Abstract
Carum carvi L. (Apiaceae family) or caraway is a common household plant grown around the world including Iran. Caraway fruits are used as flavoring agent in foods and beverages, and have various traditional uses in ethnomedicine. Anti-inflammatory, spasmolytic, antimicrobial, antioxidant, carminative and immunomodulatory properties of caraway suggest that it might exert beneficial effects on inflammatory bowel disease (IBD). Therefore, this study was carried out to investigate the effects of caraway hydroalcoholic extract (CHE) and its essential oil (CEO) in an immunological model of colitis in rats induced by trinitrobenzene sulfonic acid (TNBS). Different doses of CHE (100, 200, 400 mg/kg) and CEO (100, 200, 400 μl/kg) were administered orally (p.o.) and also doses of CHE (100, 400 mg/kg) and CEO (100, 400 μl/kg) were given intraperitoneally (i.p.) to the separate groups of male Wistar rats (n=6). Administration of the doses started 6 h after induction of colitis and continued daily for 5 consecutive days. Wet colon weight/length ratio was measured and tissue damage scores as well as indices of colitis were evaluated both macroscopically and histopathologically. CHE and CEO at all doses tested were effective in reducing colon tissue lesions and colitis indices and the efficacy was nearly the same when different doses of plant fractions were administered p.o. or i.p. Administration of prednisolone (p.o., 4 mg/kg), Asacol® (mesalazine microgranules, p.o., 100 mg/kg) and hydrocortisone acetate (i.p., 20 mg/kg) as references were effective in reducing colon tissue injures as well. These data suggest that caraway fractions are both effective and possess anti-colitic activity irrespective of the dose and route of administration.
Keywords: Colitis, Carum carvi L., Hydroalcoholic extract, Essential oil, Trinitrobenzene sulfonic acid (TNBS), Rats
INTRODUCTION
The inflammatory bowel diseases (IBD) refer essentially to two different but closely related conditions, Crohn's disease and ulcerative colitis. Although the etiology of IBD remains unclear, there are evidences that it involves immune, genetic and environmental factors, which are, in turn, related to the initiation and progression of colitis (1). Intestinal mucosal inflammation as a characteristic feature of IBD is induced by increasing in the activity of some mucosal immune cells where the T-helper cells play an important role (2). Indeed, it is a loss of homeostasis of immune cells that results in intestinal inflammation (3). Main current therapies of IBD are aminosalicylate derivatives and corticosteroids being used through various routes (4), but several side effects and/or lack of full effectiveness are common problematic features of current regular treatments. Therefore, alternative and traditional therapies are largely considered in the IBD patients for several years (5,6). Carum carvi L. (C. carvi, Caraway) as an important medicinal plant from Apiaceae family has been cultivated for long time in the north and center of Europe, Egypt, Australia, China and Iran (7). It is traditionally used as a spice in foods and beverages and as an alternative herbal medicine for GI ailments including dyspepsia, and various spasmodic conditions, bloating, diarrhea and in flatulent colic (8). It is diuretic and expectorant and used for increasing maternal milk and dysmenorrhea (9,10). Caraway has both anti-hyperglycemic and anti-hyperlipidemic activities in STZ-induced diabetic rats (11). Its volatile oil probably has a protective role in kidney tissue against oxidative injury in advanced stages of sepsis (12) and it is hepatoprotective against carbon tetrachloride-induced liver toxicity in rats (13).
Caraway has also been used in bronchopulmonary disorders as cough remedy and demulcent (14). Vapors from caraway seeds have been reported to be effective in relieving pain and inflammation in patients suffering from lumbar pains and rheumatism (15). Additionally there are numerous studies about the treatment of gastrointestinal disorders like Helicobacter pylori - induced gastritis (16) and relaxant effect of ethanol extract of caraway on isolated intestinal smooth muscle cells of the guinea pig (17). This response may explain, in part, the beneficial effects of caraway in relieving gastrointestinal symptoms associated with dyspepsia (18). Two important natural compounds of caraway, carvone and limonene are mucoprotective on duodenal peptic ulcer and gastroduodenitis and have antiulcerogenic effect (19). Due to the antiulcerogenic (20), antioxidative (21), antispasmodic (22) and immunomodulatory (23) properties of caraway, it is suggested that it has a high therapeutic potential for IBD conditions.
