Abstract
Flower of Rosa damascena Mill is widely used in Iran for gastrointestinal (GI) disorders. However, its pharmacological action on ileum contraction has not been studied. In this research we have investigated ileum motility effect of essential oil of flower petals of R. damascena growing in Kashan, Iran, and two of its constituents. The essential oils obtained by hydrodistillation were investigated by a combination of GC and GC/MS. More than 34 compounds have been identified. The main constituents of the essential oil were β-citronellol (23%), nonadecane (16%), geraniol (16%) and heneicosane (5%). A portion of rat isolated ileum was suspended under 1g tension in Tyrode's solution at 37°C and gassed with O2. Effect of the R. damascena essential oil (2.5-160 μg/ml), geraniol (0.2-3.2 μg/ml) and citronellol (0.8-6.4 μg/ml) were studied on ileum contractions induced by KCl, acetylcholine (ACh) and electrical field stimulation (EFS) and compared with standard drugs atropine and loperamide. The contractile response of EFS was mediated mainly through the intramural nerve plexuses, because its response was inhibited by loperamide and partially reduced by atropine. The essential oil concentration dependently inhibited the response to KCl (IC50=67 ± 8.4μg/ml) and EFS (IC50=47 ± 10.6 μg/ml). Geraniol (IC50=1.7 ± 0.15 μg/ml for KCl) and citronellol (IC50=2.9 ± 0.3 μg/ml for KCl) also had inhibitory effect of ileum contraction and both were more potent than the essential oil. It was concluded that R. damascena essential oil mainly had an inhibitory effect on ileum contractions and geraniol and citronellol had a major role in inhibitory effect of the essential.
Keywords: Rosa damascena, Essential oil, Geraniol, Citronellol, Ileum
INTRODUCTION
Rosa damascena Mill belong to Rosaceae family which grow in some part of Europe and Asia particularly in Middle East (1,2). R. damascena, more commonly known as the Damask rose, the Damascus rose, or sometimes as the Rose of Castile, is a rose hybrid, derived from Rosa gallica and Rosa moschata (3). The flower of R. damascena is enriched in essential oil (1,3). The flowers are renowned for their fine fragrance, and are commercially harvested for rose oil and to make rose water (1,4). The essential oil of the R. damascena flower is precious and is used in perfumery (5). It is stimulating and elevating to the mind creating a sense of well being. Damascus rose oil also has therapeutic properties that sooth the mind and helps with depression, nervous tension and stress (6). Rose is antiseptic, antispasmodic, antiviral, and antibacterial (6). Rose water and the dried flower of R. damascena are being used as folk medicine (7,8) and are believed to assist conditions like frigidity, chronic bronchitis, asthma, skin disease, cancer, ulcers, wounds, wrinkles, infections, as well as constipation (for review see reference 6). On the other hand, rose water (called Golab in Iran) sometimes used as antispasmodic for abdominal pain (8). However, the pharmacological effect of R. damascena essential oil on GI tract is not well understood. Therefore, the aim of this research was to investigate the effect of R. damascena essential oil and two of its components on ileum contractions, in order to find any excitatory or inhibitory effect they may have on intestinal motility.
MATERIALS AND METHODS
Plant materials
The essential oil was prepared by hydrodistillation according to European Pharmacopoeia (9) in Barij Essence Co. in Mashhad-Ardehal (Kashan, Iran).
Analysis of the essential oil
For analysis of the essential oil, gas chromatography (GC) analysis was carried out on a Perkin-Elmer 8500 gas chromatograph with FID detector and a BP-1 capillary column as previously described (10).
The mass spectra (MS) were recorded on a Hewlett-Packard 5983A mass selective detector coupled with a Hewlett-Packard 6890 gas chromatograph, equipped with a HP-5MS capillary column as previously described (10).
Drugs and Solutions
The following drugs were used in this research: R. damascena essential oil, geraniol (Roth, Germany), citronellol (Roth, Germany), atropine sulfate, loperamide hydrochloride (Sigma, Germany), acetylcholine hydrochloride (Sigma, Germany).
The essential oil, geraniol and citronellol were made up as 10 mg/ml stock solution in dimethyl sulfoxide (DMSO), dilution being made in 50% DMSO.
