Abstract
Genome-wide association studies (GWAS) have identified many variants that influence high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and/or triglycerides. However, environmental modifiers, such as smoking, of these known genotype–phenotype associations are just recently emerging in the literature. We have tested for interactions between smoking and 49 GWAS-identified variants in over 41,000 racially/ethnically diverse samples with lipid levels from the Population Architecture Using Genomics and Epidemiology (PAGE) study. Despite their biological plausibility, we were unable to detect significant SNP × smoking interactions.
Short report
Candidate gene and genome-wide association studies (GWAS) have identified numerous common variants associated with high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG). However, examination of possible interactions with environmental factors such as smoking is still lacking (Ordovas et al. 2011). Smoking has been associated with a poor lipid profile, including decreased HDL-C and increased triglycerides (Chelland et al. 2008). Here, we assess the influence of smoking as a modifier of known lipid-related genotype–phenotype associations across four racial/ethnic groups.
Study samples were drawn from the Population Architecture Using Genomics and Epidemiology (PAGE) study, which consists of four population-based studies and numerous racial/ethnic populations, including those examined here: European Americans (n = 24,700), African Americans (n = 9,782), American Indians (n = 3,607), and Mexican Americans/Hispanics (n = 3,357) (Matise et al. 2011). Mean lipid levels by population and self-reported smoking status (dichotomized into current and former/never smokers) for all PAGE participants are listed in Table 1. Study specific demographics are presented in Table S1.
Table 1.
Characteristics of PAGE study participants
| Trait | European Americans | African Americans | American Indians | Mexican Americans/Hispanics | ||||
|---|---|---|---|---|---|---|---|---|
| Former/never smokers |
Current smokers |
Former/never smokers |
Current smokers |
Former/never smokers |
Current smokers |
Former/never smokers |
Current smokers |
|
| Nmax (%) | 19,975 (80.9) | 4,725 (19.1) | 7,241 (74.0) | 2,541 (26.0) | 2,390 (66.3) | 1,217 (33.7) | 2,788 (83.1) | 569 (16.9) |
| HDL-C (mg/dl) | 53.7 ± 15.9 | 49.3 ± 15.9 | 56.1 ± 15.7 | 54.3 ± 17.1 | 46.6 ± 13.6 | 45.3 ± 14.3 | 50.1 ± 13.6 | 47.9 ± 14.5 |
| LDL-C (mg/dl) | 129.9 ± 36.1 | 133.9 ± 38.6 | 129.3 ± 39.6 | 123.4 ± 40.8 | 115.5 ± 33.6 | 120.4 ± 34.0 | 122.3 ± 34.3 | 123.0 ± 34.5 |
| TG (mg/dl) | 137.2 ± 83.2 | 136.5 ± 80.8 | 100.3 ± 56.6 | 104.6 ± 65.0 | 146.6 ± 100.5 | 144.6 ± 97.7 | 160.6 ± 101.1 | 165.6 ± 112.5 |
All values reported as mean ± SD unless otherwise indicated
A total of 49 SNPs (Table S2) previously associated with one or more lipid trait in published (as of 2008) candidate gene and GWA studies were selected and successfully genotyped in PAGE (Dumitrescu et al. 2011). Regression modeling was used to assess the effect of a multiplicative interaction between each variant and smoking status on HDL-C, LDL-C, and ln(TG) levels. Race-specific models were adjusted for age, sex, and marginal effects. Analyses were performed by each PAGE study site and summary statistics were meta-analyzed using METAL (Willer et al. 2010). Given that the lipid traits are correlated and the associations tested are not assumed to be completely independent, significance was defined as p < 1.0E−03 to account for the 49 SNPs tested (=0.05/49 SNPs). Effect sizes needed to detect significant interactions with 80 % power were calculated using Quanto (Gauderman and Morrison 2006). Variant main effect sizes used in the power calculation were drawn from a previous single-SNP association analysis for LDL-C (Dumitrescu et al. 2011).
