Abstract
Objective:
To determine, in patients identified as sero-positive for neuronal voltage-gated potassium channel (VGKC) complex autoantibodies, the spectrum of clinical presentations and frequency of leucine-rich glioma-inactivated protein 1 (LGI1) and contactin-associated protein-like 2 (CASPR2) as defined antigenic neuronal targets in the VGKC macromolecular complex.
Design:
Retrospective cohort study.
Setting:
Clinical practice, Mayo Clinic Neuroimmunology Laboratory and Department of Neurology.
Patients:
A total of 54853 patients were evaluated, of whom 1992 were found to be VGKC complex IgG positive.
Results:
From June 1, 2008, to June 30, 2010, comprehensive service serologic evaluation performed on 54 853 patients with unexplained neurologic symptoms identified 1992 patients (4%) who were positive for VGKC complex IgG (values ≥0.03 nmol/L). Among 316 seropositive patients evaluated clinically at our institution, 82 (26%) were seropositive for LGI1 IgG and/or CASPR2 IgG. Of these 82 patients, 27% had low (0.03-0.09 nmol/L), 51% had medium (0.10-0.99 nmol/L), and 22% had high (≥1.00 nmol/L) VGKC complex IgG values. Leucine-rich glioma-inactivated protein 1 IgG positivity was associated with higher VGKC complex IgG values (P<.001) and cortical presentations (P<.001); CASPR2 IgG was associated with peripheral motor excitability (P=.009). However, neither autoantibody was pathognomonic for a specific neurologic presentation or correlated significantly with cancer. Neurologic phenotypes were diverse. Cerebrocortical manifestations (including cognitive impairment and seizures) were recorded in 76% of patients with LGI1 IgG alone (n=46) and 29% with CASPR2 IgG alone (n=28). Peripheral motor hyperexcitability was found in 21% of patients with CASPR2 IgG alone and 6.5% of patients with LGI1 IgG alone.
Conclusions:
The study emphasizes diverse and overlapping neurologic phenotypes across a range of VGKC complex IgG values and varying LGI1 IgG and CASPR2 IgG specificities. The frequent occurrence of LGI1 IgG and CASPR2 IgG in serum samples with low and medium VGKC complex IgG values supports the clinical significance of low values in clinical evaluation. Additional antigenic components of VGKC macromolecular complexes remain to be defined.
VOLTAGE-GATED POTASSIUM channel (VGKC) complex autoimmunity was initially described with acquired neuromyotonia and hyperhidrosis (Isaacs syndrome)1,2 and subsequently with limbic encephalitis, neuromyotonia, insomnia, and autonomic dysfunction (Morvan syndrome).3,4 The restriction of autoantibody testing to patients with defined neurologic syndromes precludes appreciation of the full phenotypic spectrum of VGKC complex autoimmunity. Identification of VGKC complex antibody–positive patients through comprehensive serologic evaluation for any suspected autoimmune neurologic disorder (thus avoiding prejudgment of the clinical spectrum of VGKC complex autoimmunity) has extended the clinical spectrum to include miscellaneous sleep disorders, neuropsychiatric presentations, seizures, mimics of Creutzfeldt-Jakob disease, and frontotemporal dementia.5-10 Importantly, in these reports, robust improvement often followed immunotherapy.
It was recently reported that VGKC complex autoantibodies detected by radioimmunoprecipitation assays generally do not bind to VGKC channel proteins per se, but they bind instead to synaptic and axonal neuronal proteins that coprecipitate with detergent-solubilized VGKCs.11,12 The principal autoantigens defined most commonly were the leucine-rich glioma-inactivated protein 1 (LGI1) in the central nervous system and contactin-associated protein-like2 (CASPR2, which associates with TAG1, PDZ, and the ankyrin-spectrin protein) in both the peripheral and central nervous system.11,12 Contactin-associated protein-like 2 IgG was reported to associate with peripheral presentations,12,13 poor prognosis, and risk for tumor,12 and LGI1 IgG with limbic encephalitis and better prognosis.11,12 To our knowledge, the clinical associations of VGKC complex IgG and of LGI1 and CASPR2 autoantibody subspecificities to date have been reported from investigations involving patients with syndromic presentations of limbic encephalitis, Morvan syndrome, or neuromyotonia. The full neurologic spectrum defined by LGI1 IgG and CASPR2 IgG is under-appreciated.
