Abstract
Stress is an equated response to constant adverse stimuli. At one point or another everybody suffers from stress. Stress is compatible with good health, being necessary to cope with the challenges of everyday life. Problems start when the stress response is inappropriate to the intensity of the challenge. Psychological stress can down regulate the cellular immune response. Communication between the central nervous system and the immune system occurs via a complex network of bidirectional signals linking the nervous, endocrine, and immune systems. Stress disrupts the homeostasis of this network, which in turn, alters immune function. Direct association between periodontal disease and stress remains to be proven, which is partly due to lack of an adequate animal models and difficulty to quantifying the amount and duration of stress and also there are many factors influencing the incidence and severity of periodontal disease. Nevertheless, more recent studies indicate that psychosocial stress represents a risk indicator for periodontal disease and should be addressed before and during treatment. This paper discusses how stress may modulate host response to bacteria and influence the course and progression of periodontal disease.
Keywords: Immunosuppression, inflammatory periodontal disease, psychosocial stressors, stress
The psycho-physiological response of the organism to perceived challenge or threat is referred to as stress. It originates from a Latin word “stringere” which means “tight,” “strained” and the concept was first introduced in the life science by Hans Selye in 1936. Stress can best be understood as part of a complex and dynamic system of transaction between individuals and their environment. Over the last two decades, steady progress has been made in the development of stress theory, both in terms of our understanding of the psychological and social characteristics of situations, which elicit the experience of stress, and in terms of the psycho-physiological mechanisms, which underpin the response to stress and attempts to cope with it. Stress is compatible with good health, being necessary to cope with the challenges of everyday life. Problems start when the stress response is inappropriate to the intensity of the challenge and it has been reported that periodontal disease is more widespread and severe in those with higher levels of stress. Psychological disturbances can lead patients to neglect oral hygiene with resultant unfavorable effects on the periodontal tissues. The association of stress with periodontal disease is difficult to prove as there are many factors influencing the incidence and severity of periodontal disease, some of which are assumed and have not been identified. Nevertheless, more recent studies indicate that psychosocial stress represents a risk indicator for periodontal disease and should be addressed before and during the treatment.
PSYCHOSOCIAL STRESSORS
While much more is known about the role of disease processes such as infection and cancer as stressors capable of inducing far-flung and prolonged inflammatory and classic stress syndromes, it is now considered likely that emotional, behavioral, and psychosocial stressors are also capable of activating the stress system, along with associated immune system effects.
Psychosocial stressors are generally classified:[1]
Major stressful life events
Minor daily stressors or “hassles.”
Holmes (1967)[2] developed a scale to measure stress in terms of life changes. In this scale, the life events are ranked in order, from the most stressful (death of a spouse) to the least stressful (minor violations of the law) [Table 1].
Table 1.
Life change scale

Another set of psychosocial stressors are well-known behavioral and emotional responses to common sequela of advancing periodontal disease, which include such negative and dysphoric conditions as pain, bleeding, unpleasant tastes, and odors emanating from the mouth and unsightly appearance of the teeth and surrounding hard and soft supporting structures.
Other signs and symptoms such as abscess formation with pathogenic exudates and intense pain, loosening of teeth and the perceived threat of losing one's teeth in early adulthood are also often highly worrisome, hence serving as potentially powerful negative emotional stressors. Moreover, treatment of periodontal disease is often associated with pain and discomfort as well as being time-consuming and often expensive. All these perceptions, attributions, and emotions associated with illness can themselves come to constitute and act as an important set of stressors that may induce stress system responses that are further deleterious to periodontal health.[3]
STRESS AND IMMUNE SYSTEM
Studies had clarified that major negative life events are more dependably occurred in close proximity to the onset or exacerbation of illness, and the relationship between important negative life events and disease was mediated by the immune system (two-edged sword). Research has shown that emotional stress can modulate the immune system through the neural and endocrine systems in at least three different ways:[1,2,3,4,5,6]
Through the autonomic nervous system path ways
Through the release of neuropeptides
Through the release of hypothalamic and pituitary hormones.