Loperamide (10 mM), atropine (1 mM) and KCl (2 M) stock solutions were made up in distilled water. Acetylcholine hydrochloride (Sigma, Germany) was made up as 100 mM stock solution and acidified by 1% acetic acid, and further serial dilutions were made in distilled water.
Tyrode's solution composed of (mM): NaCl, 136.9; KCl, 2.68; CaCl2, 1.8; MgCl2, 1.05; NaHCO3, 11.9; NaH2PO4, 0.42 and glucose, 5.55, was made up in distilled water. Unless stated, all chemicals and drugs were from Merck (Germany).
In vitro contractility assessment
Experiments were conducted on adult male Wistar rats (Bred in School of Pharmacy animal house, Isfahan, Iran), weighing 180-250 g. The rats were housed in groups of 4-6 per cage with free access to food and water at room temperature. All animals were handled in accordance with the internationally accepted principles for laboratory animal use and care, as recommended by university authority (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2010) (11). On the day of experiment, rats were killed by a blow on the head, followed by exsanguination. The ileum was removed and placed in oxygenated Tyrode's solution at room temperature. Longitudinal strips, 2-3 cm long, were prepared from ileum and mounted in 50 ml organ bath (Harvard, England) filled with Tyrode's solution at 37°C and continuously bubbled with O2. The tissue was then given 3 successive washes with fresh Tyrode's solution and allowed to relax to a stable base line. Following a resting period of about 15 min, contraction was induced by KCl (80 mM), acetylcholine (ACh, 2 μM) or electrical field stimulation (EFS) (12,13). Smooth muscle contraction of the ileum was measured using a Harvard isotonic transducer under 1 g resting force and recorded on a Harvard Universal Oscillograph (England) pen recorder device.
In one group of experiments, contraction was induced in rat ileum by addition of KCl (80 mM) into the bath. After equilibration period of 15 min, drugs were added cumulatively into the bath and their effects were evaluated after at least 10 min contact with the tissue. The essential oil, geraniol and citronellol were added at 5 min intervals.
In second group of experiments, contraction was induced by single concentration of acetylcholine (2 μM). Acetylcholine was in contact with the tissue for 20 s before it was washed off with fresh Tyrode's solution.
In the third group of experiments, EFS was performed with trains of rectangular pulses from a stimulator (made in Isfahan School of Pharmacy workshop) 6 V and 50 Hz for 1 s duration. The EFS was delivered at 15 min intervals to the ileum via a couple of platinum electrodes wires sandwiching the tissue preparation.
Effect of the essential oil, geraniol and citronellol were determined on above induced contractions using two fold increments in concentration until a full concentration response effect was achieved. Experiments were performed alongside time-matched vehicle treated controls with the tissue from the same animal. Similarly, effect of atropine (0.2-50 μM) and loperamide (20 nM-12.8 μM), as standard drugs, were examined on contraction induced by KCl, acetylcholine and EFS using four folds concentration increments for comparison with the essential oil.
Measurements and statistical analysis:
Contractile response to KCl, acetylcholine and EFS were measured as maximum amplitude from the baseline, just before addition of each concentration of the drugs and expressed as the percentage of the initial response in the absence of drugs for each tissue. All the values are quoted as mean ± standard error of the mean (SEM).
Statistical significance was assessed using One-way analysis of variance (ANOVA) for repeated measures and when appropriate were compared with the control groups using unpaired Student's t-test. Differences was considered statistically significant for P<0.05.
Whenever appropriate, the IC50 value (drug concentration causing 50% of maximum response), was calculated. Sigma plot (version11) computer program was used for statistical analysis, drawing the graphs and calculation of IC50 values.