Herein we report clinical correlations and other autoantibody accompaniments of VGKC complex IgG detected in nonselected patients undergoing comprehensive autoimmune serologic evaluation for unexplained neurologic symptoms as well as the frequency of LGI1 IgG and CASPR2 IgG in these patients.
METHODS
The study was approved by the Mayo Clinic institutional review board. Subjects were Mayo Clinic patients who were identified as VGKC complex autoantibody positive in serologic evaluation for evidence of neurologic autoimmunity from June 1, 2008, to June 30, 2010. Two or more study neurologists (C.J.K, P.A.A., A.M., A.Q., O.O., S.J.P.) reviewed demographic, clinical, and laboratory data for each patient.
VGKC COMPLEX AND OTHER SEROLOGIC EVALUATIONS
Comprehensive neural autoantibody testing included a standardized immunofluorescence assay to detect antibodies specific for Purkinje cell cytoplasmic antigens (types 1, 2, and Tr), antineuronal nuclear antibody (types 1, 2, and 3), amphiphysin, collapsin response-mediator protein–5, and antiglial/neuronal nuclear antibody type 1.14 Positive results (Purkinje cell cytoplasmic autoantibody types 1 and 2; antineuronal nuclear antibody types 1, 2, and 3; and CRMP-5 IgGs) were confirmed by native or recombinant Western blot. Antibodies specific for VGKC complex, voltage-gated calcium channels (P/Q-type and N-type), muscle and ganglionic (α3) nicotinic acetylcholine receptors, and glutamic acid decarboxylase-65 were detected by radioimmunoprecipitation assay, and striational antibodies by enzyme-linked immunosorbent assay.14 To eliminate false-positive toxin reactivity, all serum samples yielding positive results for VGKC complex IgG were retested with 125I-α-dendrotoxin alone (radioligand for Kv1.1, Kv1.2, and Kv1.6 channels).15
LGI1 AND CASPR2 AUTOANTIBODY EVALUATION
Serum samples yielding positive results for VGKC complex IgG were tested further for IgG reactive with LGI1 or CASPR2 proteins by a cell-based immunofluorescence assay (Euroimmun), clinically validated in our laboratory and incorporating as substrate fixed HEK 293 cells that were nontransfected or transfected with plasmid-encoding human LGI1 or CASPR2 proteins. We detected bound IgG by use of fluorescein isothiocyanate conjugated goat IgG specific for human IgG. Negative control serum samples were from 126 healthy subjects (64 female; median age, 51 years [range, 18-82 years]). Positive control serum samples were from patients known to be positive for LGI1 IgG or CASPR2 IgG. Each assay was scored independently by at least 2 experienced laboratory consultants (C.J.K., V.A.L., A.M., S.J.P.).
STATISTICAL ANALYSES
Association studies between categorical variables were investigated using the 2-tailed Fisher exact test (P<.05 was considered significant). The Kruskal-Wallis test was used for association analysis of LGI1 IgG positivity with VGKC complex IgG values. Associations between VGKC complex IgG values and clinical characteristics were investigated by 2×3 Fisher exact test, with patients stratified into 3 serologic subgroups based on serum VGKC complex IgG values: low (0.03-0.09 nmol/L), medium (0.10-0.99 nmol/L), and high (≥1.00 nmol/L). We also investigated the frequencies of LGI1 IgG and CASPR2 IgG seropositivity and the respective clinical presentations for each subgroup using 2×3 Fisher exact test. To determine whether variables other than VGKC complex IgG values might contribute to the neurologic presentation, we performed a multivariate logistic regression analysis for significant association with neurologic presentations, coexisting autoantibodies, sex, or age.
