Abstract
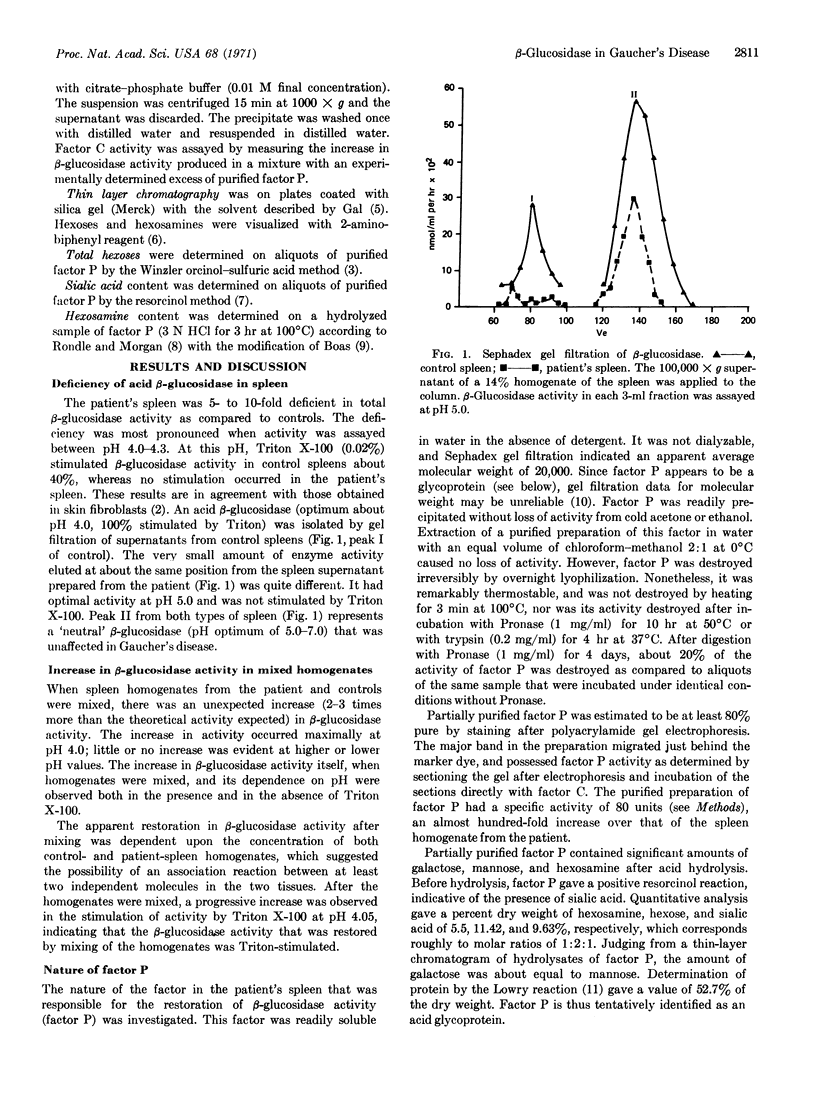
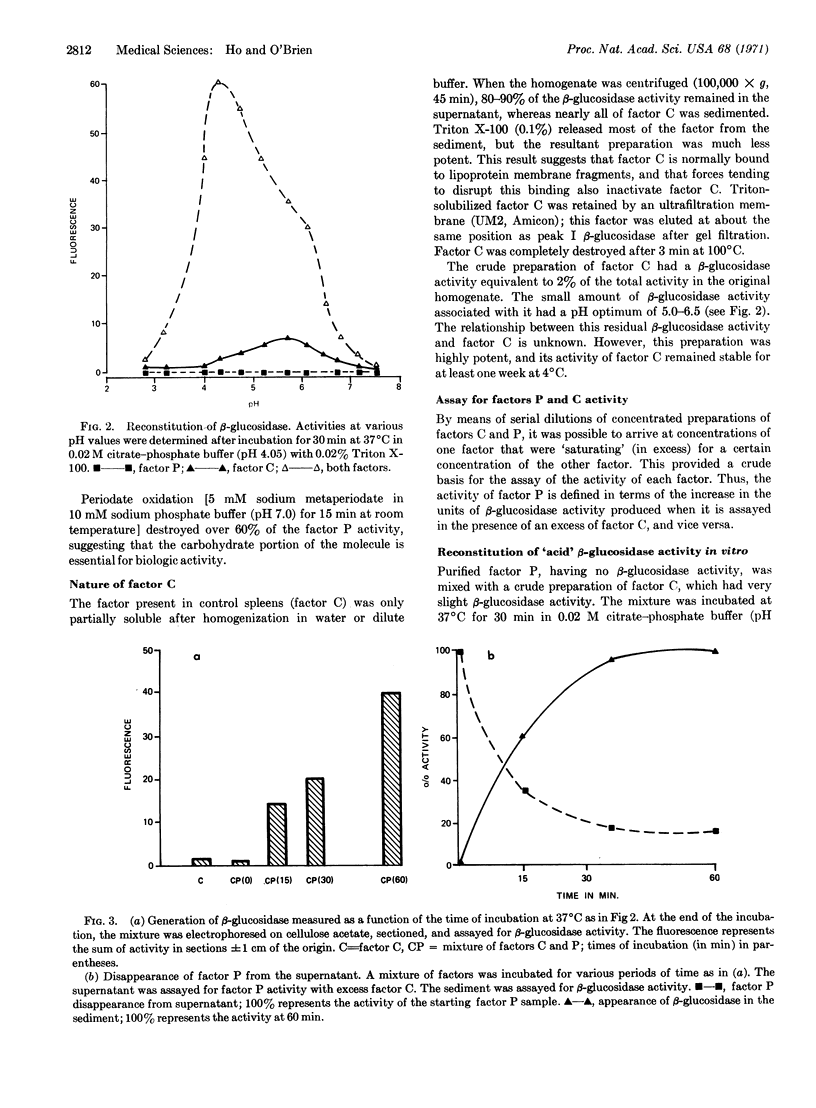
The spleen from a patient with adult Gaucher's disease was shown to be deficient in a β-glucosidase (EC 3.2.1.21) isoenzyme that has optimal activity at pH 4.0-4.3, and is stimulated by 0.02% Triton X-100. A mixture of spleen homogenates from a control and from the patient contained β-glucosidase activity equivalent to 2-3 times the theoretical expected activity. The increase in enzyme activity occurred at pH 4.0-4.3; the magnitude of the increase was proportional to the amount of each homogenate added. Two factors, one called factor P from the patient's spleen, the other called factor C from the control spleen, were responsible for a reconstitution of β-glucosidase activity in vitro. Factor P is tentatively identified as an acid glycoprotein.
Keywords: spleen, components of enzyme
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Detection of the defect of Gaucher's disease and its carrier state in peripheral-blood leucocytes. Lancet. 1970 Mar 21;1(7647):612–613. doi: 10.1016/s0140-6736(70)91646-6. [DOI] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A. E. Separation and identification of monosaccharides from biological materials by thin-layer chromatography. Anal Biochem. 1968 Sep;24(3):452–461. doi: 10.1016/0003-2697(68)90152-8. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Differential effect of chloride ions on -galactosidase isoenzymes: a method for separate assay. Clin Chim Acta. 1971 May;32(3):443–450. doi: 10.1016/0009-8981(71)90446-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]