RESULTS
Plant materials analysis
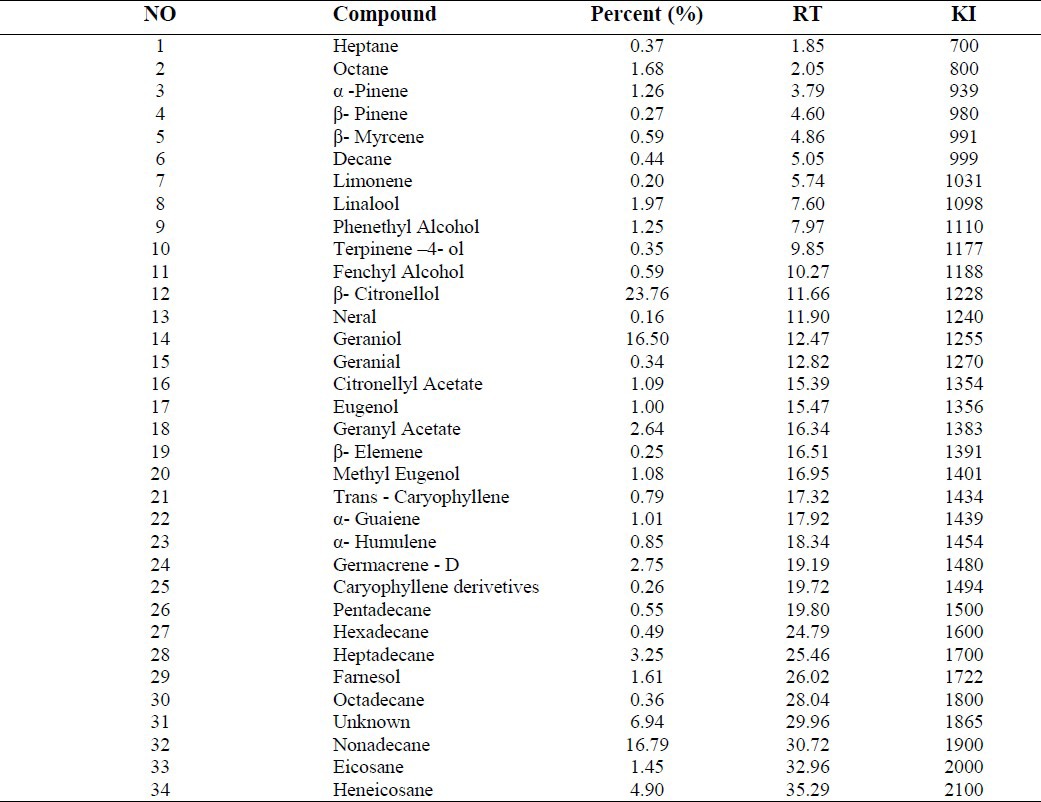
At least 34 compounds were identified in the essential oil of R. damascena, representing about 97% of the total oil. The results of GC-MS analysis of the R. damascena essential oil are shown in Table 1. The main components were β-citronellol (23%), nonadecane (16%), geraniol (16%) and heneicosane (5%).
Table 1.
Constituents of essential oil of R. damascena Mill flower collected from Kashan (Iran). The compounds are listed in order of their retention time (RT) on the HP-5MS. Kovates Indices (KI) were calculated using the Kovates equation.
In vitro contractility assessment
Rat isolated ileum suspended in the organ bath, usually showed spontaneous activity which subsided gradually over the time. KCl (80 mM) caused tonic contraction which maintained for the duration of the study. Acetylcholine caused a rapid contraction of rat ileum reaching its peak within 20 s of the contact time. Rat ileum contracted rapidly to EFS, reaching a peak within 10 s followed by partial relaxation which was then followed by a slower second peak with variable magnitude and then relaxed towards the baseline. The EFS responses were similar to EFS biphasic response reported by Ekblad & Sundler (14). Atropine (200 nM to 50 μM), at concentrations which blocked acetylcholine responses in rat ileum, had no effect on KCl induced contraction but only partially attenuated the EFS responses at its highest used concentration.
Loperamide (80 nM to 12.8 μM) concentration dependently reduced contractile responses to KCl. At similar concentration ranges, the initial responses to EFS were also attenuated by loperamide. The secondary evoked EFS response was inhibited in similar manner as the initial EFS response. Loperamide at higher concentration (0.8 μM) start to inhibit the ACh contraction and at its highest used concentration (12.8 μM), where the response to KCl and EFS was totally removed, still 30% of the response remained (Fig. 1).
Fig. 1.
