Abstract
Abrupt cessation of morphine leads to withdrawal signs and cognitive deficits. Endocannabinoid system is activated during withdrawal; therefore, the aim of the present study was to assess the effects of AM281, cannabinoid antagonist/inverse agonist, on memory deficit following spontaneous morphine withdrawal. Cognition was evaluated by using the object recognition task. The novel object recognition task was tested in a square wooden open-field box using objects. The test was consisting of three sections: 15 min exploration, first trial for 12 min and second one for 5 min. In the second trial the difference in exploration between a previously seen object and a novel one, was considered as an index of memory performance (recognition index - RI). Male mice were made dependent by increasing doses of morphine (30-90 mg/kg) subcutaneously twice daily for 3 days. AM281 (0.62, 1.25 and 2.5 mg/kg) were used in chronic form concurrent with morphine i.p. or acutely (2.5, 5 and 10 mg/kg) on the last day. RI was evaluated on the third day 4 h after the last dose of morphine. Chronic administration of AM281 at 2.5 mg/kg improved RI to the 22.1 ± 4.8 and single dose of AM281 at 5 mg/kg improved the memory impairment to the 8.5 ± 4, as compared with vehicle-treated which was 4.8 ± 2.5. The results suggested that administration of AM281 at a dose of 2.5 mg/kg in chronic form and 5 mg/kg in acute dose improved memory.
Keywords: AM281, Cannabinoid antagonist, Memory performance, Spontaneous morphine withdrawal
INTRODUCTION
Opiates are amongst the most helpful medicines; although used as recreational drugs (1). Chronic misuse of these compounds gives rise to the tolerance and physical dependence known as opiate addiction. Withdrawal syndrome is likely to appear as physiological and behavioral symptoms after prolonged exposure to morphine (2).
Prolonged exposure to opiates also disrupts brain function (3). Cognitive deficit exists even after subsiding somatic withdrawal signs that may arise from impairment of brain function during chronic misuse (4). In human, memory deficit leads to impairment of every day function, therefore, it would be rational to prevent memory impairment.
Prolonged misuse of morphine enhances the density of cannabinoid CB1 receptor mRNA in brain areas activated during morphine withdrawal (5). Cannabinoids exert their actions through two main receptors CB1, mainly in brain, CB2, located in immune cells; both of them inhibit adenylate cyclase (6). It has been suggested that chronic exposure to the cannabinoid agonist disrupts memory (7). Additionally, it is proposed that acute use of natural canabinoids such as Δ9-tetrahydrocannabinol or synthetic cannabinoid analogues impairs cognition in laboratory animals (8) and humans (9). Cannabinoid antagonists reverse cognitive deficit memory induced by MDMA (3, 4 methyl enedioxy methamphetamine) withdrawal (10). Also it has been suggested that long term-treatment with CB1 receptor antagonist attenuates morphine withdrawal signs too (11). We previously reported that AM281 ameliorates memory deficit induced by naloxone withdrawal (12) , but the severity of signs are different between spontaneous and naloxone induced morphine withdrawal. Spontaneous withdrawal is more similar to human withdrawal (13). Therefore, this study was designed to assess the effect of AM281, cannabinoid antagonist, on mice performance in object recognition task during spontaneous morphine withdrawal.
MATERIALS AND METHODS
Animals
Male NMRI mice (Pasteur institute, Tehran, Iran) with the weight of 25-30 g were used. Mice were kept under controlled conditions and a 12 h light/dark cycle. In order to reduce the effect of circadian rhythm in a noise-free room, all experiments were carried out between 08:00 and 13:00 h. At least six mice were used in each group. All experimental procedures were approved by the Ethical Committee of the Isfahan University of Medical Sciences, and in accordance with the international principles for use and care of laboratory animals.
Object recognition task
The object recognition was designed as explained by Bertaina-Anglade and coworkers (14). The apparatus was made of a square wooden open-field with base dimensions of 35 × 35 cm and walls of 40 cm. The test was carried out in a dark room under the dim light. To avoid the effect of odor in trials, the apparatus and objects were cleaned by water between the trials. Objects were logos with different shapes and colors, equidistant from the sides of the apparatus. The day before the test, mice were placed in the box individually and allowed to explore the arena barely for 15 min. On the second day, mice were submitted to two trials with 20 min interval. During the first trial (acquisition trial, T1), each animal was placed in the arena containing two copies of objects and allowed them to explore the open field freely until spent 20 s exploring the both objects, total time exploration of two objects were recorded. Mice which not explore the objects during 12 min were excluded.