In this study, we evaluated the anticolitic effects of extract and essential oil of C. carvi fruits at various doses and via two administration routes in animal rat model of experimental TNBS-induced colitis.
MATERIALS AND METHODS
Plant material
Fruits of C. carvi L. procured from Istanbul, Turkey were donated on October, 2008 by the Mashhad School of Pharmacy, Mashhad, Iran.
Preparation and analysis of extract
C. carvi fruits (300 g) were air-dried and finely powdered, soaked in enough volume of ethanol:water (70:30) for 1 h and extracted using percolation apparatus which was maintained for 48 h to accomplish a complete extraction. The extract was shaken, filtered and evaporated in a rotary evaporator under reduced pressure until a semisolid extract was obtained (7,24). According to the British Pharmacopoeia the concentrated extract was freeze-dried to obtain a dry powdered extract. Test doses were eventually prepared by reconstitution of this dried extract in water (25,26).
Preparation and analysis of essential oil
Caraway essential oil was obtained by hydro-distillation method according to the method described by the Iranian Herbal Pharmacopoeia. The essential oil was then analyzed by thin layer chromatography (TLC). The analysis was carried out on silica gel 60 F254 precoated plates, layer thickness: 250 μm (Merck, Darmstadt, Germany) and developed in different systems where Toluene:EtOAc (93:7) gave the highest resolution. The spots were visualized by UV illumination and vanilline/H2SO4 reagent (7).
Animals
Male Wistar rats weighting 200 ± 20 g bred in animal house of Isfahan School of Pharmacy were housed in wire-bottomed cages of three animals in each cage at room temperature and ambient humidity, fed with normal rat chow and water ad libitum. Experiments were performed only after the rats had acclimated to the above environment. Each animal was only used once for the experiments. All experiments were carried out in accordance with international guideline outlined in the Guide for the Care and Use of Laboratory Animals (27).
Chemicals
Prednisolone and hydrocortisone acetate were purchased from Iran Hormone Pharmaceutical Co. (Tehran, Iran). Asacol® (mesalazine microgranules) was obtained from Iran Darou Co. (Tehran, Iran). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma (St. Louis, MO). All of the organic solvents were of analytical grade and Merck brand (Darmschtdat, Germany).
Test samples including suspension of reference drugs and caraway extract or emulsion of essential oil were freshly prepared using 0.2% tween 80 in distilled water as vehicle for oral (p.o.) and intraperitoneal (i.p.) administration.
Grouping
Seventeen groups of animals were randomly assigned to sham (normal), negative control, test, and reference (positive control) groups of at least 6 rats as following:
Sham groups, treated with vehicle p.o. (5 ml/kg) and i.p. (2 ml/kg) without induction of colitis.
Negative control groups, treated with vehicle p.o. (5 ml/kg) and i.p. (2 ml/kg), after induction of colitis.
Extract groups, treated orally with C. carvi hydroalcoholic extract (CHE) at doses of 100, 200, and 400 mg/kg, and intraperitoneally at doses of 100 and 400 mg/kg.
Essential oil groups, treated orally with C. carvi essential oil (CEO) at doses of 100, 200, and 400 μl/kg, and intraperitoneally at doses of 100 and 400 μl/kg.
Reference groups, treated with prednisolone (4 mg/kg, p.o.), Asacol® (mesalazine microgranules, 100 mg/kg, p.o.) and hydrocortisone acetate (20 mg/kg, i.p.), respectively.
All the treatments were started 6 h after colitis induction and continued daily for 5 days.
Induction of Colitis
Rats were fasted for 36 h with free access to water prior to induction of colitis. When the health of animals was confirmed, the rats were lightly anesthetized with diethylether. A flexible plastic rubber catheter with an outside diameter of 2 mm was inserted rectally into the colon; and its tip was kept 8 cm proximal to the anus. TNBS (100 mg/kg) dissolved in 50% ethanol (v/v) was instilled into the colon lumen through the rubber catheter (0.5 ml) while normal saline was instilled as control (28).
