Abstract
Reteplase is a potent thrombolytic agent which is widely used in the management of acute myocardial infarction and stroke. It belongs to the third generation of the thrombolytic drugs and has been derived from native human tissue plasminogen activator by removing three domains of it and keeping the Kringle 2 and Serine protease domains. However, the high cost of this drug, has limited the application of this drug especially in the developing and third world countries. The most laborious steps in the bacterial production of this drug is its purification and refolding steps which keep the process yield low and the cost high. Therefore, in the present study we evaluated the expression of reteplase by a non-lytic insect cell expression system. Following cloning and transfection procedures, recombinant Sf9 insect cell clones expressing the reteplase protein were selected. Primarily, the expression was verified by dot-blot analysis and subsequently it was confirmed by Western Blotting showing a band of about 45 kD on nitrocellulose membrane. The biological activity of the expressed protein was also evaluated and showed to be about 29 IU/ml. This confirmed the possibility of expression and the correct folding of the expressed protein. Hence, optimization of the expression followed by purification of the protein could be the next steps of the study.
Keywords: Reteplase, Insect cell expression system, pMIB/V5-His, Thrombolysis
INTRODUCTION
Thrombolytic therapy has been recognized as a significant improvement in the management of patients with acute myocardial infarction, pulmonary embolism, deep vein thrombosis, arterial thrombosis, acute thrombosis of retinal vessel, extensive coronary emboli, and peripheral vascular thromboembolism. Hence, several plasminogen activators (PA) have been developed to treat thrombotic diseases (1). Tissue-plasminogen activator (t-PA) is a thrombolytic agent of choice for the treatment of acute myocardial infarction (2,3). It is a 68 kDa glycoprotein consisted of 527 amino acids, which in the presence of fibrin promotes the conversion of plasminogen to plasmin. Its structure consists of five domains including finger domain (F) followed by a growth factor domain (EGF) close to the N-terminal region and the two kringle 1 (K1) and kringle 2 (K2) domains. Next to the kringle 2 domain is the serine protease domain with the catalytic site at its C terminus. Binding of both K2 and finger domains to fibrin accelerates activation of plasminogen by t-PA (4). t-PA has the advantage of causing no side effects such as systemic hemorrhaging and fibrinogen depletion (5). Although treatment with t-PA appears beneficial, there are some limitations. Short half life and rapid clearance from plasma due to the recognition of structural elements on first three N-terminal domains by certain hepatic receptors is the major limitation in the use of t-PA (6). Furthermore, it has been reported that t-PA may have certain side effects in clinic use, including enrichment of platelets and strong resistance to lysis (7). In addition, prokaryotic production and refolding process of its full length form is challenging (8). These drawbacks resulted in the design and application of a truncated form of t-PA which is called reteplase (9). This recombinant human plasminogen activator derivative (rPA) is a single chain protein containing 355 amino acid residues with molecular mass of 39 kDa and consists of the Kringle 2 and protease domains of t-PA (10,11). The enzymatic activity of reteplase is comparable to the native t-PA in vitro. It is a potent thrombolytic agent in clinical settings, due to its decreased affinity to fibrin which allows rPA to diffuse more freely into clots instead of binding only on the surface, and prolonged half-life resulting from removal of binding domain for hepatic cells involved in hepatic metabolic clearance of t-PA (7). Although rPA is one of the most effective agents for clinical thrombolysis, thousands of dollars per therapy dose make it still expensive, especially in developing countries (12). So far, the expression of rPA has been mostly reported in Escherichia coli. However, in this system, the enzymatic activity is obtained only following effective refolding of the purified inclusion bodies (13,14,15). Hence, the expression of rPA and its derivatives has been evaluated by some eukaryotic systems such as Chinese hamster ovary (CHO) cells (16) , and seaweed Laminaria japonica (12). One of the most applicable eukaryotic expression systems is the insect cell-based expression system which includes both the baculovirus expression system and non-viral insect cell expression systems (17,18). Insect cells perform most, if not all, of the post translational modifications (19). In addition, they do not contain pyrogens or endotoxins from microbes or contaminants from mammalian sources (20). Therefore, the baculovirus/insect cell expression systems could be efficiently and safely used for the production of recombinant proteins with therapeutic applications. Considering previous reports on the successful expression of fully active recombinant t-PA by the baculovirus-insect cell expression systems (21,22,23) , in the present study we evaluated the possibility of expression of reteplase by a non-lytic secretory insect cell expression system.