Exploration was described as looking at, touching or placing the nose on the objects. For the second trial (retrieval, T2), one of the objects was replaced with the novel one, each mouse was placed back in the task for 5 min and exploration time for the novel (N) and familiar (F) objects were recorded. Times were finally analyzed.
Drugs
Morphine sulfate (Temade, Tehran, Iran), and AM281 (Sigma, USA) were used. Morphine was dissolved in saline 0.9%, whilst AM281 was dissolved in dimethyl sulfoxide (DMSO) 4% and saline 0.9%, all the drugs were prepared freshly. AM281 was injected intraperitoneally (i.p.), however, morphine was injected subcutaneously (s.c.). Test drug concentrations were adjusted as each animal received a volume of 10 ml/kg.
Drug treatments
Animals were made dependent by increasing doses of morphine, twice daily. Mice were injected with 30 and 45 mg/kg morphine on the first day, 60 and 90 mg/kg on the second day, and 90 mg/kg on the third day, with 12 h intervals (15). Control group received normal saline only twice daily for three days. Vehicle group received morphine and vehicle for three days. AM281 (0.62, 1.25 and 2.5 mg/kg) was administrated i.p. every day concurrently with morphine except the day of experiment. To assess the acute effect of AM281 (2.5, 5 and 10 mg/kg), it was singly injected 40 min before second trial (test trial). Cognition was evaluated 4 h after the last dose of morphine on the third day (16).
Data processing and statistical analysis
Time required for exploration the identical objects (T1) in the first trial and time spent for exploration of the N and F objects in the second trial (T2) were calculated. Cognition memory was evaluated by means of a recognition index (RI). RI was calculated for each animal using the following formula: difference between the time exploring the N and F object, divided by total time exploring both objects: N-F/N+F by Ennaceur and coworkers. Results were analyzed with one way ANOVA. P<0.05 was considered as a significant result. Results were expressed as the mean ± S.E.M. Sigma Stat Ver. 3.5 was used as computing software.
RESULTS
Effect of acute administration of AM281 on memory performance
The dependent mice received AM281 at doses of 2.5, 5 and 10 mg/kg 45 min prior to T2. These results suggest that treatment at a dose of 5 mg/kg was able to improve acquisition time and change the exploration time but not to the saline values (Fig. 1).
Fig. 1.
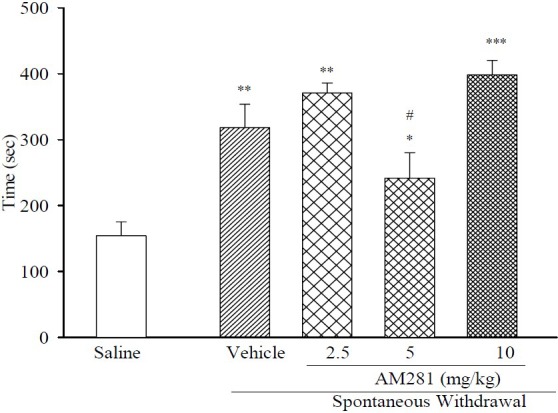
Effect of acute administration of AM281 on duration of T1 (time required to achieve 20 s of object exploration) during spontaneous morphine withdrawal in all groups (n=6). Results are expressed as mean ± S.E.M. ***P<0.001, **P<0.01 and *P<0.05 in comparison to normal saline, #P<0.05 in comparison to vehicle.
Fig. 2 shows that acute administration of AM281 at a dose of 5 mg/kg, significantly augmented RI. However, the RI for this treatment was less than that of saline. We also observed that AM281 at the dose of 2.5 and 10 mg/kg exerted no influence upon RI.
Fig. 2.