Evaluation of the colonic damage
Rats were euthanized using over-dose ether anesthesia at the day of six. The abdomen was opened and the colon, 8 cm in length and 3 cm proximal to the anus, was excised and incised longitudinally and washed with normal saline. Wet colon was then weighed and weight/length ratio was determined for each specimen. Macroscopic mucosal damage was evaluated using the validated grading scale according to Morris and coworkers (29). Scores were: 0=no ulcer, 1=mucosal erythema only, 2=mild mucosal edema, slight bleeding or slight erosion, 3=moderate edema, bleeding ulcers or erosions, 4=severe ulceration, erosions, edema and tissue necrosis and/or perforation. Ulcer area was determined using 3M® scaled surgical transparent tape, which was fixed on a light and transparent sheet. Each cell on the tape was 1 mm2 in area and the number of cells was counted for measuring the ulcerated areas for each specimen. Ulcer index was measured by summing the ulcer score and the ulcer area for each colon (30,31).
For histological examination, colon tissues were individually fixed in 10% formalin, processed (dehydrated, cleared, impregnated with paraffin, blocked, sectioned in 4 μm thick slices) and stained with haematoxylin and eosin (H&E). Inflammation severity and extent as well as crypt damage were evaluated on H&E-stained and coded sections while a modification of a validated scoring system described by Cooper and coworkers(32) and Dieleman and coworkers (33) was used. Total colitis index was measured by summing 3 subscores (inflammation severity, inflammation extent, crypt damage). Coworker pathologist unaware of treatments recorded macroscopic and histological injuries. It was implemented by using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging.
Statistical Analysis
Analysis of data was performed by SPSS (version 10) statistical software. Non-parametric data were analyzed by Kruskal-Wallis and Mann-Whitney U tests. Results were expressed as mean ± standard error of mean (S.E.M). Differences between groups were determined using one-way analysis of variance (ANOVA) with Scheffe as post hoc test. The minimal level of significance was identified at P<0.05.
RESULTS
Yield value of the extract
The concentrated extract yielded 14% w/w and freeze-dried CHE yielded 7%.
Analysis of the essential oil
The plant fruits yielded 2.9% (v/w) of a pale-yellowish essential oil with a fresh pleasant odor. Carvone as the most important part of CEO showed hot red spot in the middle of TLC chromatogram (Rf = 0.5). It is a main parameter to identify and standardize caraway oil as noted in Iranian Herbal Pharmacopoeia (7).
Macroscopic assessment
As it is shown in Fig. 1 and Table 1, macroscopic tissue damage parameters manifested severe inflammation; hemorrhage, ulcer, and necrosis as well as thickening of colon wall in colitis control groups compared to sham groups in which no changes were observed.
Fig. 1.
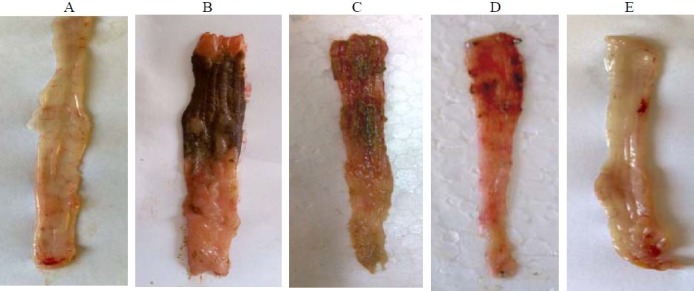
Macroscopic presentation of TNBS-induced colitis in rats. A) Sham, normal colon treated with vehicle, 2 ml/kg; B) Control colitis treated with vehicle, 2 ml/kg; C) Caraway hydroalcoholic extract treated colitis, 400 mg/kg; D) Caraway essential oil treated colitis, 400 μl/kg; E) Prednisolon treated colitis, 4 mg/kg.
Table 1.