MATERIALS AND METHODS
Strain, plasmid and reagents
pMIB/V5-His C vector (Fig. 1) was obtained from Invitrogen (Carlsbad, CA). blasticidine S. HCl was purchased from Invivogen (San Diego, California, USA) and used for the selection of stable cell lines. GeneJet™ Gel extraction kit, GeneJet™ Plasmid Miniprep kit, pFu DNA polymerase and FastDigest™ restriction endonucleases were purchased from Fermentas (Fermentas; Vilnius, Lithuania). Construction of the recombinant plasmid and molecular cloning procedure was performed in Top 10 E. coli strain. All other chemicals and reagents were obtained from other commercial sources and were of the molecular biology grade.
Fig. 1.
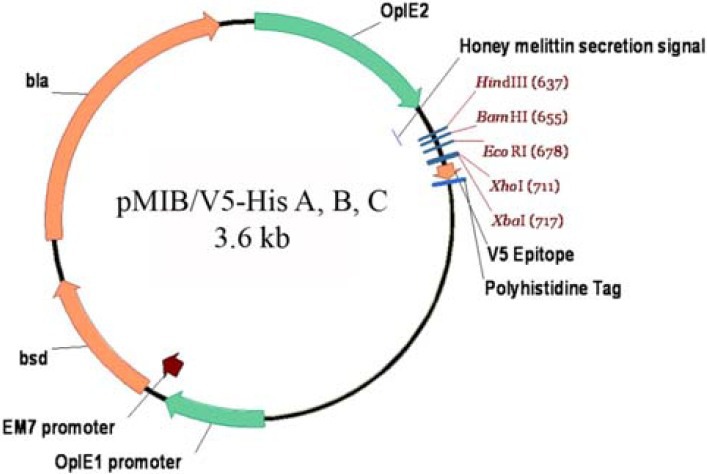
Schematic representation of the pMIB/V5-His plasmid. The version C of this plasmid was used in the present study.
PCR amplification and recombinant plasmid construction
The reteplase coding sequence was amplified using a recombinant plasmid obtained from our previous study (24) as PCR template. For PCR amplification, suitable primers were designed so that the expressed protein could be in frame with the upstream melittin signal sequence and the downstream V5 and 6X-His tags of the plasmid. The sequence of forward primer was 5’-CCCAAGCTTCATGTCTTACCAAGGAAAC-3’ and contained a HindIII recognition site (underlined). The sequence of reverse primer was 5’-CGCTCGAGCCGGTCGCATGTTGTC-3’ and contained an XhoI recognition site (underlined). PCR condition included a primary denaturation step of 5 min at 95°C, followed by 30 cycles of 45s at 94°C, 45s at 55°C and 80s at 72°C, and a final extension step of 10 min at 72°C. Following amplification of the desired fragment, it was gel purified, restriction endonuclease digested and ligated into similarly digested ends of the pMIB/V5-His plasmid, then used to transform Top10 E. coli cells. Following an overnight incubation of the transformed cells on LB-agar plates containing 100 μg/ml ampicillin, some colonies were selected and subjected to colony PCR to choose recombinant clones. The PCR condition was as above, and the 5´-CGCAACGATCTGGTAAACAC-3´ (OpIE2Fr) & 5´-GACAATACAAACTAAGATTTAGTCAG-3´ (OpIE2Rv) primers which flank the cloned gene were used. After selection of recombinant colonies, they were subjected to overnight cultivation in LB broth medium containing 100 μg/ml ampicillin for the preparation of plasmids. Finally, the fidelity of the cloning was verified by restriction enzyme digestion and DNA sequencing.