RESULTS
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF VGKC COMPLEX IgG–POSITIVE PATIENTS
During the 25-month study, 54 853 patients had a comprehensive neural autoantibody evaluation in the Mayo Clinic Neuroimmunology Laboratory. Voltage-gated potassium channel complex IgG was detected in 1992 patients (4%); 316 underwent neurologic evaluation at our institution, of whom 55% were female. The median age at initial symptom onset was 57 years (range, 2-88 years). Table 1 and Table 2 summarize the diverse neurologic manifestations. Table 1 illustrates the diverse neurologic accompaniments of low, medium, and high VGKC complex IgG value ranges. Only cognitive impairment and seizures were more likely to be associated with higher values. Coexisting neural autoantibodies identified in 33 patients included antineuronal nuclear antibody type 1 (n=1), collapsin response-mediator protein–5 (n=1), nicotinic ganglionic acetylcholine receptor (α3; n=17), nicotinic muscle acetylcholine receptor (n=10), and P/Q-type voltage-gated calcium channel (n=7) (Table 3). Coexisting neural antibodies did not associate with occurrence of cancer (Table 3). Fifty-five percent of the cohort was evaluated for cancer (including by whole-body computed tomographic–positron-emission tomographic imaging, colonoscopy, or mammography) and cancer was found in 15%. No statistically significant association was found between the evidence of malignancy and VGKC complex autoantibody subtypes (Table 3).
Table 1.
Neurologic Accompaniments Stratified Serologically by High, Medium, and Low Serum VGKC Complex IgG Values
| Neurologic Accompaniment Level Involved or Manifestation |
Patients With Specific Neurologic Accompaniment, No. (%) |
P Valuea | |||
|---|---|---|---|---|---|
| Value |
|||||
| Total (n = 316) |
High (≥1.00 nmol/L) (n = 28) |
Medium (0.10-0.99 nmol/L) (n = 179) |
Low (0.03-0.09 nmol/L) (n = 109) |
||
| Cerebral cortex | 144 (45.6) | 19 (67.9) | 87 (48.6) | 38 (34.9) | .007 |
| Cognitive impairment | 70 (22.2) | 13 (46.4) | 33 (18.4) | 24 (22.0) | .004 |
| Seizure | 33 (10.4) | 8 (28.6) | 20 (11.2) | 5 (4.6) | <.001 |
| Frontosubcortical | 10 (3.2) | 0 | 8 (4.5) | 2 (1.8) | >.05 |
| Depression/anxiety/agitation | 52 (16.5) | 4 (14.3) | 36 (20.1) | 12 (11.0) | >.05 |
| Hypothalamic | |||||
| Hyponatremia | 6 (1.9) | 3 (10.7) | 3 (1.7) | 0 | >.05 |
| Dyssomnia | 15 (4.7) | 4 (14.3) | 6 (3.4) | 5 (4.6) | |
| Hypersomnia | 9 (2.8) | 1 (3.6) | 4 (2.2) | 4 (3.7) | >.05 |
| Insomnia | 6 (1.9) | 3 (10.7) | 2 (1.1) | 1 (0.9) | >.05 |
| Extrapyramidal | 11 (3.5) | 2 (7.1) | 5 (2.8) | 4 (3.7) | >.05 |