The sympathetic nervous system also regulates immune cell activities. When the body is in an acute stress or alarm state, there is a marked increase of immune cells in the plasma mobilized from lymphoid organs. Emotional stress results in the release of adrenalin and noradrenaline from cells of the adrenal medulla. Through interaction with adrenergic receptors, noradrenaline and adrenaline mediate cardiovascular and metabolic effects. In blood samples collected immediately before and after an emotional stress situation, such as a parachute jump, the circulating concentration of T-helper lymphocytes (Cluster of Differentiation + T-cells), cytotoxic T-cells (CD8+), and natural killer cells (NK cells), is dramatically increased, but 1 h later, it is lowered to the baseline values. Furthermore, the plasma levels of Immunoglobulin (Ig) IgM, IgG, and complement component C3 are elevated after an acute stress situation (a lifeboat launched in free fall from an oil platform).
Also the release of neuropeptides such as substance P (SP), somatostatin, the endogenous opioid peptides (beta-endorphin and enkephalins), Vasoactive intestinal peptide VIP and nerve growth factor from peptidergic sensory nerves also modulate the activity of the immune system and the release of cytokines. They are also present in gingival and periodontal tissues in close contact with the vascular plexus and penetrate into the epithelium. Experimental studies suggest long lasting emotional stress may increase SP release, resulting in enhanced and imbalanced inflammatory reactions, which may promote tissue damage. These neuropeptides selectively regulate Th1/Th2 cytokine secretion and may regulate immune responses in, for example, granulomatous infections. Thus, multiple nervous and endocrine factors tend to drive the immune response toward Th2 cell dominance, and therefore emotional stress may be an important predisposing factor in severe and progressive chronic infections.
Furthermore, the hypothalamus-pituitary-adrenal (HPA) axis probably plays a key role in stress responses and can serve as a prototype for coordination of psychological information into physiological responses and immune modulation. During a stress response, the higher center of the central nervous system causes the release of corticotrophin-releasing factor (CRF) and arginine vasopressin from the hypothalamus, which further stimulates adrenal cortex and causes the production and release of glucocorticoid hormones [Figure 1]. These glucocorticoids exert its major suppressive effects by reducing the number and activity (chemotaxis, secretion, and degranulation) of circulating inflammatory cells including lymphocytes, monocytes macrophages, neutrophils, eosinophils, and mast cells and also inhibits the production of proinflammatory mediators, cytokines (interleukin [IL] IL-1, IL-2, IL-3, IL-6, tumor necrosis factor (TNF), interferon gamma, and granulocyte and monocyte colony stimulating factors) and cascade of the immune response by inhibiting macrophage-antigen presentation, lymphocyte proliferation, and lymphocyte differentiation to effectors cell types such as helper lymphocytes, cytotoxic lymphocytes, NK-cells, and antibody-forming B cells.
Figure 1.
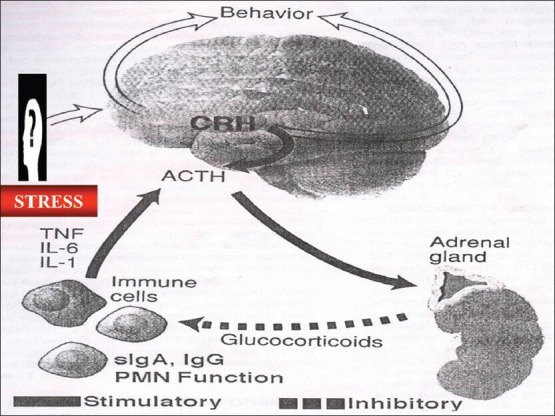
Hypothalamus-pituitary-adrenal-axis
The two other hormones of the HPA axis, CRF and adrenocorticotrophic hormone ACTH, also separately modulate the immune system activity by regulating production of signal substances from immune cells (cytokines) such as IL-1 by monocytes and blocking the activation of macrophages. They also promote B-cell proliferation, but inhibit antibody production.
Recent studies of an interdisciplinary nature in the field of psychoneuroimmunology make the lowered host response as a potential mediator of the putative relationship between psychosocial factors and inflammatory periodontal diseases. These studies have produced data to support the premise that excessive stress associated with life-change events and psychological responses to them can alter host defenses and increase vulnerability to certain illnesses, especially those intimately associated with immunologic mechanisms, such as infection, autoimmune disease, and malignancy.[7]
STRESS AND PERIODONTAL DISEASE
Chronic diseases are the result of long-term interactions between a host and its environment and are multifactorial in nature. A number of mechanisms have been proposed, which could mediate the putative relationship between psychosocial conditions and inflammatory periodontal diseases.