Insect cell culture and transfection
Spodoptera frugiperda (Sf9) insect cell line was obtained from Invitrogen and used as expression host. The cells were cultivated in Grace's insect cell medium (Invitrogen) supplemented with 10% Fetal bovine serum, or Ex-cell 420 serum free medium (Sigma, Germany), both media containing 100 U/ml penicillin and 100 mg/ml streptomycin (Biosera, UK). Transfection of the Sf9 cells with the authenticated recombinant pMIB plasmid was performed using cellfectin II transfection reagent (Invitrogen) as instructed by the manufacturer. Four days post transfection, the cell culture medium was replaced with fresh medium containing 80 μg/ml blasticidin S. HCl, to induce the integration of the plasmid elements into the genomic DNA of the transfected cells. The medium refreshment was continued every four days until isolated stable cell colonies were obtained. The stable cells were expanded to larger cell monolayers stepwise, and the reteplase producer cells were selected through dot-blot analysis.
Then the producer cells were cultivated in the presence of 10 μg/ml blasticidin S. HCl to maintain the integrated genes, and expression of the recombinant reteplase was evaluated by Western blot analysis.
Evaluation of reteplase expression by Dot-blot and Western blot analyses
In order to evaluate the expression of reteplase by the transfected insect cells dot blot analysis was performed. In this regard, the cell culture medium of the propagated stable cell lines were concentrated and spotted on nitrocellulose membrane. Then the membrane was blocked with 5% (v/v) skimmed milk containing 0.1% Tween 20 for 90 min.
Afterwards, the membrane was washed three times with PBS buffer containing 0.1% Tween 20 (wash buffer) and incubated with HRP-conjugated anti V5 tag antibody (Genescript, Germany) for 2 h. Finally the membrane was washed three times with the wash buffer and the reaction was developed with DAB reagent.
In order to evaluate the expression of reteplase by Western blot analysis, the protein bands were separated by 15% SDS-PAGE and then electrotransfered to nitrocellulose membrane. Then the remaining steps were performed as described for dot-blot analysis. In order to detect the expression of the reteplase protein, HRP conjugated anti His-tag antibody (Roche, Germany) was used.
Assessment of biological activity
The biological activity of the expressed reteplase was evaluated using AssaySense Human tPA Chromogenic Activity Assay Kit (Assaypro, MO, USA) according to the manufacturer's instructions. The assay measures the ability of tPA to convert the plasminogen to plasmin in coupled or indirect assays that contain tPA, plasminogen and a plasmin specific synthetic substrate. Briefly, an assay mix containing 50 μl assay diluents, 10 μl plasminogen and 20 μl plasmin substrate per each sample or standard was prepared. Then, 80 μl of the prepared mixture was added to wells of a 96-well plate. Afterwards, 20 μl of tPA standard or insect cell culture supernatant were added to each well and the plate was incubated at 37°C humidified incubator for 24 h. Finally the plate was subjected to absorbance read at 405 nm and the activity of the sample was measured according to the activity of the standard samples.
RESULTS
PCR amplification and cloning
Agarose gel electrophoresis of the PCR products confirmed the correctness of amplification by revealing a band of about 1100 bp (Fig. 2). Cloning of the gel purified fragments into the pMib/V5-His plasmid resulted in some ampicillin resistant bacterial colonies. Colony PCR using OpIE2Fr and OpIE2Rv primers confirmed some positive colonies by showing a band of about 1300 bp (Fig. 3-A).
Fig. 2.
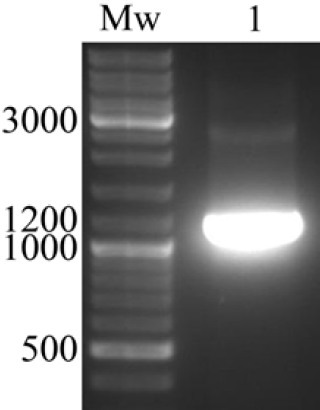
PCR amplification of reteplase cDNA. Agarose gel electrophoresis of the PCR product confirmed the amplification of reteplase cDNA by revealing a band of about 1100 bp. Mw; Molecular weight marker.