Effects of Carum carvi hydroalcoholic extract and essential oil on the macroscopic parameters of TNBS- induced colitis in rats.
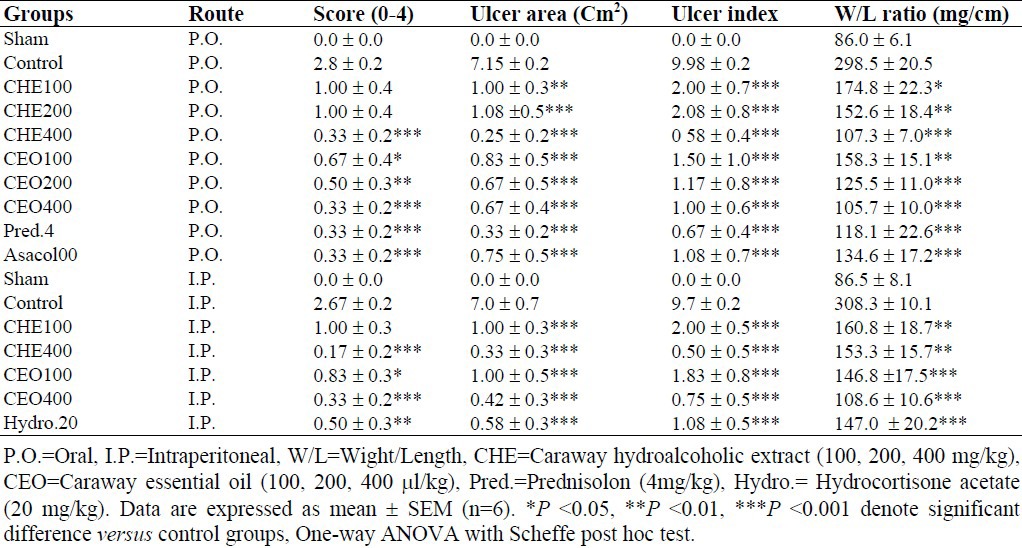
All the treatments with increasing doses of CHE and CEO were effective to reduce weight/length ratio and ulcer index (ulcer area + ulcer severity) in colon samples compared to control groups (at least P<0.05) (Table 1). CHE and CEO also diminished ulcer features after both routes of P.O. and I.P. administration.
On the other hand, prednisolone and Asacol® significantly (P<0.001) diminished macroscopic scores and weight/length ratio in colitis rats after P.O. administration. Hydrocortisone acetate had also desirable effect on macroscopic features of colitis lesions (P<0.001) intraperitoneally.
Histological assessment
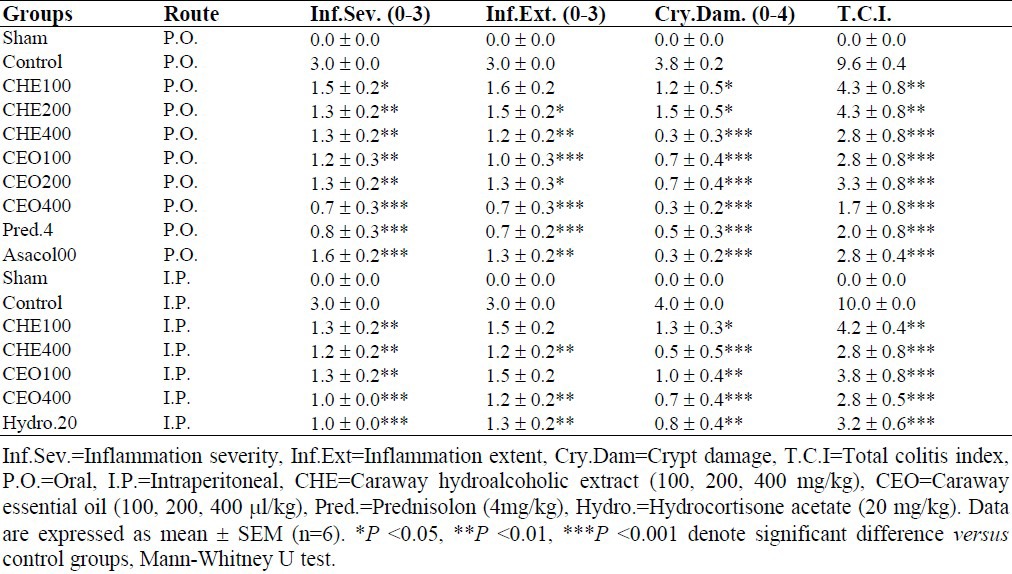
No histological damage was observed in sham groups. In control groups, microscopic assessments revealed highly severe inflammation and infiltration of white blood cells in mucus and sub-mucosal layers (Fig. 2). P.O. treatment with prednisolone and Asacol® as well as parentreal hydrocortisone acetate were all effective to reduce total colitis index (inflammation severity + inflammation extent + crypt damage) in injurious colons (at least P<0.05) (Table 2). Similar findings were obtained by increasing doses of CEO (100-400 μl/kg) and CHE (100-400 mg/kg) in comparison with control groups and there was no meaningful difference between test doses and/or plant fractions administered by different routes (P > 0.05).