Endocrine changes
Although interactions between stress-endocrine-periodontal changes are not yet well- understood, some hypotheses have been proposed. It has been suspected that periodontal status is related to alterations in the concentration of adrenal corticoids and by altering the response of oral tissues to bacterial toxins and other hormones involved in the general adaptation syndrome.[8]
Model 1 [Figure 2][9] offered a schematic model, which demonstrates the potential role that psychosocial stressors may play in initiating a cascade of events in the corticotrophin releasing hormone/HPA axis, the autonomic nervous system and the central nervous system, the physiological consequences of which are to depress immunity, enhancing the likelihood of infection and specifically, periodontal disease. Recent studies had confirmed the fact that the concentration of cytokines (IL-6, IL-1 β etc.,) cortisol in GCF is higher in person showing depression sign.[10,11,12,13,14]
Figure 2.
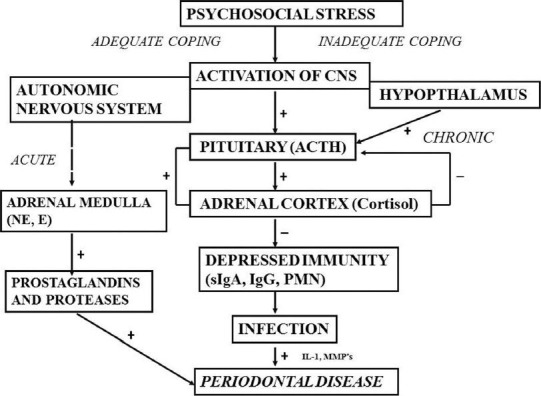
Model-1 for the effects of stress on periodontal disease
Neglect of oral hygiene
It is obvious that proper oral hygiene is partially dependent on the mental health status of the patient. It has been reported that psychological disturbances can lead patients to neglect oral hygiene and that the resultant accumulation of plaque is detrimental to the periodontal tissue. Academic stress was reported as a risk factor for gingival inflammation with increasing crevicular interleukin-b levels and a diminution of quality of oral hygiene.[15,16,17,18,19,20]
Changes in dietary intake
Emotional conditions are thought to modify dietary intake, thus indirectly affecting periodontal status. Psychological factors affect the choice of foods, the physical consistency of the diet, and the quantities of food eaten. This can involve, for instance, the consumption of excessive quantities of refined carbohydrates and softer diets requiring less vigorous mastication and therefore predisposing to plaque accumulation at the approximal risk site.[21] Model-2, [Figure 3][9] hypothesized that stress leads to other behavioral changes such as overeating, especially a high-fat diet, which then can lead to immunosuppression through increased cortisol production.
Figure 3.
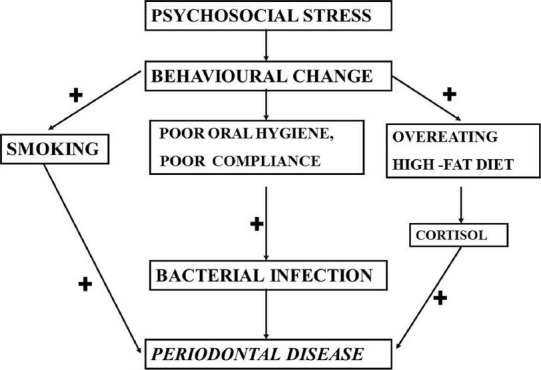
Model-2 explaining the role of stress and its effects on behavior resulting in periodontal disease
Smoking and other harmful habits
Among the many harmful oral habits, which are believed to be induced by emotional disturbances, smoking is possibly the most important in relation to worsened periodontal conditions.[22] Circulating nicotine results in (i) vasoconstriction, produced by the release of adrenaline and noradrenaline, which is supposed to result in a lack of nutrients for the periodontal tissue; (ii) suppression of in vitro secondary antibody responses and (iii) inhibition of oral neutrophil function.