Fig. 3.
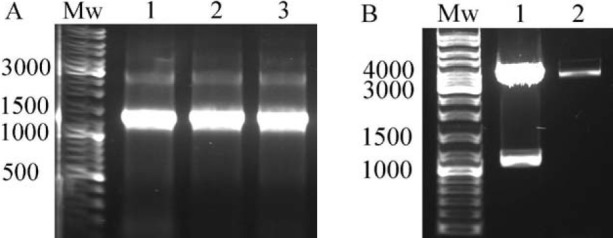
Verification of cloning by colony PCR and restriction enzyme digestion. (A) Some colonies showed to be recombinant by revealing a band of about 1300 bp following colony PCR using OpIE2Fr and OpIE2Rv primers. (B) Restriction endonuclease digestion of the prepared plasmid by HindIII and XhoI enzymes confirmed the cloning by releasing a band of about 1100 bp in some clones (1), while the other clones showed to be not recombinant (2). Mw; Molecular weight marker.
Extraction of the recombinant plasmids from positive colonies followed by their restriction endonuclease digestion with HindIII and XhoI enzymes revealed two fragments with sizes of about 1100 and 3600 bp confirming the correctness of cloning (Fig. 3). Finally, DNA sequencing of the recombinant plasmids validated the fidelity of the cloning. The authenticated recombinant plasmid was designated as pMIB-RPA plasmid and used in the next steps of the study.
Production of stable insect cells expressing reteplase
Following transfection of Sf9 cells with the pMIB-RPA plasmid, blasticidin S HCl screening continued until sufficiently large isolated resistant cell colonies were obtained. The colonies were continuously expanded to wells of a 6-well cell culture plate. Primary assessment of the concentrated medium of the cells using Dot-blot analysis confirmed their ability to express reteplase gene (Fig. 4). Finally, the reteplase producer stable cells were subjected to high yield expression of the protein in larger monolayers.
Fig. 4.
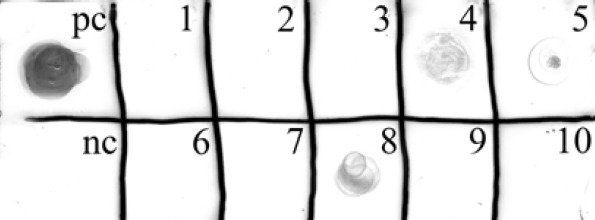
Dot-blot analysis of the recombinant stable clones of the Sf9 insect cells. Evaluation of the concentrated cell culture medium of the obtained stable cell lines revealed the expression of reteplase by some clones (4, 5 and 8) while the other clones were not capable of its expression. (pc) positive control for anti V5 tag; (nc) concentrated cell culture medium of cells transfected with non-recombinant pMIB/V5-His plasmid as negative control.
Expression of reteplase
Expression of reteplase was performed by previously authenticated stable lines of Sf9 cells in T25 and T75 cell culture flasks. Western blot analysis of 5X concentrated cell culture medium confirmed the expression of the reteplase protein by revealing a band of about 45 kD (Fig. 5). Biological activity of the reteplase expressed by the insect cells was also evaluated and showed to be about 29 IU/ml.
Fig. 5.
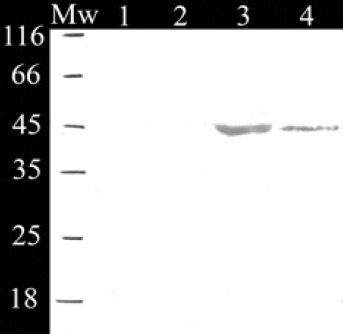
Western blot analysis of concentrated cell culture medium confirmed the expression of reteplase. (1) Not transfected insect cells; (2) Insect cells transfected with non-recombinant pMIB/V5-His plasmid; (3) recombinant stable cells, clone 8; (4) recombinant stable cells, clone 4. Mw; Molecular weight marker.