Fig. 2.
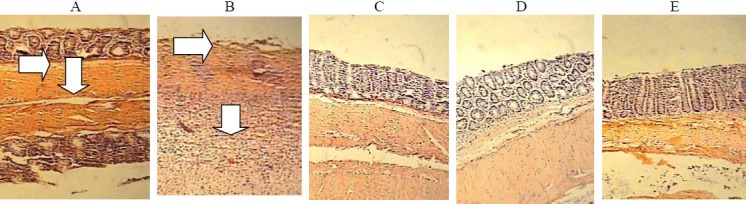
microscopic presentation of TNBS-induced colitis in rats. A) Sham, normal colon treated with vehicle, 2 ml/kg; mucus layer and crypts are normal and leucocyte infiltration is absent. B) Control colitis treated with vehicle, 2 ml/kg; mucosal and submucusal inflammation as well as crypt damage and leucocyte infiltration are evident; C) Caraway hydroalcoholic extract treated colitis, 400 mg/kg; D) Caraway essential oil treated colitis, 400 μl/kg; E) Prednisolon treated colitis, 4 mg/kg. H & E staining with low power.
Table 2.
Effects of Carum carvi hydroalcoholic extract and essential oil on the histopathologic parameters of TNBS- induced colitis in rats.
DISCUSSION
It has been described that TNBS model of experimental colitis is beneficial for the screening of drugs with anticolitic activity and has several similarities to pathological and clinical features of the human ulcerative colitis (34).
The CHE and CEO inhibited the ulcerative lesion index both macroscopically and pathologically in accordance with the previous study reported antiulcerogenic activity for CHE in gastric ulcer (35). Khayyal and coworkers demonstrated that different CHE produced anti-ulcerogenic effect against indomethacin-induced gastric ulcer accompanied by reduction in gastric acid output and leukotriens synthesis while mucus secretion and prostaglandin E2 release were enhanced. This was attributed to the radical scavenging, spasmolytic and immunomodultory properties of compounds found in the CHE and CEO (20).
Our results showed that the activity of caraway test fractions was independent of the administration routes and the dose. The lower doses of CHE and CEO were efficient like the greater ones indicating high potency components within the fractions. So it seems that caraway is a valuable candidate to be considered and designed as a dosage form for marketing. Lower dose efficiency also indicates that the components are more abundant in caraway would be more responsible for these beneficial effects. For example in caraway CEO; carvone is the most dominant and important component (36).
The results of the current study showed that administration route did not exert a significant influence on the therapeutic efficacy and activity of CHE and CEO. Two suggestions might be presented to describe this finding; Firstly, it is supposed that treatment of animals with P.O. administration of caraway fractions for a period of 5 days provided a suitable condition for systemic absorption and availability of active plant constituents as it is comparable with parenteral route. Secondly, the active constituents of CHE that has high lipid solubility similar to volatile oil constituents of CEO could be absorbed readily from GI tract and even after applying intraperitoneally are effective on colitis.