| Tremor | 7 (2.2) | 2 (7.1) | 4 (2.2) | 1 (0.9) | >.05 |
| Parkinsonism | 3 (0.9) | 0 | 1 (0.6) | 2 (1.8) | >.05 |
| Chorea | 2 (0.6) | 0 | 1 (0.6) | 1 (0.9) | >.05 |
| Cranial nerve brainstem | 2 (0.6) | 1 (3.6) | 0 | 1 (0.9) | >.05 |
| Cerebellum | 6 (1.9) | 0 | 4 (2.2) | 2 (1.8) | >.05 |
| Spinal cord | 9 (2.8) | 0 | 7 (3.9) | 2 (1.8) | >.05 |
| Peripheral autonomic nerve | 95 (30.1) | 7 (25.0) | 51 (28.5) | 37 (33.9) | >.05 |
| Gastrointestinal | 29 (9.2) | 1 (3.6) | 14 (7.8) | 14 (12.8) | >.05 |
| Genitourinary | 4 (1.3) | 0 | 4 (2.2) | 0 | >.05 |
| Blood pressure | 23 (7.3) | 2 (7.1) | 12 (6.7) | 9 (8.3) | >.05 |
| Sudomotor | 41 (12.3) | 4 (14.3) | 22 (12.3) | 15 (13.8) | >.05 |
| Peripheral somatic nerve | 109 (34.5) | 6 (21.4) | 63 (32.9) | 40 (33.0) | >.05 |
| Hyperexcitability, motor | 46 (14.5) | 2 (7.1) | 26 (14.5) | 18 (16.5) | >.05 |
| Neuropathy, sensorimotor | 52 (16.5) | 4 (14.3) | 30 (16.8) | 18 (16.5) | >.05 |
| Small fiber sensory | 11 (3.5) | 0 | 7 (3.9) | 4 (3.7) | >.05 |
| Motor neuron | 7 (2.2) | 1 (3.6) | 3 (1.7) | 3 (2.8) | >.05 |
| Myoclonus | 4 (1.3) | 1 (3.6) | 1 (0.6) | 2 (1.8) | >.05 |
| Stiff-person phenomena | 2 (0.6) | 0 | 1 (0.6) | 1 (0.9) | >.05 |
| Morvan syndrome | 1 (0.3) | 0 | 1 (0.6) | 0 | >.05 |
| Headache | 13 (4.1) | 0 | 10 (5.6) | 3 (2.8) | >.05 |
| Gait disorder not specified | 19 (6.0) | 0 | 13 (7.3) | 6 (5.5) | >.05 |
| Vision loss | 8 (2.5) | 0 | 6 (3.4) | 2 (1.8) | >.05 |
| Pain only | 45 (28.0) | 4 (14.3) | 27 (15.1) | 14 (12.8) | >.05 |
Abbreviation: VGKC, voltage-gated potassium channel.
Comparison of 3 serologic subgroups.
Table 2.
Neurologic Accompaniments of LGI1 IgG, CASPR2 IgG, or Both
| Neurologic Accompaniment Level Involved or Manifestation |
No. (%) |
P Valuea | ||
|---|---|---|---|---|
| Patients Positive for LGI1 IgG Only (n = 46) |
Patients Positive for CASPR2 IgG Only (n = 28) |
Patients Positive for LGI1 IgG and CASPR2 IgG (n = 8) |
||
| Cerebral cortex | 35 (76.1) | 8 (28.5) | 2 (25.0) | <.001 |
| Cognitive impairment | 21 (45.7) | 5 (17.9) | 0 | .005 |
| Seizure | 17 (37.0) | 3 (10.7) | 1 (12.5) | .03 |
| Frontosubcortical | 2 (4.3) | 0 | 1 (12.5) | >.05 |
| Depression/anxiety/agitation | 8 (17.4) | 2 (7.1) | 1 (12.5) | >.05 |
| Hypothalamic | ||||
| Hyponatremia | 4 (8.7) | 1 (3.6) | 0 | >.05 |
| Dyssomnia | 3 (6.5) | 0 | 1 (12.5) | >.05 |
| Hypersomnia | 1 (2.2) | 0 | 0 | >.05 |
| Insomnia | 2 (4.3) | 0 | 1 (12.5) | >.05 |
| Extrapyramidal | 1 (2.2) | 1 (3.6) | 0 | >.05 |
| Tremor | 1 (2.2) | 1 (3.6) | 0 | >.05 |
| Parkinsonism | 0 | 0 | 0 | >.05 |
| Chorea | 0 | 0 | 0 | >.05 |