DISCUSSION
The aim of the present study was to express recombinant reteplase (rPA) as an effective thrombolytic agent by a non-lytic insect cell expression system. The expression of reteplase by the E. coli expression system has been reported by various groups (5,13,25). However, the main step in prokaryotic expression which keeps the price of this medication as high as $ 2000 or even more per dose is the purification step (12,15). Usually, rPA is produced as inactive inclusion bodies in E. coli and requires in vitro refolding to become active. Since the structural stability and biological activity of rPA depends on the correct formation of nine disulfide bonds, the renaturation ratio is still low (5). Furthermore, the Lipopolysaccharide (LPS) contamination, which is a special drawback perticularly related to the E. coli expression system, interferes with the medical application of recombinant proteins and should be declined to acceptable levels during production process which could also lower the production yield (26,27). Considering the disadvantages of the prokaryotic expression system and because of the previous reports on successful expression of full length human t-PA by the baculovirus expression system (21,22,23) , in the present study we evaluated expression of the recombinant reteplase by a non-lytic insect cell expression system. The non-lytic insect cell expression system takes advantage of a strong immediate-early baculovirus promoter which is activated independent of viral infection. Therefore, the expression, modification and secretion of a recombinant protein are not affected by the viral infection as is seen in case of baculovirus expression system (21,28). In addition, expression of the recombinant protein could be obtained continuously following insertion of the coding sequence into the genomic DNA of the host cells by some means like antibiotic screening (17). In the present study we cloned the reteplase coding sequence into the pMIB/V5-His plasmid which harbors melittin signal sequence for effective secretion of the recombinant proteins into the cell culture medium. It also carries the bsd (blasticidin S deaminase) gene which induces genomic integration of the recombinant plasmid elements through screening with the blasticidin S. HCl antibiotic. The cloning of reteplase gene into the pMIB/V5-His plasmid was confirmed by restriction endonuclease digestion and subsequent DNA sequencing. Then, the stable cell lines were obtained by blasticidin S. HCl screening and selection of the high producer cells was performed. Expression of retepalse was finally confirmed by revealing a band of about 45 kD in Western blot analysis. Since the theoretically predicted size of the expressed protein was 42 kD (3 kD regarding the V5-His tags added to the 39 kD of reteplase), it seemed that any post translational modification had been occurred, because insect cells perform almost all post translational modifications which are performed by mammalian cells (19). This kind of weight variations have been also reported by other groups for various proteins expressed by the insect cell expression systems (29,30). The biological activity of the preliminary expressed reteplase protein was also measured and revealed to be almost similar to that of the standard of the kit. This shows that the expressed and secreted reteplase successfully underwent a proper post-translational modification and structural folding. Zhang et al reported the expression of reteplase in gametophyte of seaweed Laminaria japonica (12). However, its intracellular expression could cause some difficulties in its purification process because of the many concomitant proteins existing in the cells. But, in the present study, the expressed reteplase is secreted into the cell culture medium which contains less concomitant proteins and the purification step could be much more easier specially in case of using serum free insect cell culture media.
CONCLUSION
As we are aware, this is the first report on the expression of the reteplase by an insect cell expression system. Considering the capability of this system to express and secret biologically active reteplase, in the next steps of the study the expression yield must be further improved by optimization of the expression conditions such as cell density, time course, addition of FBS to the medium and changing other effective factors.
ACKNOWLEDGMENT
We are grateful to thank Ms. F. Khodabakhsh for her technical assistance in evaluation of the biological activity of the expressed protein. This work has been financially supported by the Research Council of Isfahan University of Medical Sciences and Health Services.