Gingival circulation
The tonus of the smooth muscle of blood vessels may be altered by the emotions by way of the autonomic nervous system. Furthermore, in long or continued emotions, a constant constriction of blood vessels could alter the supply of oxygen and nutrients to the tissues.[23]
Alteration in salivary flow and components
It is assumed that both increase and decrease in salivary flow, induced by emotional disturbance, may affect the periodontium adversely. Emotional distress may also produce changes in saliva pH and chemical composition like IgA secretion. These relationships between salivary physiology and psychological status do not necessarily demonstrate causation of periodontal disease, but they show a pathway in which periodontal health is influenced by salivary changes.[24]
Oral habits
Neurotic needs find oral expression. The mouth may be used to obtain satisfaction, to express dependency or hostility, and to inflict or receive pain. Sucking, biting, sensing, and feeling may become habitual as in thumb sucking, tongue thrusting, infantile swallowing, and biting of tongue, lip, cheek or fingernail. These actions also figure in bruxing, clenching, tooth doodling, and smoking. Such habits may lead to tooth migration, occlusal traumatism, and occlusal wear.
Lowered host resistance
As outline previously, stress and its biochemical mediators may modify the immune response to microbial challenge, which is an important defense against inflammatory periodontal disease. Under stress, the release of adrenaline and noradrenaline may not only induce a decrease in blood flow, but possibly also in those blood elements necessary for maintaining resistance to disease-related microbes. It may be that glucocortiocoids, released during the stress prolong this vascular response.
Bruxism
Bruxism is the clenching or grinding of teeth when individual is not chewing or swallowing. Bruxism has been considered a multifactorial psychosomatic phenomenon with individuals displaying aggressive, controlling precise energetic personality type on one hand (non-stress bruxists) and anxious tense types on the other (stress bruxists).[25] Evaluations from psychometric and health inventories suggest that stress bruxists have more muscular symptoms and seemed more emotionally disturbed. Bruxism has been considered of etiological importance in chronic inflammatory periodontal disease. However, it is difficult to find scientific evidence to substantiate this claim, which seems to be basically supported only by clinical observations.[26]
Stress and acute necrotizing ulcerative gingivitis
Possibly because of its nature (acute painful onset, short lived infection, ease of diagnosis, and multiple predisposing factors), ANUG is most studied periodontal disorder in relation to psychosocial predisposing factors. A psychogenic origin has been suggested for ANUG. Psychogenic factors probably predispose to the disease by favoring bacterial overgrowth and/or weakening host resistance.[27]
Host tissue resistance may be changed by mechanisms acting through the autonomic nervous system and endocrine glands resulting in elevation of corticosteroid and catecholamine levels. This may reduce gingival microcirculation and salivary flow and enhance nutrition of Prevotella inter-media, and at the same time also depress neutrophil and lymphocyte functions, which facilitate bacterial invasion and damage. It has been reported that ANUG patients as compared to controls presented: (i) depressed polymorphonuclear leukocyte chemotaxis and phagocytosis; and (ii) reduced proliferation of lymphocytes upon stimulation by a nonspecific mitogen. Because ANUG patients were also more stressed than controls, data suggest that depression of some host defense mechanisms, under stress conditions, may be necessary in the pathogenesis of ANUG. It is not uncommon to have outbreaks of ANUG among college students during examinations and people during military service.[28]
Stress and aggressive periodontitis
There is a link existing between aggressive periodontitis and psychosocial factors and loss of appetite.[29] A case-control study on 1196 subjects showed people with aggressive periodontitis were more depressed and socially isolated than people with chronic periodontitis or control group.[30] The clinical and microbiological status evaluation of patients with early onset periodontitis who had received supportive periodontal care every 3-6 months for a period of 5 years after active periodontal treatment showed stress as one of the variables for progression of periodontal disease at few sites in few patients.[31]
Stress and systemic inflammatory disease
A number of chronic recurrent conditions, in addition to periodontal disease, are characterized by a fluctuating course, with ongoing disease punctuated by bouts of greater severity. It is well-established that cardiovascular disease, diabetes mellitus, preterm delivery, osteoporosis, rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus etc., are related to stress either as a physiological response to stress or as a behavioral response. It may be that stress is a significant common risk factor for diabetes mellitus, cardiovascular disease, preterm delivery, and osteoporosis, as well as periodontal disease [Figure 4]. Of course, different stressors and different responses to stress may be operative in each disease. Alternately or simultaneously, stress that is modified by perceptions in coping can give rise to at-risk health behaviors, which then could affect the same spectrum of chronic diseases. The more severe bouts of all these conditions involve activation of the immune response and an associated increase in inflammation. The body of evidence on the relationship of stress to disease activity appears to be greatest for rheumatoid arthritis; however, because of the types of tissues affected, information on inflammatory bowel disease may be particularly pertinent to periodontal disease.[32,33,34]
Figure 4.