| Cranial nerve brainstem | 1 (2.2) | 0 | 0 | >.05 |
| Cerebellum | 0 | 1 (3.6) | 0 | >.05 |
| Spinal cord | 1 (2.2) | 0 | 0 | >.05 |
| Peripheral autonomic nerve | 8 (17.4) | 3 (10.7) | 3 (37.5) | >.05 |
| Gastrointestinal | 3 (6.5) | 1 (3.6) | 1 (12.5) | >.05 |
| Genitourinary | 0 | 0 | 0 | >.05 |
| Blood pressure | 3 (6.5) | 0 | 1 (12.5) | >.05 |
| Sudomotor | 6 (13.0) | 6 (21.4) | 3 (12.5) | >.05 |
| Peripheral somatic nerve | 5 (10.9) | 14 (50.0) | 4 (50.0) | <.001 |
| Hyperexcitability, motor | 3 (6.5) | 10 (21.4) | 2 (12.5) | .004 |
| Neuropathy, sensorimotor | 2 (4.3) | 5 (17.9) | 1 (12.5) | >.05 |
| Small fiber sensory | 0 | 2 (7.1) | 2 (25.0) | >.05 |
| Motor neuron | 0 | 1 (3.6) | 0 | >.05 |
| Myoclonus | 2 (4.3) | 0 | 0 | >.05 |
| Stiff-person phenomena | 0 | 0 | 0 | >.05 |
| Morvan syndrome | 0 | 0 | 0 | >.05 |
| Headache | 3 (6.5) | 3 (10.7) | 0 | >.05 |
| Gait disorder not specified | 5 (10.9) | 5 (10.9) | 0 | >.05 |
| Vision loss | 2 (4.3) | 3 (10.7) | 0 | >.05 |
| Pain only | 2 (4.3) | 4 (14.3) | 3 (37.5) | .02 |
Abbreviations: CASPR2, contactin-associated protein-like 2; LGI1, leucine-rich glioma-inactivated protein 1.
Comparison of LGI1 IgG, CASPR2 IgG, or both; P < .05 was considered significant.
Table 3.
Frequency of Coexisting Neural Autoantibodies and Neoplasia in 316 Seropositive Patients According to VGKC Complex IgG Subspecificitya
| Coexisting Neural Antibody Detected, No. (%) |
Negative for LGI1 IgG and CASPR2 IgG (n = 234) |
Positive for LGI1 IgG Only (n = 46) |
Positive for CASPR2 IgG Only (n = 28) |
Positive for LGI1 IgG and CASPR2 IgG (n = 8) |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
|
| ||||||||
| 26 (11) | 208 (89) | 3 (6.5) | 43 (93) | 4 (14) | 24 (86) | 0 | 8 (100) | |
| ANNA-1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CRMP-5 IgG | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nicotinic ganglionic acetylcholine receptor (α3) IgG |
13 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Nicotinic muscle acetylcholine receptor IgG |
9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| P/Q-type voltage-gated calcium channel IgG |
4 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Neoplasm, No. (%)b | 4 (1.7) | 28 (12.0) | 2 (4.3) | 8 (17.0) | 0 | 4 (14.0) | 0 | 2 (25.0) |
Abbreviations: ANNA-1, antineuronal nuclear antibody 1; CASPR2, contactin-associated protein-like 2; CRMP-5, collapsin response-mediator protein–5; LGI1, leucine-rich glioma-inactivated protein 1; VGKC, voltage-gated potassium channel.
No significant association identified between cancer, VGKC complex IgG subspecificity, and coexisting neural autoantibodies.