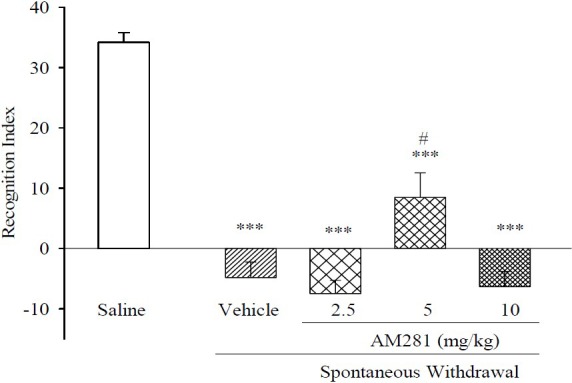
Effect of acute administration of AM281 on memory performance on two trial object recognition task, during spontaneous morphine withdrawal. In all groups (n=6). ***P<0.001 in comparison to normal saline, #P<0.05 in comparison to vehicle.
Effect of chronic administration of AM281 on memory performance
As depicted in Fig. 3, the simultaneous daily administration of AM281 with morphine significantly shortened the exploration time (T1), as compared with morphine-dependent mice receiving vehicle. Moreover, in contrast to T1 in vehicle group, AM281 treatment at doses of 1.25 and 2.5 mg/kg ameliorated T1, whilst AM281 at 0.62 mg/kg had no effect on T1.
Fig. 3.
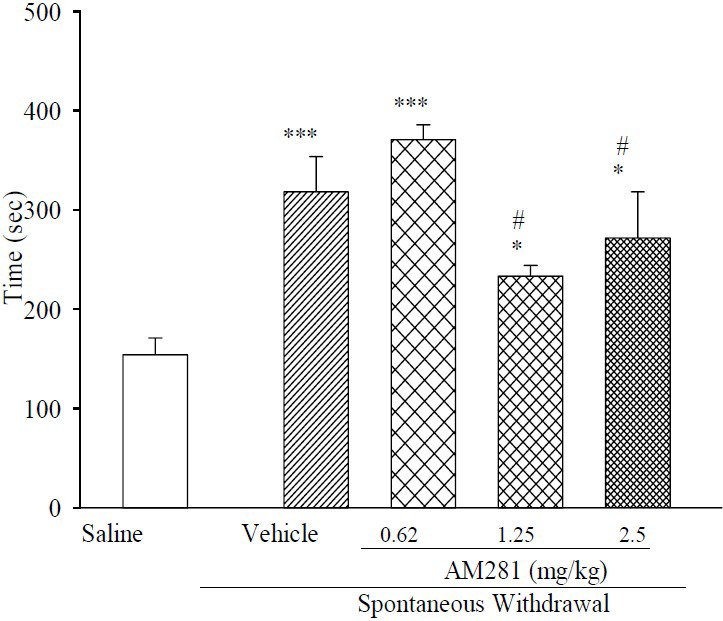
Effect of chronic administration of AM281 on duration of T1 (time required to achieve 20 s of object exploration in the first trial) in morphine dependent mice. In all vehicle groups n=6. Results are expressed as mean ± S.E.M. ***P<0.001 and *P<0.05 in comparison to normal saline, #P< 0.05 in comparison to vehicle.
Fig. 4 illustrates RI scores following the concurrent treatments. We found that treatments made their impacts dose-dependently. Additionally, compared with RI in morphine-treated animals, the RI score rose as a consequence of chronic concurrent administrations of AM281 (2.50 mg/kg) and morphine. AM281 at 0.62 and 1.25 mg/kg did not improve the RI score.
Fig. 4.
