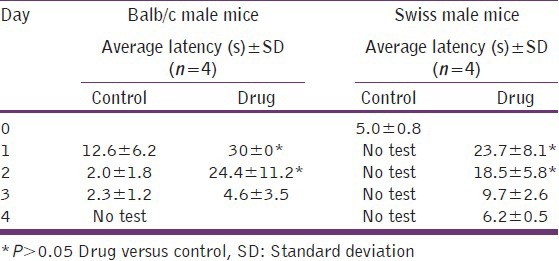
Table 4 shows the response times of Balb/c and SWR/J mice injected with 0.05 mL of the long-acting buprenorphine drug suspension for up to 96 h. The Balb/c mice were tested by observers blinded to the treatment group at 48°C. Because of the difficulty in managing the highly active SWR/J mice, the drug group was tested only at 55°C. The vehicle group was used as the day-0 control. The data in Table 4 demonstrates that the long-acting buprenorphine suspension provided significant levels of analgesic activity in male mice as measured by a hot water tail-flick response assay. The duration of the effect was for at least 48 h in both strains. SWR/J and Balb/c female mice showed the same response.
Table 4.
Effects of a long-acting buprenorphine suspension on tail-flick response in Balb/c and Swiss mice
Discussion
Straightforward and sensitive efficacy tests play a key role in research efforts aimed at new veterinary pharmaceutical products. The development of analgesics presents a challenge because pain is a complex summation of multiple physiological processes, many of which are poorly understood. Numerous studies have shown that mice treated with opioids respond to thermal sensitivity tests, which may be surrogate tests of pain. The drug-treated mice are slower to process the thermal stimuli and move away from them. As a result thermal assays using a hot plate procedures and tail-flick assays have been widely used as models of nociception.[10] Carbone et al., used this paradigm to study the duration of bolus and sustained-release formulations of buprenorphine in two strains of mice using a hot plate test. Videos of the procedure were used to confirm the end point behavior.[11] In the present study, we elected to use a Hargreaves system to test opiate analgesia in mice because previous research in rats demonstrated that the system provided rapid and robust measures of pain therapy in rats and required no special operator training.
The results presented here demonstrate the limitations on using Hargreaves systems to study opiate analgesia in mice. In the period following SC injections of 0.5 or 2.0 mg/kg buprenorphine, the Hargreaves test could not be used because the drug induced a locomotor response that lasted for approximately 4 h. Because the duration of the response was similar at both doses, it appears likely that it is based on the rapid interaction of buprenorphine with its opioid receptors.[12] Previous observations of locomotor activity in opiate treated rodents have been reported. Activity appears to be species dependent.[13] Cowan et al. noted locomotor activity in male albino mice and Sprague-Dawley rats treated with SC doses of buprenorphine.[14] Subsequent reports have associated locomotor activity in studies of buprenorphine in combination with other drugs,[15,16] and for post-surgical analgesia.[17,18,19]
The sensitivity of Hargreaves tests to detect extended-release opiate activity in mice was examined using osmotic pumps and a long-acting buprenorphine suspension. Osmotic pumps have been standard tools to investigate analgesic drug efficacy and toxicity.[20,21] As shown in Table 1, mice with pump-delivered infusions had about 15% increased latency times in the Hargreaves tests. Statistical analyses showed that significant latency times were achieved in one of 5 test days. Similar results were seen in Balb/c mice injected with a long-acting buprenorphine suspension. Hargreaves sensitivity tests with a long-acting preparation of buprenorphine also produced only modest evidence for drug efficacy. A significant latency time was observed in one of the 5 test days. This limited result cannot be attributed to under-dosing. Buprenorphine blood levels greater than 0.5 ng/mL have been associated with clinical efficacy in mice and rats.[22] Mice treated with the long-acting suspension had blood levels of buprenorphine of 50-1 ng/mL for the first 4 days of the study.
Studies have demonstrated that tail-flick tests provide dose and temperature-sensitive responses to buprenorphine in mice.[23] However and perhaps because the method seems intuitive, there is scarce detail in published reports describing mouse restraint procedures. Light restraint using a towel has been reported. Plexiglas tubes have been described and several designs are commercially available. We observed that towel use did not eliminate variation in the amount of stress to restrain mice and we would not recommend its use for surgically-treated mice. Commercially available restraint apparatus can be expensive if multiple devices are used. They require rigid cleaning protocols. We examined the utility of disposable centrifuge tubes for restraining mice. These tubes often are used to restrain mice for tail vein phlebotomy. Plastic tubes are low cost, disposable and easily sterilized if required. We found no indication that mice require acclimation to the laboratory, the tubes for test purposes, or become conditioned by previous exposure. The restraining tubes completely managed the obtunded response of the opiate injection.
The tail-flick assay described in this report was sensitive to short-term studies starting approximately 30 min after bolus injections. Saline treated mice generally failed to respond to room temperature water. Male and female mice left their tail in the 21°C water bath up to 1-2 s before the 30 s cut-off time. The removal time was approximately four-fold faster at 48°C and eight-fold faster at 55°C. Buprenorphine injected SC at 0.1 mg/kg decreased the response time 7.7-23.9 s at 48°C. The same dose decreased response time of 1.5-5.3 s at 55°C.
The tail-flick assay was also sensitive to a long-acting buprenorphine suspension. We examined two strains of mice because genetic variations to opiate tolerance have been identified.[24] The long-acting buprenorphine suspension afforded at least 48 h of analgesic activity in Balb/c mice and SWR/J mice.