REFERENCES
- 1.Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: a comparison. Vascul Pharmacol. 2006;44:1–9. doi: 10.1016/j.vph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Moser M, Nordt T, Peter K, Ruef J, Kohler B, Schmittner M, et al. Platelet function during and after thrombolytic therapy for acute myocardial infarction with reteplase, alteplase, or streptokinase. Circulation. 1999;100:1858–1864. doi: 10.1161/01.cir.100.18.1858. [DOI] [PubMed] [Google Scholar]
- 3.Ellis K, Brener S. New fibrinolytic agents for MI: as effective as current agents, but easier to administer. Cleve Clin J Med. 2004;71:23–30. doi: 10.3949/ccjm.71.1.20. [DOI] [PubMed] [Google Scholar]
- 4.van Zonneveld AJ, Veerman H, MacDonald ME, van Mourik JA, Pannekoek H. Structure and function of human tissue-type plasminogen activator (t-PA) J Cell Biochem. 1986;32:169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]
- 5.Manosroi J, Tayapiwatana C, Gotz F, Werner RG, Manosroi A. Secretion of active recombinant human tissue plasminogen activator derivatives in Escherichia coli. Appl Environ Microbiol. 200;7:2657–2664. doi: 10.1128/AEM.67.6.2657-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper J, Van’t Hof A, Otter M, Biessen EA, Rijken DC, van Berkel TJ. Interaction of mutants of tissue-type plasminogen activator with liver cells: effect of domain deletions. Biochem J. 1996;313:775–780. doi: 10.1042/bj3130775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao L, Zhang C, Li L, Liang L, Deng X, Wu W, et al. Construction, expression and refolding of a bifunctional fusion protein consisting of C-terminal 12-residue of hirudin-PA and reteplase. Protein J. 2012;31:328–336. doi: 10.1007/s10930-012-9407-8. [DOI] [PubMed] [Google Scholar]
- 8.Datar RV, Cartwright T, Rosen CG. Process economics of animal cell and bacterial fermentations: a case study analysis of tissue plasminogen activator. Biotechnology (N Y) 1993;11:349–357. doi: 10.1038/nbt0393-349. [DOI] [PubMed] [Google Scholar]
- 9.Yenari MA, Lee LK, Beaulieu C, Sun GH, Kunis D, Chang D, et al. Thrombolysis with reteplase, an unglycosylated plasminogen activator variant, in experimental embolic stroke. J Stroke Cerebrovasc Dis. 1998;7:179–186. doi: 10.1016/s1052-3057(98)80004-9. [DOI] [PubMed] [Google Scholar]
- 10.Martin U, Fischer S, Kohnert U, Lill H, Rudolph R, Sponer G, et al. Properties of a novel plasminogen activator (BM 06.022) produced in Escherichia coli. Z Kardiol. 1990;79(Suppl 3):167–170. [PubMed] [Google Scholar]
- 11.Dormiani K, Khazaie Y, Sadeghi HM, Rabbani M, Moazen F. Cloning and expression of a human tissue plasminogen activator variant: K2S in Escherichia coli. Pak J Biol Sci. 2007;10:946–949. doi: 10.3923/pjbs.2007.946.949. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Jiang P, Gao J, Liao J, Sun S, Shen Z, et al. Recombinant expression of rt-PA gene (encoding Reteplase) in gametophytes of the seaweed Laminaria japonica (Laminariales, Phaeophyta) Sci China C Life Sci. 2008;51:1116–1120. doi: 10.1007/s11427-008-0143-4. [DOI] [PubMed] [Google Scholar]
- 13.Obukowicz MG, Gustafson ME, Junger KD, Leimgruber RM, Wittwer AJ, Wun TC, et al. Secretion of active kringle-2-serine protease in Escherichia coli. Biochemistry. 1990;29:9737–9745. doi: 10.1021/bi00493a033. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, Ishii Y, Sasaki H, Hayashi M, Fujimura T, Imai Y, et al. Production and characterization of a novel tissue-type plasminogen activator derivative in Escherichia coli. Biotechnol Prog. 1994;10:472–479. doi: 10.1021/bp00029a004. [DOI] [PubMed] [Google Scholar]