Cancer evaluation was performed in 173 VGKC complex IgG–positive patients (55%); 133 cases (42%) underwent positron emission tomographic body scans. A neoplasm was identified after VGKC complex IgG detection in 11 patients (carcinomas of lung, n = 3; colon, n = 2; prostate, n = 1; thyroid, n = 2; vagina, n = 1; lymphoma, n = 1; thymoma, n = 1) and prior to autoantibody detection in 37 patients (carcinomas of lung, n = 3; kidney, n = 2; bladder, n = 2; colon or rectum, n = 4; prostate, n = 5; breast, n = 3; cervix, n = 1; skin, n = 1; hematologic malignancy, n = 5; lymphoma, n = 1. Ten patients had multiple malignancies).
THE NEUROLOGIC ACCOMPANIMENTS OF LGI1 IgG AND CASPR2 IgG
Voltage-gated potassium channel complex IgG values for the 126 healthy control subjects’ serum samples ranged from 0.00 to 0.02 nmol/L; none was positive for LGI1 IgG or CASPR2 IgG. Table 2 compares the frequency of each neurologic accompaniment recorded in 46 LGI1 IgG only–positive patients, 28 CASPR2 IgG only–positive patients, and 8 LGI1 and CASPR2–positive patients. The associations of LGI1 IgG with cognitive impairment and seizures (P < .05) and of CASPR2 IgG with peripheral motor excitability (P = .004) were the most striking differences noted. However, neither autoantibody was pathognomonic for a specific neurologic presentation. For example, cerebral cortical manifestations (including cognitive impairment and seizures) were recorded in 76% of patients with LGI1 IgG alone and in 29% of patients with CASPR2 IgG alone. Peripheral motor hyperexcitability was found in 21% of patients with CASPR2 IgG alone and in 6.5% of patients with LGI1 IgG alone.
VGKC COMPLEX IgG DIRECTED AT LGI1 OR CASPR2
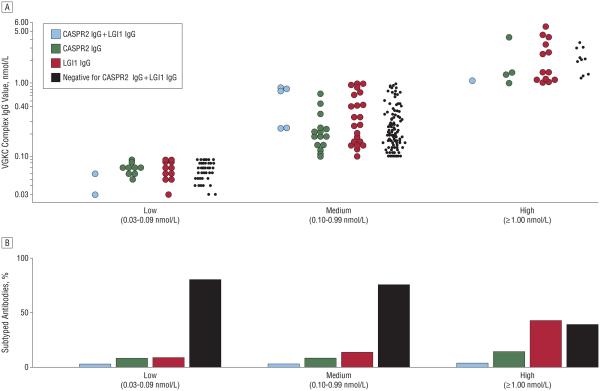
The Figure summarizes the frequencies of LGI1 IgG and CASPR2 IgG detection by cell-binding assay stratified into 3 subgroups based on VGKC complex IgG values. Of 109 patients with low values, 10% were seropositive for LGI1 IgG alone, 8% for CASPR2 IgG alone, and 2% for both. Of 179 patients with medium values, 12% were seropositive for LGI1 IgG alone, 8% for CASPR2 IgG alone, and 3% for both. Of 28 patients with high values, 43% were seropositive for LGI1 IgG alone, 14% for CASPR2 IgG alone, and 4% for both. Only 39% of patients with high values were negative for both CASPR2 IgG and LGI1 IgG. High values associated significantly with LGI1 IgG positivity (P < .001), but CASPR2 IgG frequency did not relate significantly to VGKC complex IgG values. The lack of association for LGI1 IgG–negative patients (n = 262) between VGKC complex IgG value and cerebral cortex abnormalities supports a role for LGI1 IgG in the pathogenesis of autoimmune encephalopathies.
Figure.