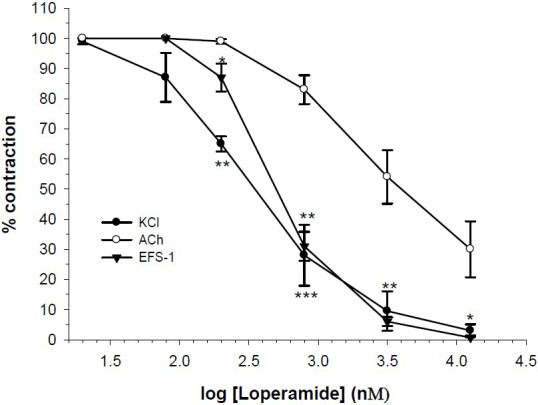
Effect of loperamide on tension development to potassium chloride (KCl, 80 mM), acetylcholine (ACh, 2 μM) and first contractile responses to electrical field stimulation (EFS-1, 6V, 50Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of loperamide. Lines drawn through the points, using four fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6). There are statistically differences in percent of inhibition of ACh in comparison to KCl and EFS, Keys: *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
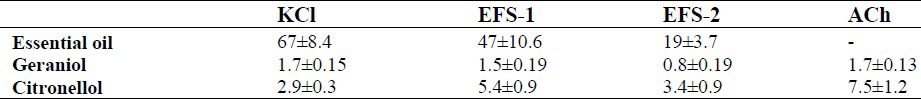
The essential oil of R. damascena (10-160 μg/ml), concentration dependently inhibited contraction induced by 80 mM KCl (Fig. 2). The essential oil at concentration which removed contractile response to KCl, only reduced the acetylcholine response by 7.0 ± 5.9%. The IC50 values are compared in Table 2. The vehicle had no significant effect on ileum contraction induced by acetylcholine, EFS or KCl (ANOVA).
Fig. 2.
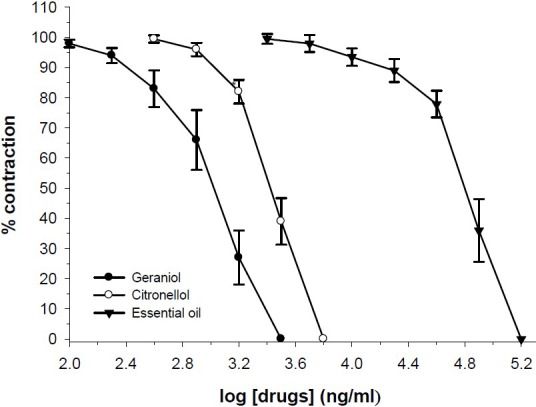
Effect of R. damascena essential oil, geraniol and citronellol on tension development to KCl, (80 mM) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of test compounds. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM (n=6).
Table 2.
Inhibitory concentrations (μg/ml) causing 50% inhibition of rat ileum contraction (IC50) induced by KCl (80 mM), electrical field stimulations (EFS) and acetylcholine (ACh, 2 μM).
Relaxant effect of the essential oil of R. damascena at concentration ranges which inhibited the KCl responses were also examined on biphasic contractions induced by EFS. The essential oil of R. damascena at concentration ranges of 10 μg/ml to 160 μg/ml had a similar pattern of inhibition on EFS responses (Fig. 3). The IC50 value of the essential oil for the initial and the secondary contractile response are shown in Table 2. The percentage inhibitory effect of the essential oil was very similar on both the initial (EFS1) and the secondary contraction induced by EFS (EFS2). There was no statistically difference in vehicle treated time-matched control tissues contractions over the course of study (ANOVA). The inhibitory effect of the essential oil on KCl, ACh and EFS responses was reversible following washing the tissue with fresh Tyrode's solution.
Fig. 3.