- 15.Gerami SM, Farajnia S, Mahboudi F, Babaei H. Optimizing refolding condition for recombinant tissue plasminogen activator. Iran J Biotechnol. 2011;9:253–259. [Google Scholar]
- 16.Davami F, Sardari S, Majidzadeh AK, Hemayatkar M, Barkhrdari F, Omidi M, et al. Expression of a novel chimeric truncated t-PA in CHO cells based on in silico experiments. J Biomed Biotechnol 2010. 2010 doi: 10.1155/2010/108159. 108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouveia RM, Morais VA, Peixoto C, Sousa M, Regalla M, Alves PM, et al. Production and purification of functional truncated soluble forms of human recombinant L1 cell adhesion glycoprotein from Spodoptera frugiperda Sf9 cells. Protein Expr Purif. 2007;52:182–193. doi: 10.1016/j.pep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Masroori N, Halabian R, Mohammadipour M, Roushandeh AM, Rouhbakhsh M, Najafabadi AJ, et al. High-level expression of functional recombinant human coagulation factor VII in insect cells. Biotechnol Lett. 2010;32:803–809. doi: 10.1007/s10529-010-0227-7. [DOI] [PubMed] [Google Scholar]
- 19.Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 20.Hu YC, Yao K, Wu TY. Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev Vaccines. 2008;7:363–371. doi: 10.1586/14760584.7.3.363. [DOI] [PubMed] [Google Scholar]
- 21.Farrell PJ, Behie LA, Iatrou K. Transformed Lepidopteran insect cells: new sources of recombinant human tissue plasminogen activator. Biotechnol Bioeng. 1999;64:426–433. [PubMed] [Google Scholar]
- 22.Jarvis DL, Weinkauf C, Guarino LA. Immediate-early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Protein Expr Purif. 1996;8:191–203. doi: 10.1006/prep.1996.0092. [DOI] [PubMed] [Google Scholar]
- 23.Steiner H, Pohl G, Gunne H, Hellers M, Elhammer A, Hansson L. Human tissue-type plasminogen activator synthesized by using a baculovirus vector in insect cells compared with human plasminogen activator produced in mouse cells. Gene. 1988;73:449–457. doi: 10.1016/0378-1119(88)90509-4. [DOI] [PubMed] [Google Scholar]
- 24.Sadeghi HM, Rabbani M, Rismani E, Moazen F, Khodabakhsh F, Dormiani K, et al. Optimization of the expression of reteplase in Escherichia coli. Res Pharm Sci. 2011;6:87–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Mattes R. The production of improved tissue-type plasminogen activator in Escherichia coli. Semin Thromb Hemost. 2001;27:325–336. doi: 10.1055/s-2001-16886. [DOI] [PubMed] [Google Scholar]
- 26.Magalhaes PO, Lopes AM, Mazzola PG, Rangel-Yagui C, Penna TC, Pessoa A., Jr Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci. 2007;10:388–404. [PubMed] [Google Scholar]
- 27.Daneshian M, Guenther A, Wendel A, Hartung T, von Aulock S. In vitro pyrogen test for toxic or immunomodulatory drugs. J Immunol Methods. 2006;313:169–175. doi: 10.1016/j.jim.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Jahanian-Najafabadi A, Bouzari S, Oloomi M, Roudkenar MH, Mayr LM. Attempts to express the A1-GMCSF immunotoxin in the baculovirus expression vector system. Biosci Biotechnol Biochem. 2012;76:749–754. doi: 10.1271/bbb.110862. [DOI] [PubMed] [Google Scholar]
- 29.Gouveia R, Kandzia S, Conradt HS, Costa J. Production and N-glycosylation of recombinant human cell adhesion molecule L1 from insect cells using the stable expression system. Effect of dimethyl sulfoxide. J Biotechnol. 2010;145:130–138. doi: 10.1016/j.jbiotec.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Jahanian-Najafabadi A, Bouzari S, Oloomi M, Habibi Roudkenar M, Shokrgozar MA. Assessment of selective toxicity of insect cell expressed recombinant A1-GMCSF protein toward GMCSF receptor bearing tumor cells. Res Pharm Sci. 2012;7:133–140. [PMC free article] [PubMed] [Google Scholar]