Voltage-gated potassium channel (VGKC) values and frequency of contactin-associated protein-like 2 (CASPR2) IgG and leucine-rich glioma-inactivated protein 1 (LGI1) IgG detection. Voltage-gated potassium channel complex IgG binds to LGI1 or CASPR2 in only a minority of cases. The frequency of LGI1 IgG and CASPR2 IgG detection by cell-binding assay stratified into 3 subgroups based on VGKC complex IgG values shows a range of values associated with CASPR2 IgG and LGl1 IgG detection. A, The VGKC complex IgG values (y-axis) of patients identified as positive for CASPR2 IgG (green circles), LGI1 IgG (red circles), both CASPR2 IgG and LGI1 IgG (blue circles), or negative for both (black dots). The dashed line indicates the frequency of LGI1 IgG and/or CASPR2 IgG in seropositive patients whose VGKC complex IgG values exceed or are less than 0.40 nmol/L. B, The percentage of subtyped antibodies based on VGKC complex IgG values.
COMMENT
This large clinicoserologic study of VGKC complex autoimmunity makes several important observations. First, both LGI1 IgG and CASPR2 IgG are associated with diverse neurologic manifestations that commonly overlap. Second, only a minority of seropositive patients have antibodies that recognize LGI1 and/or CASPR2. It remains to be determined whether IgG in patients lacking those antibody specificities binds to a VGKC channel subunit or to another molecular component of the neuronal VGKC complex. Third, provided that false-positive results attributable to IgG binding to 125I-α-dendrotoxin are subtracted,15 the findings support the clinical significance of relatively low VGKC complex IgG values. Of 82 serum samples that were reactive with LGI1 and/or CASPR2, 64 (78%) had VGKC complex IgG values in the range of 0.03 to 0.99 nmol/L. Several earlier studies have suggested that values less than 100 pM or 400 pM (ie, 0.10 nmol/L or 0.40 nmol/L) are negligible.12,16 Had we used 0.40 nmol/L as the cutoff value in this study, we would have missed detecting 61% of the patients (50 of 82) whom reflexive cell-binding assay identified as seropositive for LGI1 IgG, CASPR2 IgG, or both (Figure). Even with a cutoff value of 0.10 nmol/L, we would have missed 27% of the patients (22 of 82). In accord with previous reports,11,12 among the 26 patients who were diagnosed as having limbic encephalitis and whose VGKC complex IgG values exceeded 0.40 nmol/L, 81% were positive for LGI1 IgG or CASPR2 IgG or both (65%, 8%, and 8%, respectively). For patients with VGKC complex IgG values of 1.00 nmol/L or greater, only a minority were negative for LGI1 IgG or CASPR2 IgG. The nonsignificant association between cortical presentations and the serum level of VGKC complex IgG among LGI1 IgG–seronegative patients suggests that LGI1 IgG plays a role in the pathogenesis of autoimmune encephalopathy.
Because our study’s design did not restrict antibody testing to patients prejudged to have a pertinent syndromic presentation, it permitted broader determination of the neurologic spectrum of VGKC autoimmunity. Study patients were identified through comprehensive neural autoantibody testing of thousands of patients undergoing service evaluation for any suspected autoimmune neurologic disorder, generally of subacute onset, with or without historic or familial stigmata of autoimmunity and not readily explained by alternative diagnoses. The distribution of clinical presentations among the 3 stratified VGKC complex IgG values highlights the associated neurologic diversity and justifies testing for VGKC complex IgG in a broader clinical context. It is noteworthy that none of the 10 CASPR2 IgG–positive patients whose VGKC complex IgG values exceeded 0.40 nmol/L had Morvan syndrome (fibrillary chorea, encephalopathy, autonomic instability, and neuromuscular hyperexcitability).
Of practical importance, VGKC complex IgG values were low or medium in most cases in whom CASPR2 IgG or LGI1 IgG were identified. Therefore, our data support the clinical significance of low VGKC complex IgG values detected in a validated radioimmunoprecipitation screening assay. The high proportion of VGKC complex IgG–seropositive patients whose serum samples lack LGI1 IgG and CASPR2 IgG specificities suggests that other VGKC complex molecular targets remain to be discovered.