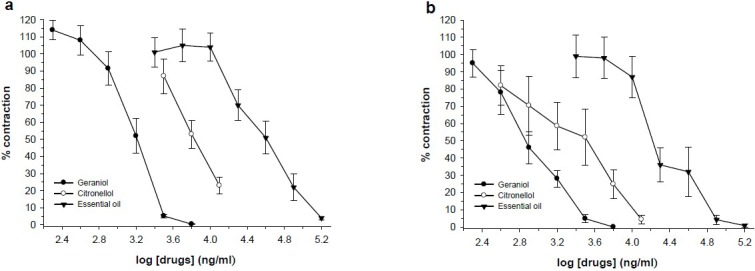
Effect of R. damascena essential oil (n=6), geraniol (n=6) and citronellol (n=5) on tension development to first (a) and second (b) contractile responses to electrical field stimulation (EFS, 6V, 50Hz, 1 s duration) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the test compounds. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM.
Geraniol (0.2-3.2 μg/ml) and citronellol (0.8-6.4 μg/ml) in concentration dependent manner inhibited ileum contraction induced by KCl (Fig. 2). Both geraniol and citronellol inhibited EFS responses (Fig. 3). However, geraniol and citronellol were less effective on ACh response and inihibition of ACh response was observed at higher bath concentration in comparison with inhibition of KCl response (Fig. 4). The IC50 values for comparison with the essential oils are presented in Table 2.
Fig. 4.
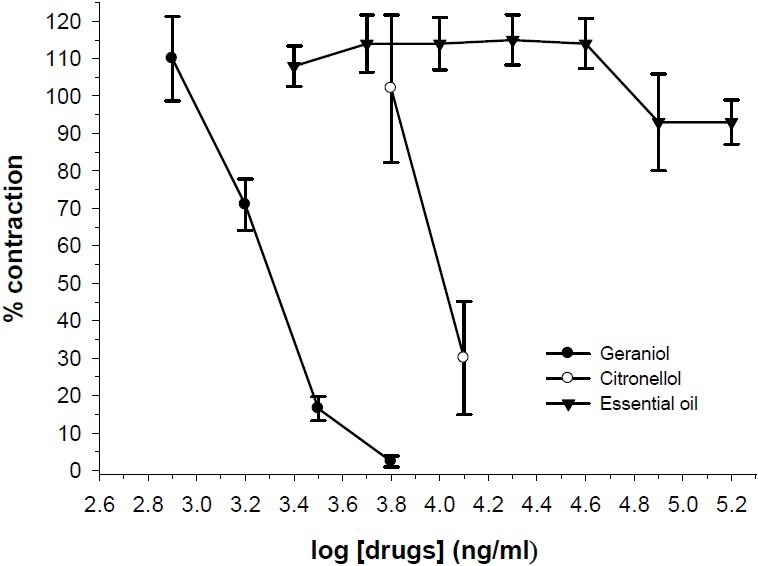
Effect of R. damascena essential oil (n=6), geraniol (n=6) and citronellol (n=5) on tension development to acetylcholine (ACh, 2 μM) in isolated ileum of rats. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of the test compounds. Lines drawn through the points, using two fold increments in concentration. The points are mean and the vertical bars show the SEM.
DISCUSSION
Dried flower petals of R. damascena traditionally are used to alleviate constipation while rose water containing traces of R. damascena essential oil are believed to alleviate bowel spasm (7,8). Drugs that affect lower GI function may act on smooth muscles and/or work by modulating the activity of the enteric nervous system (ENS). The ENS is embedded in the lining of the GI system. The neurotransmitters acetylcholine (ACh), serotonin (5HT), and a number of peptides including opioid peptide are the important regulators of motility and water absorption (15).
The essential oil had an inhibitory effect on contraction induced by KCl, and EFS. The inhibitory effect of the essential oil of R. damascena is in parallel with people's belief that rose water (or Golab as called in Iran) is useful for abdominal spasm (8,16). From identified components in the essential oil, geraniol and citronellol were examined on ileum and it was found to be 40 and 20 times more potent than the essential oil respectively in inhibiting KCl response. Therefore, they ought to have major contribution to the inhibitory effect seen with the essential oil. Other constituents, β-caryophyllene (17), methyleugenol (18), eugenol (19), limonene (20), α-pinene and β-pinene (10) are also known to have anti-spasmodic effect on ileum. Farnesol, a constituent of the essential oil, also known to inhibit L-type Ca2+ channels in vascular smooth muscle cells (21), which could explain the inhibition of KCl response. Linalool, another constituent, is reported to reduce the ACh evoked release in neuromuscular junction (22). Similar mechanism may explain the reduction in EFS response. The essential oil, however, only partially reduced the ACh response, which may indicate that the inhibitory effect of the essential oil is mainly mediated via cell membrane rather than intracellular mechanisms.