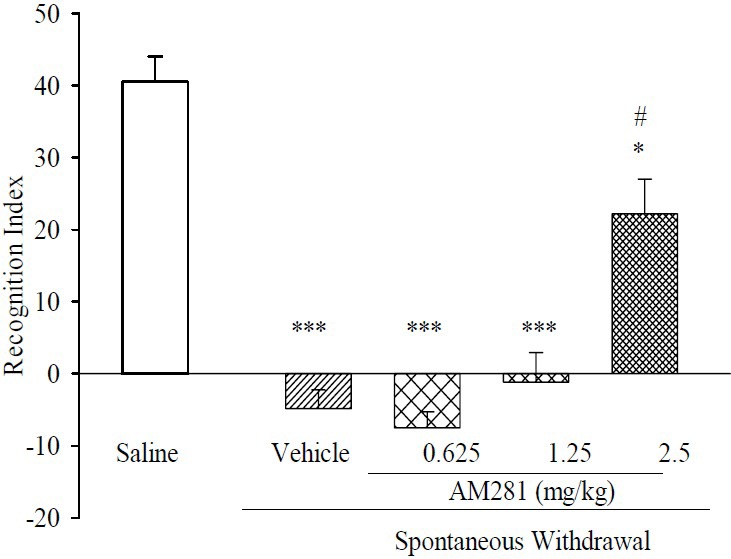
Effect of chronic administration of AM281 on memory performance on two trial object recognition task, during spontaneous morphine withdrawal. In all groups n=6. Results are expressed as mean ± S.E.M. ***P<0.001 and *P<0.05, in comparison to normal saline, #P< 0.05 in comparison to vehicle.
DISCUSSION
Memory impairment was evaluated during spontaneous morphine withdrawal, 4 h following the last dose of morphine while the influence of AM281 was investigated in the object recognition task. Acute administration of AM281 shortened exploration time and improved memory performance, as did chronic administration of AM281. Object recognition test includes a rapid assessment of cognition predicated upon rodents’ propensity to explore new objects (17).
This test assesses memory performance under normal environmental conditions such as low stress. Therefore, this model is very similar to human (18).
A previous research demonstrated that spontaneous morphine withdrawal gives rise to impairment of memory, 14 h subsequent to the last dose of morphine in mice (19). Mesripour and coworkers showed that mifepristone and metyrapone reversed recognition memory loss induced by spontaneous morphine withdrawal (16). Manzanares and coworkers found that cannabinoid CB1 receptors were changed in opiate dependence (20). In spite of the fact that association between morphine withdrawal and endocannabinoid release has not yet been completely worked out, anandamide (an endocannabinoid) release was shown during spontaneous morphine withdrawal in the brain (21). This may refer to the fact that AM404 (anandamide transport inhibitor) exerts no impact upon naloxone-precipitated withdrawal. In addition, aforesaid decrease in anandamide secretion may highlight the increased expression of striatal cannabinoid CB1 receptors in opiate-dependent rodents (22). It was demonstrated that progressive neuro adaption related to spontaneous withdrawal brought about a normalization of anandamide release, as reflected by the attenuation of spontaneous withdrawal through the administration of the AM404, a carrier inhibitor. In fact, AM404 is likely to improve the inhibition of this endogenous cannabinoid on locomotors activity and anxiety. This may be as a result of restricting the uptake of anandamide (23). What we know mostly about cannabinoids and activity-dependent changes in synaptic strength is largely based upon studies investigating excitatory synapses by means of acute hippocampal slices as the experimental model (24). Induction of long term potential (LTP) was inhibited by cannabinoid receptor activation in the hippocampal slice. Using endocannabinoid such as anandamide, inhibition of LTP was shown in field potentials in the CA1 region (25,26). Akirav and coworkers found that cannabinoid antagonists such as SR141716A block the impairment in the induction of LTP of the CA1 (27). Rubino and coworkers argued that cannabinoid receptor antagonists exerted a therapeutic effect upon attenuating morphine withdrawal syndrome as long term-treatment with CB1 receptor antagonist attenuates somatic withdrawal sign also (11).
CONCLUSION
The most striking result to emerge from this study is that impairment of recognition memory in spontaneous morphine withdrawal arises from the activation of cannanbinoid CB1 receptors. AM281 at the doses of 2.5 mg/kg in chronic form and 5 mg/kg in acute form improved RI in spontaneous morphine withdrawal higher doses had negative effect perhaps because of their anxiety-like effect (28). We highlight the positive role of CB1 antagonist in ameliorating spontaneous morphine withdrawal syndrome, which was consisting with our previous finding (12). Finally, further investigation and experimentation into the role of CB1 receptors is strongly recommended.