Acknowledgments
Funding/Support: The study was supported by the Mayo Foundation and the National Institutes of Health (grant K08NS065007) and Mayo Clinic CTSA through grant UL1RR024150 from the National Center for Research Resources, a component of the National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: Klein, Lennon, and Pittock. Acquisition of data: Klein, Aston, O’Toole, Quek, and Pittock. Analysis and interpretation of data: Klein, Lennon, McKeon, O’Toole, Quek, and Pittock. Drafting of the manuscript: Klein, O’Toole, and Pittock. Critical revision of the manuscript for important intellectual content: Klein, Lennon, Aston, McKeon, O’Toole, Quek, and Pittock. Statistical analysis: Klein and Quek. Obtained funding: Klein. Administrative, technical, and material support: Klein, Lennon, Aston, and O’Toole. Study supervision: Klein and Pittock.
Conflict of Interest Disclosures: Dr Pittock is a named inventor on patents (12/678 350 filed 2010 and 12/573 942 filed 2008) that relate to functional aquaporin-4/neuromyelitis optica–IgG assays and neuromyelitis optica–IgG as a cancer marker. Dr Pittock receives research support from Alexion Pharmaceuticals Inc, the Guthy Jackson Charitable Foundation, and the National Institutes of Health. Mayo Medical Laboratories/Mayo Collaborative Services Inc receive revenue for performing voltage-gated potassium channel complex and other autoantibody testing.
Additional Contributions: We thank Vickie Mewhorter, Amy Moses, BA, Jade Zbacnik, BA, and Debby Cheung, BS, for their technical assistance.
References
- 1.Shillito P, Molenaar PC, Vincent A, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38(5):714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 2.Hart IK, Waters C, Vincent A, et al. Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol. 1997;41(2):238–246. doi: 10.1002/ana.410410215. [DOI] [PubMed] [Google Scholar]
- 3.Liguori R, Vincent A, Clover L, et al. Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124(pt 12):2417–2426. doi: 10.1093/brain/124.12.2417. [DOI] [PubMed] [Google Scholar]
- 4.Josephs KA, Silber MH, Fealey RD, Nippoldt TB, Auger RG, Vernino S. Neurophysiologic studies in Morvan syndrome. J Clin Neurophysiol. 2004;21(6):440–445. doi: 10.1097/00004691-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. [published online March 26, 2012] doi:10.1001/archneurol.2011.2985. [DOI] [PMC free article] [PubMed]
- 6.Somers KJ, Lennon VA, Rundell JR, et al. Psychiatric manifestations of voltage-gated potassium-channel complex autoimmunity. J Neuropsychiatry Clin Neurosci. 2011;23(4):425–433. doi: 10.1176/jnp.23.4.jnp425. [DOI] [PubMed] [Google Scholar]
- 7.Cornelius JR, Pittock SJ, McKeon A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68(6):733–738. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85(10):881–897. doi: 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol. 2008;65(10):1341–1346. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeon A, Marnane M, O’connell M, Stack JP, Kelly PJ, Lynch T. Potassium channel antibody associated encephalopathy presenting with a frontotemporal dementia like syndrome. Arch Neurol. 2007;64(10):1528–1530. doi: 10.1001/archneur.64.10.1528. [DOI] [PubMed] [Google Scholar]
- 11.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster E, Huijbers MG, Bar V, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69(2):303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56(5):715–719. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 15.Apiwattanakul M, McKeon A, Pittock SJ, Kryzer TJ, Lennon VA. Eliminating false-positive results in serum tests for neuromuscular autoimmunity. Muscle Nerve. 2010;41(5):702–704. doi: 10.1002/mus.21653. [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Buckley C, Lang B, Irani S. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2009;72(1):99–100. doi: 10.1212/01.wnl.0000339405.94708.8d. 99-author reply. [DOI] [PubMed] [Google Scholar]