The main pharmacological agents that decrease GI motility are opioids and muscarinic receptor antagonists (23). Atropine and loperamide were standard compounds in this study. Although atropine completely abolished the contractile effect of ACh on the ileum, it only incompletely inhibited the effect of EFS. The remaining contraction is most likely due to release of other excitatory neurotransmitters from the enteric plexus such as serotonin, histamine, vasoactive intestinal peptide, substance P, etc (24). This indicates that excitatory transmitters other than ACh are also important in normal function of myenteric plexus. Removal of ACh response by atropine, indicate that ACh action is mainly mediated through muscarinic receptors, whose activation result in release of Ca2+ ions from intracellular stores mediated through M3 receptors (25). Inhibition of KCl response by the essential oil indicates that the inhibitory effect is post-synaptic but it is not like muscarinic receptor antagonists. The extract of Rosa damascena also is reported to have inhibitory effect on guinea-pig tracheal smooth muscle (6,26,27) but so far the effect of extract on isolated ileum had not been reported.
Loperamide is one of the opioids used in diarrhoea because it has relatively selective action on the GI tract (28). The effect of loperamide on neurons of myenteric plexus was inhibitory, associated with reduction in neurotransmitter release mediated through pre-synaptic opioid μ- receptors (29). Opioid receptors also exists on ileum smooth muscles, whose activation may reduced influx of Ca2+ through voltage gated calcium channels (30). This could explain the inhibitory action of loperamide on KCl response.
CONCLUSION
In conclusion we have shown that R. damascena essential oil exhibited inhibitory effect on rat isolated ileum. These experiments support the use of rose water (Golab) as antispasmodic remedy for treatment of abdominal spasm. Geraniol and citronellol are two major components which were responsible for the inhibitory effect of R. damascena essential oil.
ACKNOWLEDGMENT
We would like to thanks the research department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Evans WC. London: WB Sunders company Ltd; 1996. Trease and Evans’ Pharmacognosy; pp. 151–163. [Google Scholar]
- 2.Ghahreman A. Tehran: Institute of forests and rangelands Publications; 2001. Flora of Iran; p. 2465. [Google Scholar]
- 3.Zargari A. 5th ed. Vol. 2. Tehran: Tehran University Publications; 1992. Medicinal Plants; pp. 281–284. [Google Scholar]
- 4.Baydar H, Baydar NG. The effects of harvest date fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.) Ind Crop Prod. 2005;21:251–255. [Google Scholar]
- 5.Widrlechner MP. History and utilization of Rosa damascene. Econ Bot. 1981;35:42–58. [Google Scholar]
- 6.Boskabady M, Shafei M, Saberi Z, Amini S. Pharmacological effects of Rosa damascena. Iranian J Basic Med Sci. 2011;14:295–307. [PMC free article] [PubMed] [Google Scholar]
- 7.Sharafkandy A. Tehran: Ministry of Guidance publication; 1990. Ave-sina Law in Medicine; pp. 129–131. [Google Scholar]
- 8.Mirheydar H. 1st ed. Vol. 3. Iran: Islamic Culture Press; 1993. Plant Science; pp. 392–396. [Google Scholar]
- 9.European Pharmacopoeia. Council of Europe Strasbourg. 2002:183–184. [Google Scholar]
- 10.Sadraei H, Asghari G, Hajhashemi V, Kolagar A, Ebrahimi M. Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine. 2001;8:370–376. doi: 10.1078/0944-7113-00052. [DOI] [PubMed] [Google Scholar]
- 11.Washington DC: The National Academies Press; 2010. Committee for the update of the guide for the care and use of laboratory animals National Research Council. Guide for the Care and use of Laboratory animals. [Google Scholar]