ACKNOWLEDGMENT
This work was financially supported by research council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Mirzaii-Dizgah I, Ojaghi R, Sadeghipour-Roodsari HR, Karimian SM, Sohanaki H. Attenuation of morphine withdrawal signs by low level laser therapy in rats. Behav Brain Res. 2009;196:268–270. doi: 10.1016/j.bbr.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Feng P, Meissler JR, Adler MW, Eisenstein TK. Morphine withdrawal sensitizes mice to lipopolysaccharide: elevated TNF-alpha and nitric oxide with decreased IL-12. J Neuroimmunol. 2005;164:57–65. doi: 10.1016/j.jneuroim.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Miller L. Neuropsychological assessment of substance abusers: review and recommendations. J Subst Abuse Treat. 1985;2:5–17. doi: 10.1016/0740-5472(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 4.O’Brian CP. Drug addiction and drug abuse. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill companies; 2006. pp. 607–626. [Google Scholar]
- 5.Navarro M, Chowen J, Rocio M, Del Arco J, Villanua MA, Martin Y, Roberts AJ, et al. CB1 cannabinoid receptor antagonist-induced opiaite withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology. 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- 8.Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 10.Nawata Y, Hiranita T, Yamamoto T. A canabinoid CB 1 receptor antagonist ameliorates impairment of recognition memory on withdrawal from MDMA. Neuropsychopharmacology. 2010;35:515–520. doi: 10.1038/npp.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubino T, Massi P, Vigano D, Fuzio D, Parolaro D. Long-term treatment with SR141716, the CB1 receptor antagonist, influence morphine withdrawal syndrome. Life Sci. 2000;66:2213–2221. doi: 10.1016/s0024-3205(00)00547-6. [DOI] [PubMed] [Google Scholar]
- 12.Vaseghi G, Rabbani M, Hajhashemi V. The CB1 receptor antagonist, AM281, improves recognition loss induced by naloxone in morphine withdrawal mice. Bas Clin Pharmacol Toxicol. doi: 10.1111/j.1742-7843.2012.00881.x. In press. [DOI] [PubMed] [Google Scholar]
- 13.Zelena D, Barna I, Mlynarik M, Gupta OP, Jezova D, Makara GB. Stress symptoms induced by repeated morphine withdrawal in comparison to other chronic stress models in mice. Neuroendocrinology. 2005;81:205–215. doi: 10.1159/000087034. [DOI] [PubMed] [Google Scholar]
- 14.Bertaina-Anglade V, Enjuanes E, Morillon D, Drieu la Rochelle C. The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Meth. 2006;54:99–105. doi: 10.1016/j.vascn.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Broseta I, Rodriguez-Arias M, Stinus L, Minarro J. Ethological analysis of morphine withdrawal with different dependence programs in male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:335–347. doi: 10.1016/s0278-5846(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 16.Mesripour A, Hajhashemi V, Rabbani M. Metyrapone and Mifepristone reverse recognition on memory loss induced by spontaneous morphine withdrawal. Bas Clin Pharmacol Toxicol. 2008;102:377–381. doi: 10.1111/j.1742-7843.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Meer P, Raber J. Mouse behavioural analysis in systems biology. Biochem J. 2005;389:593–610. doi: 10.1042/BJ20042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuda S, Roozendaal B, Mc Gaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci (USA) 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabbani M, Hajhashemi V, Mesripour A. Increase in brain corticosterone concentration and recognition memory impairment following morphine withdrawal. Stress. 2009;12:451–456. doi: 10.1080/10253890802659612. [DOI] [PubMed] [Google Scholar]
- 20.Manzanares J, Corchero J, Romero J, Fernández-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 21.Giuffrida A, Parsons LH, Kerr TM, Rodriguez F de Fonseca, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 22.Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Arco I, Navarro M, Bilbao A, Ferrer B, Piomelli D, Rodrıguez De Fonseca F. Attenuation of spontaneous opiate withdrawal in mice by the anandamide transport inhibitor AM404. Eur J Pharmacol. 2002;454:103–104. doi: 10.1016/s0014-2999(02)02483-4. [DOI] [PubMed] [Google Scholar]
- 24.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 25.Nowicky AV, Teyler TJ, Vardaris RM. The modulation of long term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull. 1987;19:663–672. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 26.Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212–2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- 27.Akirav I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front Behav Neurosci. 2011;5:34. doi: 10.3389/fnbeh.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic andpharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]