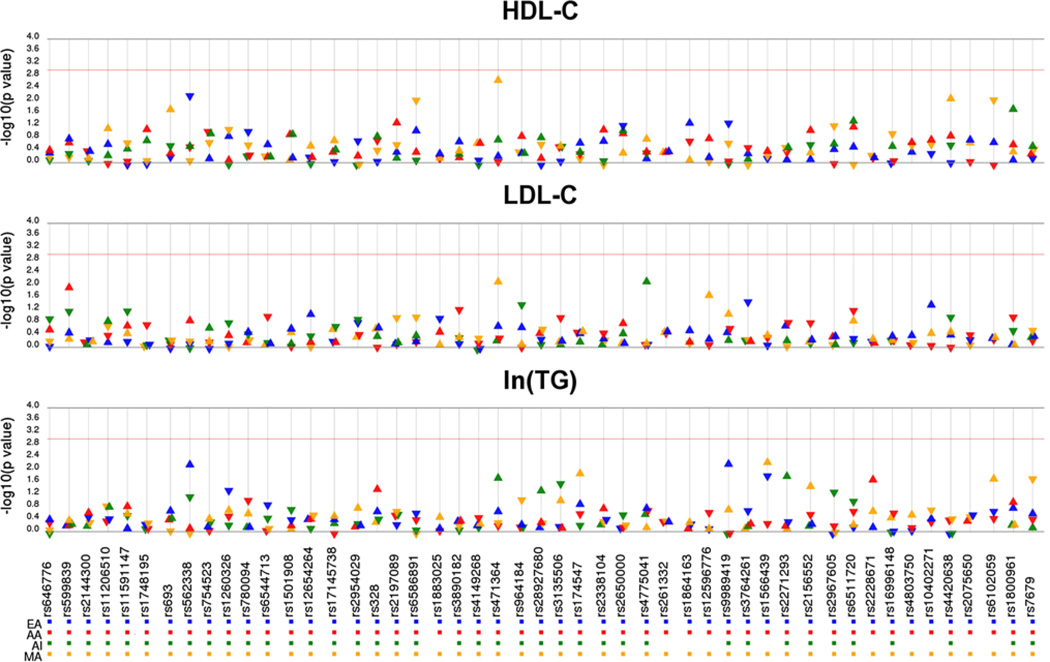
No significant SNP × smoking interactions were detected (Fig. 1). Indeed, only 28 interactions (out of 588 tested) had p values <0.05, consistent with chance alone. The most significant interaction was TTC39B rs471364x-smoking (p = 2.55E−03) for HDL-C levels among Mexican Americans/Hispanics. Only one interaction (CETP rs1566439 for TG) was nominally associated in more than one population; however, the direction of effect was inconsistent (p = 1.35E−02, β = −0.031 in European Americans; p = 6.84E−03, β = 0.106 in Mexican Americans/Hispanics).
Fig. 1.
SNP × smoking interaction results by lipid trait and population. Each SNP × smoking interaction was tested for an association with the indicated lipid trait after adjustment for age and sex. p values (−log10 transformed) of the meta-analysis are plotted along the y-axis. SNPs are ordered on the x-axis based on chromosomal location. Each triangle represents a meta-analysis p value for each population. The direction of the arrows corresponds to the direction of the beta coefficient. Populations are color-coded as denoted in the legend: European Americans (EA), African Americans (AA), American Indians (AI), and Mexican Americans/Hispanics (MA). The significance threshold (p = 1.0E−03) is indicated by the red line
Several reasons may underlie the lack of significant interactions. First, not all PAGE study sites collected sufficient data to assess smoking status as recommended by harmonization work groups such as the consensus measures for phenotypes and eXposures [PhenX; (Hamilton et al. 2011)]. Additionally, quantitative measures of smoking exposure such as serum cotinine levels or number of pack-years, were not available for all PAGE study sites. Therefore, our binary categorization of smoking (though a commonly used metric of exposure) may have inhibited our ability to detect existing interactions.