- 12.Sadraei H, Asghari G, Poorkhosravi R. Spasmolytic effect of root and aerial parts extract of Pycnocycla spinosa on neural stimulation of rat ileum. Res Pharm Sci. 2011;6:43–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Sadraei H, Shokoohinia Y, Sajjadi SE, Ghadirian B. Antispasmodic effect of osthole and Prangos ferulacea extract on rat uterus smooth muscle motility. Res Pharm Sci. 2012;7:141–149. [PMC free article] [PubMed] [Google Scholar]
- 14.Ekblad E, Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: Modulation by pituitary adenylate cyclase-activating peptide forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 15.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;17:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 16.Nikbakht A, Kafi M. A study on the relationships between Iranian people and Damask rose (Rosa damascena) and its therapeutic and healing properties. Acta Hort (ISHS) 2008;790:251–254. [Google Scholar]
- 17.Leonhardt V, Leal-Cardoso JH, Lahlou S, Albuquerque AA, Porto RS, Celedônio NR, et al. Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundam Clin Pharmacol. 2010;24:749–758. doi: 10.1111/j.1472-8206.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 18.Lima CC, Criddle DN, Coelho-de-Souza AN, Monte FJ, Jaffar M, Leal-Cardoso JH. Relaxant and antispasmodic actions of methyleugenol on guinea-pig isolated ileum. Planta Med. 2000;66:408–411. doi: 10.1055/s-2000-8594. [DOI] [PubMed] [Google Scholar]
- 19.Leal-Cardoso JH, Lahlou S, Coelho-de-Souza AN, Criddle DN, Pinto Duarte GI, Santos MA, et al. Inhibitory actions of eugenol on rat isolated ileum. Can J Physiol Pharmacol. 2002;80:901–906. doi: 10.1139/y02-117. [DOI] [PubMed] [Google Scholar]
- 20.de Sousa DP, Júnior GA, Andrade LN, Calasans FR, Nunes XP, Barbosa-Filho JM, et al. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z Naturforsch C. 2008;63:808–812. doi: 10.1515/znc-2008-11-1205. [DOI] [PubMed] [Google Scholar]
- 21.Roullet JB, Luft U, Xue H, Chapman J, Bychkov R, Roullet C, et al. Farnesol inhibits L-type Ca2+ channels in vascular smooth muscle cells. J Biol Chem. 1997;272:32240–32246. doi: 10.1074/jbc.272.51.32240. [DOI] [PubMed] [Google Scholar]
- 22.Re L, Barocci S, Sonnino S, Mencarelli A, Vivani C, Paolucci G, et al. Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction. Pharmacol Res. 2000;42:82–177. doi: 10.1006/phrs.2000.0671. [DOI] [PubMed] [Google Scholar]
- 23.Pasricha PJ. Treatment of disorders of bowel motility and water flux; antiemetics; agents used in biliary and pancreatic disease. In: Hardman J.G, Limbird L.E, editors. Goodman & Gilmans The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 983–1008. [Google Scholar]
- 24.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 25.Goyal RK. Identification localization and classification of muscarinic receptor subtypes in the gut. Life Sci. 1988;43:2209–2220. doi: 10.1016/0024-3205(88)90414-6. [DOI] [PubMed] [Google Scholar]
- 26.Boskabady MH, Kiani S, Rakhshandeh H. Relaxant effect of Rosa damascena on guinea pig tracheal chains and its possible mechanism(s) J Ethnopharmacol. 2006;106:377–382. doi: 10.1016/j.jep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Rakhshandeh H, Boskabady MH, Mossavi Z, Gholami M, Saberi Z. The differences in the relaxant effect of different fractions of Rosa damascena on guinea pig tracheal smooth muscle. Iranian J Basic Med Sci. 2010;13:126–132. [Google Scholar]
- 28.Daugherty LM. Loperamide hydrochloride. Am Pharm. 1990;NS30:45–48. doi: 10.1016/s0160-3450(15)31396-9. [DOI] [PubMed] [Google Scholar]
- 29.Kromer W. Endogenous and exogenous opioid in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 30.Reynolds IJ, Gould RJ, Snyder SH. Loperamide: Blocked of calcium channels as a mechanism for antidiarrheal effect. J Pharmacol Exp Ther. 1984;231:628–632. [PubMed] [Google Scholar]