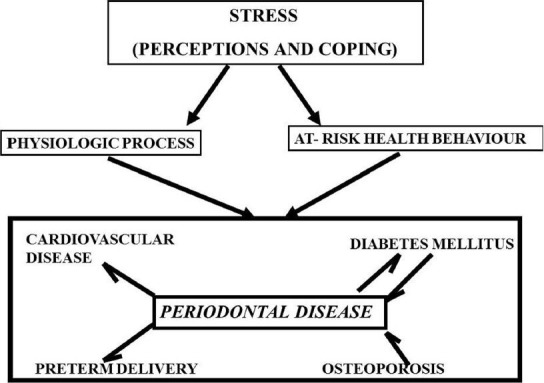
Stress correlation with systemic diseases and periodontal disease
Stress and wound healing
Patients with maladaptive coping strategies have more advanced disease and poor response to non-surgical treatment,[35] whereas positive correlation was observed in reduction of dental plaque and gingival bleeding in patients having an active coping.[36] Furthermore, the cellular immune response plays a vital role in wound healing. Not only does it protect the wound site from infection, it also prepares the wound for healing and regulates its repair. Cytokines such as IL-1, IL-8, and TNF are extremely important in recruiting phagocytic cells to clear away the damaged tissue and to regulate the rebuilding by fibroblasts and epithelial cells. A decrease in expression in any of these cytokines could theoretically impair wound healing. Stress could suppress certain aspects of the cellular immune response such as mitogen stimulation, antibody and cytokine production, and NK cell activity. Furthermore, since stress deregulates inflammatory and immune response, stress can alter the course of oral wound healing and affect the management of other oral diseases, e.g., periodontitis.[37]
CONCLUSION
Since the 1950's emotional factors have been related to periodontal disease. It is now well-established that psychological stress can down-regulate the cellular immune response. Communication between the central nervous system and the immune system occurs through a complex network of bidirectional signals linking the nervous, endocrine, and immune systems. Stress disrupts the homeostasis of this network, which in turn, alters immune function.
Direct association between periodontal disease and stress remains to be proven, which is partly due to lack of an adequate animal models and difficulty to quantifying the amount and duration of stress. Furthermore, multiple variables affect the severity of periodontal disease and there is uncertainty about the individual's onset of periodontal disease. Moreover, it is not possible to separate the effects of physical stress from emotional stress in these animal studies.
Furthermore, it is likely that systemic diseases associated with periodontal disease such as diabetes, cardiovascular disease etc., may share psychosocial stress as common risk factor. The available scientific evidence thus, does not definitively support a casual relationship between psychosocial factors and inflammatory periodontal diseases. The information reviewed above nevertheless does indicate the possible influence of psychosocial factors in the etiology of inflammatory periodontal diseases though at the moment, the more suggestive evidence relates to ANUG. These studies indicate that psychosocial stress represents a risk indicator for periodontal disease. Consequently, it is noteworthy that the practitioner is aware of these factors and taken them into consideration. The clinical management of inflammatory periodontal diseases might benefit from an exploration of these relationships, principally when disease severity cannot be explained by established etiological factors and when there is no response to periodontal treatment or when there is a sudden, marked and in explicable increase in the rate of periodontal destruction.[38,39]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.LeResche L, Dworkin SF. The role of stress in inflammatory disease, including periodontal disease: Review of concepts and current findings. Periodontol 2000. 2002;30:91–103. doi: 10.1034/j.1600-0757.2002.03009.x. [DOI] [PubMed] [Google Scholar]