In the present study, all macroscopic and microscopic lesion parameters were improved after administration of the reference drugs. Glucocorticoids in IBD inhibit the synthesis of inflammatory cytokines (IL-1, TNF-α) and chemokines (IL-8) and decrease phospholipase A2, cyclooxygenase and NF-kappa B activity. So glucocorticoids diminish all three forms of leukoterians, prostaglandins and TNF-α as inflammatory mediators are commonly involved in IBD pathogenesis; (1,4,37). Asacol® administered orally was similarly effective (compared to glucocorticoides) in TNBS-induced colitis suggesting involvement of some more molecular and/or cellular mechanisms like an important radical scavengering related mechanism, inhibition of natural killer cells (NKC), macrophages and mucosal lymphocytes (1,37). Moreover, Asacol can inhibit the cyclooxygenase and 5-lipoxygenase pathways of arachidonic acid metabolism similar to corticosteroids, in which the latter mechanism seems to be more important in IBD pathology, although the exact mechanism of Asacol® is unclear (1,4).
The terpenoid, flavonoids, fatty acids, triacylglycerols, polysaccharides, lignin and polyacetylenic compounds of caraway can reduce inflammatory cytokines and chemokines similar to glucocorticoids mechanism (38). It seems that caraway is anticolitis with reducing the production of prostaglandin E2 and increasing the production of leucotriene B4 in human polymorphonuclear leucocytes (39).
The most present component of C. carvi which is known as an anti-inflammatory agent is carvone. Carvone inhibits 5-lipoxigenase and cyclooxygenase activity so it can decrease biosynthesis of leucotriens and prostaglandins. Additionally carvone acts as a blocker of voltage dependent Ca channels resulting in probably spamolytic and anti-inflammatory effects of this active compound (7,40). The beneficial activity of C. carvi could also be attributed to its antioxidant properties of the caraway phenolic compounds (41). Caraway has potent antioxidant and antibacterial activity due to their contents of carvacrol (42). Another study showed that antioxidant activity of carvone and limonene in caraway (13). It has emerged that the major compounds occurring in caraway are carvacrol, carvone, α-pinene, limonene, γ-terpinene, linalool, carvenone, and p-cymene, carveol, camphene, fenchen (43,44). Moreover Caraway fruits have immunomodulatory activity for which monoterpenes like carvone and limonene have identified (45). It is reasonable to assume that a synergy between or within a group of compounds might be responsible for the beneficial activities of caraway for IBD therapy or prevention.
CONCLUSION
In conclusion, our results suggest an advantageous therapeutic activity for CHE and CEO as an anti-inflammatory and anti-ulcerative medicinal plant for IBD conditions. This reinforces the use of this plant as an alternative remedy for IBD conditions and/or prevention of its recurrence; especially when it is effective even with low doses and in both administration routes, orally and intraperitoneally. More studies are strongly recommended to establish the mechanisms are involved and the active constituents which are really responsible for its beneficial pharmacologic actions.
ACKNOWLEDGMENT
This research project numbered 387423 was fully sponsored by Research Council of Isfahan University of Medical Sciences, Isfahan, Iran. We thank Dr. Javad Asili from Pharmacognosy Department of Mashhad School of Pharmacy for providing the caraway fruits.