Second, our power to detect to small interaction effects was limited, especially in minority populations and for variants with low minor allele frequencies (examples in Table 2). For example, we had 80 % power to detect a minimum interaction beta of 3.5 in European Americans, 5.0 in African Americans, 7.4 in American Indians, and 9.4 in Mexican Americans/Hispanics for HMGCR rs12654264 (allele frequency = 0.55–0.62). However, the effect sizes needed to detect a significant interaction with PCSK9 rs11591147 (allele frequency = 0.004–0.02) were four to seven times larger than those needed for rs12654264, despite the fact that the main effect of rs11591147 size was very large (βG = −15.67 to 23.39 mg/dl; Dumitrescu et al. 2011).
Table 2.
Minimum interaction effect sizes needed to detect representative SNP × smoking interactions
| SNP | Allele frequency in EA, AA, AI, MA/H |
Minimum βGE needed to detect interaction with smoking | |||
|---|---|---|---|---|---|
| European Americans |
African Americans |
American Indians |
Mexican Americans/ Hispanics |
||
| rs12654264 | 0.62, 0.67, 0.62, 0.55 | 3.5 | 5.0 | 7.4 | 9.4 |
| rs599839 | 0.77, 0.28, 0.78, 0.79 | 4.0 | 5.3 | 8.4 | 11.2 |
| rs562338 | 0.18, 0.60, 0.15, 0.07 | 4.3 | 4.8 | 10.0 | 18.0 |
| rs11591147 | 0.02, 0.004, 0.01, 0.01 | 13.5 | 35.5 | 34.2 | 44.9 |
Effect sizes (βGE) were estimated for SNP × smoking interactions on LDL-C levels
Another factor that has implications for power is the range of smoking prevalence, both across (Table 1) and within (Table S1) racial/ethnic groups. All other measures being equal, increased prevalence of the environmental exposure results in increased power to detect a gene–environment interaction. The populations studied here demonstrated a range of smoking exposures, with American Indians (33.7 %) having the largest percentage of current smokers and Mexican Americans/Hispanics having the smallest (16.9 %). As one can see from Table 2, we were powered to detect smaller interaction effect sizes in American Indians compared to Mexican Americans/Hispanics for all four modeled interactions. Therefore, when designing gene–environment interaction studies, even larger sample sizes may be necessary for populations in which the environmental exposure is rare.
Last, the lack of significant interactions may simply be due to the fact that none exists for the variants and populations studied here. However, for some interactions, it is impractical to draw this conclusion given we cannot distinguish between a true negative and a false negative due to lack of statistical power.
Despite the known impact of smoking on lipids and the relevant role of the loci studied here in lipid metabolism, we were unable to identify significant SNP × smoking interactions. We demonstrate that studies of gene–environment interactions require very large sample sizes, greatly impeding the investigation of minority populations and complex environmental exposures.
Supplementary Material
Acknowledgments
The Population Architecture Using Genomics and Epidemiology (PAGE) program is funded by the National Human Genome Research Institute (NHGRI), supported by U01HG004803 (CALiCo), U01HG004798 (EAGLE), U01HG004802 (MEC), U01HG004790 (WHI), and U01HG004801 (Coordinating Center), and their respective NHGRI ARRA supplements. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The complete list of PAGE members can be found at http://www.pagestudy.org. The “Epidemiologic Architecture for Genes Linked to Environment (EAGLE)” is funded through the NHGRI PAGE program (U01HG004798 and its NHGRI ARRA supplement). Genotyping services for select NHANES III SNPs presented here were also provided by the Johns Hopkins University under federal contract number (N01-HV-48195) from NHLBI. The study participants were derived from the National Health and Nutrition Examination Surveys (NHANES), and these studies are supported by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The Multiethnic Cohort study (MEC) characterization of epidemiological architecture is funded through the NHGRI PAGE program (U01HG004802 and its NHGRI ARRA supplement). The MEC study is funded through the National Cancer Institute (R37CA54281, R01 CA63, P01CA33619, U01CA136792, and U01CA98758). Funding support for the “Epidemiology of putative genetic variants: The Women’s Health Initiative” study is provided through the NHGRI PAGE program (U01HG004790 and its NHGRI ARRA supplement). The WHI program is funded by the National Heart, Lung, and Blood Institute; NIH; and U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf. Funding support for the Genetic Epidemiology of Causal Variants Across the Life Course (CALiCo) program was provided through the NHGRI PAGE program (U01HG004803 and its NHGRI ARRA supplement). The following studies contributed to this manuscript and are funded by the following agencies: The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts: HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN 268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The Coronary Artery Risk Development in Young Adults (CARDIA) study is supported by the following National Institutes of Health, National Heart, Lung and Blood Institute contracts: N01-HC-95095; N01-HC-48047; N01-HC-48048; N01-HC-48049; N01-HC-48050; N01-HC-45134; N01-HC-05187; and N01-HC-45205. The Cardiovascular Health Study (CHS) is supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). The Strong Heart Study (SHS) is supported by NHLBI grants U01 HL65520, U01 HL41642, U01 HL41652, U01 HL41654, and U01 HL65521. The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Indian Health Service. Assistance with phenotype harmonization, SNP selection and annotation, data cleaning, data management, integration and dissemination, and general study coordination were provided by the PAGE Coordinating Center (U01HG004801 and its NHGRI ARRA supplement). The National Institutes of Mental Health also contributes to the support for the Coordinating Center. The PAGE consortium thanks the staff and participants of all PAGE studies for their important contributions.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00439-013-1375-3) contains supplementary material, which is available to authorized users.