Although the method described in this report affords a simple, robust technique to measure opiate activity, it is essential to recognize that the tests involve the measurement of a withdrawal reflexes evoked by a thermal stimulus. Pain caused by surgery or disease involves injury and inflammation. There is a significant body of clinical experience in human medicine showing that opiates can significantly mitigate surgical pain. Significantly more research is needed to define optimal analgesia and delivery systems in laboratory animals.
Acknowledgements
Funding for the present study was supplied by the Maryland Industrial Partnership, a State of Maryland fund to promote the development of products and processes through industry/university research partnerships. M. Guarnieri received additional funding from Bamvet, Inc. and holds a significant financial interest in Bamvet. Angela She and Nikita Robbins provided technical assistance. Gina Wilkerson provided manuscript editing.
Footnotes
Source of Support: Funding for the present study was supplied by the Maryland Industrial Partnership, a State of Maryland fund to promote the development of products and processes through industry/university research partnerships. M. Guarnieri received additional funding from Bamvet, Inc., and holds a significant financial interest in Bamvet. Angela She and Nikita Robbins provided technical assistance. Gina Wilkerson provided manuscript editing
Conflict of Interest: None declared.
References
- 1.Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–7. [PubMed] [Google Scholar]
- 2.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 3.Foley PL, Liang H, Crichlow AR. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci. 2011;50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 4.D’Elia M, Patenaude J, Hamelin C, Garrel DR, Bernier J. No detrimental effect from chronic exposure to buprenorphine on corticosteroid-binding globulin and corticosensitive immune parameters. Clin Immunol. 2003;109:179–87. doi: 10.1016/s1521-6616(03)00177-3. [DOI] [PubMed] [Google Scholar]
- 5.Pontani RB, Misra AL. A long-acting buprenorphine delivery system. Pharmacol Biochem Behav. 1983;18:471–4. doi: 10.1016/0091-3057(83)90472-0. [DOI] [PubMed] [Google Scholar]
- 6.Alzet osmotic pump implantation. Available from: http://www.alzet.com/resources/index .
- 7.Forbes N, Brayton C, Grindle S, Shepherd S, Tyler B, Guarnieri M. Morbidity and mortality rates associated with serial bleeding from the superficial temporal vein in mice. Lab Anim (NY) 2010;39:236–40. doi: 10.1038/laban0810-236. [DOI] [PubMed] [Google Scholar]
- 8.Cirimele V, Etienne S, Villain M, Ludes B, Kintz P. Evaluation of the One-Step ELISA kit for the detection of buprenorphine in urine, blood, and hair specimens. Forensic Sci Int. 2004;143:153–6. doi: 10.1016/j.forsciint.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Kalliokoski O, Jacobsen KR, Hau J, Abelson KS. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J. 2011;187:251–4. doi: 10.1016/j.tvjl.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Bannon AW, Malmberg AB. Models of nociception: Hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007;(Chapter 8:Unit 8.9) doi: 10.1002/0471142301.ns0809s41. [DOI] [PubMed] [Google Scholar]
- 11.Carbone ET, Lindstrom KE, Diep S, Carbone L. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci. 2012;51:815–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Tallarida RJ, Cowan A, Raffa RB. On deriving the dose-effect relation of an unknown second component: An example using buprenorphine preclinical data. Drug Alcohol Depend. 2010;109:126–9. doi: 10.1016/j.drugalcdep.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varnado-Rhodes Y, Gunther J, Terman GW, Chavkin C. Mu opioid analgesia and analgesic tolerance in two mouse strains: C57BL/6 and 129/SvJ. Proc West Pharmacol Soc. 2000;43:15–7. [PubMed] [Google Scholar]
- 14.Cowan A, Doxey JC, Harry EJ. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol. 1977;60:547–54. doi: 10.1111/j.1476-5381.1977.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filibeck U, Castellano C, Oliverio A. Differential effects of opiate agonists-antagonists on morphine-induced hyperexcitability and analgesia in mice. Psychopharmacology (Berl) 1981;73:134–6. doi: 10.1007/BF00429203. [DOI] [PubMed] [Google Scholar]
- 16.Sorge RE, Stewart J. The effects of long-term chronic buprenorphine treatment on the locomotor and nucleus accumbens dopamine response to acute heroin and cocaine in rats. Pharmacol Biochem Behav. 2006;84:300–5. doi: 10.1016/j.pbb.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Gades NM, Danneman PJ, Wixson SK, Tolley EA. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci. 2000;39:8–13. [PubMed] [Google Scholar]
- 18.Bourque SL, Adams MA, Nakatsu K, Winterborn A. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: Effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci. 2010;49:617–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, et al. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci. 2011;50:185–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: Similar analgesic profile but different effects on immune responses. Pain. 2004;110:385–92. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Canta A, Chiorazzi A, Meregalli C, Carozzi V, Oggioni N, Lauria G, et al. Continuous buprenorphine delivery effect in streptozotocine-induced painful diabetic neuropathy in rats. J Pain. 2009;10:961–8. doi: 10.1016/j.jpain.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 2012;41:337–43. doi: 10.1038/laban.152. [DOI] [PubMed] [Google Scholar]
- 23.Raffa RB, Ding Z. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as a full- or partial-agonist. Acute Pain. 2007;9:145–52. [Google Scholar]
- 24.Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: A survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–8. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]