REFERENCES
- 1.Sellin JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel diseases. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill companies; 2006. pp. 1009–1011. [Google Scholar]
- 2.Bouma C, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Murata Y, Ishiguru Y, Itoh J, Munakata A, Yoshida Y. The role of proinflammatory and immuno-regulatory cytokines in the pathogenesis of ulcerative colitis. J Gasteroenterol. 1995;30:56–60. [PubMed] [Google Scholar]
- 4.McQuaid KR. Drugs used in the treatment of gastrointestinal disease. In: Katzung BG, editor. Basic and clinical pharmacology. 10th ed. New York: McGraw Hill Company; 2007. pp. 1029–1035. [Google Scholar]
- 5.Jagtap AG, Shirke SS, Phadke AS. Effect of polyherbal formulation on experimental models of inflammatory bowel diseases. J Ethnopharmacol. 2004;90:195–204. doi: 10.1016/j.jep.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 6.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143–152. [PubMed] [Google Scholar]
- 7.Salehi Surmaghi MH, Amin GhR, Kaveh Sh. Iranian herbal pharmacopeia. 1st ed. Tehran: Iranian Ministry of Health & Medical Education Publications; 2002. Carvi fructus. In: Iranian herbal pharmacopeia scientific committee; pp. 419–424. [Google Scholar]
- 8.Johri RK. Cuminum cyminum and Carum carvi: An update. Pharmacogn Rev. 2011;5:63–72. doi: 10.4103/0973-7847.79101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110:458–463. doi: 10.1016/j.jep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Phillipson JD, Czygan FC, Frohne D, Holtzelc NA, Pfander HJ, Willuhn GB. Stuttgart: Med Pharm Scientific; 1994. Herbal drug and phytopharmaceuticals. [Google Scholar]
- 11.Haidari F, Seyed-Sadjadi N, Taha-Jalali M, Mohammed-Shahi M. The effect of oral administration of Carum carvi on weight, serum glucose, and lipid profile in streptozotocin-induced diabetic rats. Saudi Med J. 2011;32:695–700. [PubMed] [Google Scholar]
- 12.Dadkhah A, Fatemi F. Heart and kidney oxidative stress status in septic rats treated with caraway extracts. Pharm Biol. 2011;49:679–686. doi: 10.3109/13880209.2010.539618. [DOI] [PubMed] [Google Scholar]
- 13.Samojlik I, Lakić N, Mimica-Dukić N, Daković-Svajcer K, Bozin B. Antioxidant and hepato protective potential of essential oils of coriander (Coriandrum sativum L.) and caraway (Carum carvi L.) (Apiaceae) J Agric Food Chem. 2010;58:8848–8853. doi: 10.1021/jf101645n. [DOI] [PubMed] [Google Scholar]
- 14.Joshi SG. 1st ed. Delhi: Oxford and IBH Publishing Co; 2000. Medicinal plants: Family Apiaceae. [Google Scholar]
- 15.Sivarajan VV, Balachandran I. New Delhi: Oxford and IBH Publication; 1994. Ayurvedic Drugs and their Plant Sources. [Google Scholar]
- 16.Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, et al. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 17.Al-Essa MK, Shafagoj YA, Mohammed FI, Afifi FU. Relaxant effect of ethanol extract of Carum carvi on dispersed intestinal smooth muscle cells of the guinea pig. Pharm Biol. 2010;48:76–80. doi: 10.3109/13880200903046161. [DOI] [PubMed] [Google Scholar]
- 18.Thomson Coon J, Ernst E. Systematic review: Herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689–1699. doi: 10.1046/j.1365-2036.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng GQ, Kenney PM, Lam LK. Anethofuran, carvone, and limonene: potential cancer chemopreventive agents from dill weed oil and caraway oil. Planta Med. 1992;58:338–341. doi: 10.1055/s-2006-961480. [DOI] [PubMed] [Google Scholar]
- 20.Khayyal MT, el-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, et al. Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung. 2001;51:545–553. doi: 10.1055/s-0031-1300078. [DOI] [PubMed] [Google Scholar]
- 21.Lado C, Then M, Varga I, Szoke E, Azentminalyi K. Antioxidant property of volatile oils determined by ferric reducing ability. Z Naturforsch. 2004;59c:354–358. doi: 10.1515/znc-2004-5-611. [DOI] [PubMed] [Google Scholar]