- 2.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 3.Breivik T, Thrane PS, Murison R, Gjermo P. Emotional stress effects on immunity, gingivitis and periodontitis. Eur J Oral Sci. 1996;104:327–34. doi: 10.1111/j.1600-0722.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 4.Sateesh CP, Santosh Kumar R, Pushpalatha G. Relationship between stress and periodontal disease. J dent Sci Res. 2010;1:54–61. [Google Scholar]
- 5.Chandna S, Bathla M. Stress and periodontium: A review of concepts. J Oral Health Comm Dent. 2010;(Suppl 4):1. 17-22. [Google Scholar]
- 6.Ballieux RE. Impact of mental stress on the immune response. J Clin Periodontol. 1991;18:427–30. doi: 10.1111/j.1600-051x.1991.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 7.da Silva AM, Newman HN, Oakley DA. Psychosocial factors in inflammatory periodontal diseases. A review. J Clin Periodontol. 1995;22:516–26. doi: 10.1111/j.1600-051x.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis CH, Jenkins CD. Mental stress and oral disease. J Dent Res. 1962;41:1045–9. doi: 10.1177/00220345620410050601. [DOI] [PubMed] [Google Scholar]
- 9.Genco RJ, Ho AW, Kopman J, Grossi SG, Dunford RG, Tedesco LA. Models to evaluate the role of stress in periodontal disease. Ann Periodontol. 1998;3:288–302. doi: 10.1902/annals.1998.3.1.288. [DOI] [PubMed] [Google Scholar]
- 10.Axtelius B, Söderfeldt B, Nilsson A, Edwardsson S, Attström R. Therapy-resistant periodontitis. Psychosocial characteristics. J Clin Periodontol. 1998;25:482–91. doi: 10.1111/j.1600-051x.1998.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 11.Mengel R, Bacher M, Flores-De-Jacoby L. Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. J Clin Periodontol. 2002;29:1012–22. doi: 10.1034/j.1600-051x.2002.291106.x. [DOI] [PubMed] [Google Scholar]
- 12.Johannsen A, Rylander G, Söder B, Asberg M. Dental plaque, gingival inflammation, and elevated levels of interleukin-6 and cortisol in gingival crevicular fluid from women with stress-related depression and exhaustion. J Periodontol. 2006;77:1403–9. doi: 10.1902/jop.2006.050411. [DOI] [PubMed] [Google Scholar]
- 13.Deinzer R, Förster P, Fuck L, Herforth A, Stiller-Winkler R, Idel H. Increase of crevicular interleukin 1beta under academic stress at experimental gingivitis sites and at sites of perfect oral hygiene. J Clin Periodontol. 1999;26:1–8. doi: 10.1034/j.1600-051x.1999.260101.x. [DOI] [PubMed] [Google Scholar]
- 14.Deinzer R, Kottmann W, Förster P, Herforth A, Stiller-Winkler R, Idel H. After-effects of stress on crevicular interleukin-1beta. J Clin Periodontol. 2000;27:74–7. doi: 10.1034/j.1600-051x.2000.027001074.x. [DOI] [PubMed] [Google Scholar]
- 15.Ringsdorf WM, Jr, Cheraskin E. Emotional status and the periodontium. J Tenn State Dent Assoc. 1969;49:5–18. [PubMed] [Google Scholar]
- 16.Meyer MJ. Stress and periodontal disease: A review of the literature. J N Z Soc Periodontol. 1989;68:23–6. [PubMed] [Google Scholar]
- 17.Deinzer R, Rüttermann S, Möbes O, Herforth A. Increase in gingival inflammation under academic stress. J Clin Periodontol. 1998;25:431–3. doi: 10.1111/j.1600-051x.1998.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 18.Deinzer R, Hilpert D, Bach K, Schawacht M, Herforth A. Effects of academic stress on oral hygiene – A potential link between stress and plaque-associated disease? J Clin Periodontol. 2001;28:459–64. doi: 10.1034/j.1600-051x.2001.028005459.x. [DOI] [PubMed] [Google Scholar]
- 19.Deinzer R, Granrath N, Spahl M, Linz S, Waschul B, Herforth A. Stress, oral health behaviour and clinical outcome. Br J Health Psychol. 2005;10:269–83. doi: 10.1348/135910705X26858. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand HC, Epstein J, Larjava H. The influence of psychological stress on periodontal disease. J West Soc Periodontol Periodontal Abstr. 2000;48:69–77. [PubMed] [Google Scholar]