- 22.Al-Essa MK, Shafagoj YA, Mohammed Fi, Afifi FU. Relaxant effect of ethanol extract of Carum carvi on dispersed intestinal smooth muscle cells of the guinea pig. Pharm Biol. 2010;48:76–80. doi: 10.3109/13880200903046161. [DOI] [PubMed] [Google Scholar]
- 23.Raphael TJ, Kuttan G. Immunomodulatory activity of naturally occurring monoterpenes carvone, limonene, and perillic acid. Immunopharmacol Immunotoxicol. 2003;25:285–294. doi: 10.1081/iph-120020476. [DOI] [PubMed] [Google Scholar]
- 24.Sadraei H, Ghannadi A, Tak-Bavani M. Effects of Zataria multiflora and Carum carvi essential oils and hydroalcoholic extracts of Passiflora incarnata, Berberis integerrima and Crocus sativus on rat isolated uterus contractions. Int J Aromather. 2003;13:121–127. [Google Scholar]
- 25.London: The Stationary Office; 2001. British Pharmacopea; pp. 1782–1783. [Google Scholar]
- 26.Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 27.Washington DC: The National Academies Press; 2010. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council. Guide for the Care and use of Laboratory animals. [Google Scholar]
- 28.Minaiyan M, Ghannadi A, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterol. 1989;96:795–803. [PubMed] [Google Scholar]
- 30.Minaiyan M, Ghannadi A, Karimzadeh A. Antiulcerogenic effects of ginger (Zingiber officinale Roscoe) on cysteamine induced duodonal ulcer in rats. Daru. 2006;14:97–101. [Google Scholar]
- 31.Minaiyan M, Ghannadi A, Mahzouni P, Nabi-Meibodi M. Antiulcerogenic effects of ginger (rhizome of Zingiber officinale Roscoe) hydro-alcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 32.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 33.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuno. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres MI, Garcia-Martin M, Fernandez MI, Nietro N, Gil A, Rios A. Experimental colitis induced by trinitrobenzene sulfonic acid: an ultrastructural and histochemical study. Dig Dis Sci. 1999;44:2523–2529. doi: 10.1023/a:1026651408998. [DOI] [PubMed] [Google Scholar]
- 35.Borrelli F, Izzo A. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–591. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Seidler-Lozykowska K, Baranska M, Baranski R, Krol D. Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. J Agric Food Chem. 2010;58:5271–5275. doi: 10.1021/jf100298z. [DOI] [PubMed] [Google Scholar]
- 37.Varshosaz J, Emami J, Fassihi A, Tavakoli N, Minaiyan M, Ahmadi F. Effectiveness of budesonide-succinate-dextran conjugate as a novel prodrug of budesonide against acetic acid-induced colitis in rats. Int J Colorectal Dis. 2010;25:1159–1165. doi: 10.1007/s00384-010-1026-2. [DOI] [PubMed] [Google Scholar]
- 38.Ngo-Duy C, Destaillats F, Keskitalo M, Arul J, Angers P. Triacylglerols of Apiaceaae seed oils: composition and regiodistribution of fatty acids. Eur J Lipid Sci Technol. 2009;111:164–169. [Google Scholar]
- 39.Mahadevan U, Loftus EV, Jr, Tremaine WJ. Saftey of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97:910–914. doi: 10.1111/j.1572-0241.2002.05608.x. [DOI] [PubMed] [Google Scholar]
- 40.Aevang Y. New York: John Wiley and Sons; 1996. Encyclopedia of common natural ingredients used in food, drugs and cosmetics; pp. 66–67. [Google Scholar]
- 41.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 42.De Martino L, De Feo V, Fratianni F, Nazzaro F. Chemistry, antioxidant, antibacterial and antifungal activities of volatile oils and their components. Nat Prod Commun. 2009;4:1741–1750. [PubMed] [Google Scholar]
- 43.Seidler-Lozykowska K, Baranska M, Baranski R, Krol D. Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. J Agric Food Chem. 2010;58:5271–5275. doi: 10.1021/jf100298z. [DOI] [PubMed] [Google Scholar]
- 44.Reiter B, Lechner M, Lorbeer E. The fatty acid profiles-including petroselinic and cis-vaccenic acid-of different Umbelliferae seed oils. Fett/Lipid. 1998;100:498–502. [Google Scholar]
- 45.Raphael TJ, Kuttan G. Immunomodulatory activity of naturally occurring monoterpenes carvone, limonene, and perillic acid. Immunopharmacol Immunotoxicol. 2003;25:285–294. doi: 10.1081/iph-120020476. [DOI] [PubMed] [Google Scholar]