- 21.Suchday S, Kapur S, Ewart CK, Friedberg JP. Urban stress and health in developing countries: Development and validation of a neighborhood stress index for India. Behav Med. 2006;32:77–86. doi: 10.3200/BMED.32.3.77-86. [DOI] [PubMed] [Google Scholar]
- 22.Haber J. Smoking is a major risk factor for periodontitis: current opinion in periodontology. In: Williams RC, Yukna RA, Newman MG, editors. Philadelphia: Current Science; 1994. pp. 12–8. [PubMed] [Google Scholar]
- 23.Manhold JH, Doyle JL, Weisinger EH. Effects of social stress on oral and other bodily tissues. II. Results offering substance to a hypothesis for the mechanism of formation of periodontal pathology. J Periodontol. 1971;42:109–11. doi: 10.1902/jop.1971.42.2.109. [DOI] [PubMed] [Google Scholar]
- 24.Gupta OP. Psychosomatic factors in periodontal disease. Dent Clin North Am. 1966 Mar;:11–9. [PubMed] [Google Scholar]
- 25.Arnold M. Bruxism and the occlusion. Dent Clin North Am. 1981;25:395–407. [PubMed] [Google Scholar]
- 26.Olkinuora M. A psychosomatic study of bruxism with emphasis on mental strain and familiar predisposition factors. Proc Finn Dent Soc. 1972;68:110–23. [PubMed] [Google Scholar]
- 27.Reners M, Brecx M. Stress and periodontal disease. Int J Dent Hyg. 2007;5:199–204. doi: 10.1111/j.1601-5037.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 28.Cogen RB, Stevens AW, Jr, Cohen-Cole S, Kirk K, Freeman A. Leukocyte function in the etiology of acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:402–7. doi: 10.1902/jop.1983.54.7.402. [DOI] [PubMed] [Google Scholar]
- 29.Page RC, Altman LC, Ebersole JL, Vandesteen GE, Dahlberg WH, Williams BL, et al. Rapidly progressive periodontitis. A distinct clinical condition. J Periodontol. 1983;54:197–209. doi: 10.1902/jop.1983.54.4.197. [DOI] [PubMed] [Google Scholar]
- 30.Monterio Da Silva A, Oakley D, Newmann H, Nohl F, Lloyd H. Psychosocial factors and adult onset rapidly progressive periodontitis. J Clin Periodontol. 2003;30:562–72. doi: 10.1111/j.1600-051x.1996.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 31.Kamma JJ, Baehni PC. Five-year maintenance follow-up of early-onset periodontitis patients. J Clin Periodontol. 2003;30:562–72. doi: 10.1034/j.1600-051x.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: Inversion of a paradigm. Ann Periodontol. 1998;3:108–20. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 33.Lamey PJ, Linden GJ, Freeman R. Mental disorders and periodontics. Periodontol 2000. 1998;18:71–80. doi: 10.1111/j.1600-0757.1998.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 34.Peruzzo DC, Benatti BB, Ambrosano GM, Nogueira-Filho GR, Sallum EA, Casati MZ, et al. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491–504. doi: 10.1902/jop.2007.060371. [DOI] [PubMed] [Google Scholar]
- 35.Wimmer G, Köhldorfer G, Mischak I, Lorenzoni M, Kallus KW. Coping with stress: Its influence on periodontal therapy. J Periodontol. 2005;76:90–8. doi: 10.1902/jop.2005.76.1.90. [DOI] [PubMed] [Google Scholar]
- 36.Gamboa AB, Hughes FJ, Marcenes W. The relationship between emotional intelligence and initial response to a standardized periodontal treatment: A pilot study. J Clin Periodontol. 2005;32:702–7. doi: 10.1111/j.1600-051X.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 37.Rozlog LA, Kiecolt-Glaser JK, Marucha PT, Sheridan JF, Glaser R. Stress and immunity: Implications for viral disease and wound healing. J Periodontol. 1999;70:786–92. doi: 10.1902/jop.1999.70.7.786. [DOI] [PubMed] [Google Scholar]
- 38.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–9. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 39.Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61–87. doi: 10.1111/j.1875-595x.1997.tb00680.x. [DOI] [PubMed] [Google Scholar]