Contributor Information
Logan Dumitrescu, Email: logan.dumitrescu@chgr.mc.vanderbilt.edu, Center for Human Genetics Research, Vanderbilt University, 2215 Garland Avenue, 515B Light Hall, Nashville, TN 37232, USA.
Cara L. Carty, Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Nora Franceschini, Department of Epidemiology, University of North Carolina, Chapel Hill, NC, USA.
Lucia A. Hindorff, Office of Population Genomics, National Human Genome Research Institute, Bethesda, MD, USA
Shelley A. Cole, Department of Genetics, Texas Biomedical Research Institute, San Antonio, TX, USA
Petra Bůžková, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Fredrick R. Schumacher, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Charles B. Eaton, Department of Family Medicine, Warren Alpert Medical School Brown University, Providence, RI, USA
Robert J. Goodloe, Center for Human Genetics Research, Vanderbilt University, 2215 Garland Avenue, 515B Light Hall, Nashville, TN 37232, USA
David J. Duggan, Translational Genomic Science Institute, Phoenix, AZ, USA
Jeff Haessler, Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Barbara Cochran, Baylor College of Medicine, Houston, TX, USA.
Brian E. Henderson, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Iona Cheng, Cancer Research Center, University of Hawaii, Honolulu, HI, USA.
Karen C. Johnson, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN, USA
Chris S. Carlson, Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Shelly-Anne Love, Department of Epidemiology, University of North Carolina, Chapel Hill, NC, USA.
Kristin Brown-Gentry, Center for Human Genetics Research, Vanderbilt University, 2215 Garland Avenue, 515B Light Hall, Nashville, TN 37232, USA.
Alejandro Q. Nato, Department of Genetics, Rutgers University, Piscataway, NJ, USA
Miguel Quibrera, Gillings School of Public Health, University of North Carolina, Chapel Hill, NC, USA.
Ralph V. Shohet, John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, USA
José Luis Ambite, Information Sciences Institute, University of Southern California, Marina del Rey, CA, USA.
Lynne R. Wilkens, Cancer Research Center, University of Hawaii, Honolulu, HI, USA
Loïc Le Marchand, Cancer Research Center, University of Hawaii, Honolulu, HI, USA.
Christopher A. Haiman, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Steven Buyske, Department of Genetics, Rutgers University, Piscataway, NJ, USA; Department of Statistics, Rutgers University, Piscataway, NJ, USA.
Charles Kooperberg, Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Kari E. North, Department of Epidemiology, University of North Carolina, Chapel Hill, NC, USA Carolina Center for Genome Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Myriam Fornage, Division of Epidemiology, Human Genetics, and Environmental Sciences, School of Public Health, University of Texas Health Sciences Center at Houston, Houston, TX, USA; Institute of Molecular Medicine, University of Texas Health Sciences Center at Houston, Houston, TX, USA.
Dana C. Crawford, Email: crawford@chgr.mc.vanderbilt.edu, Center for Human Genetics Research, Vanderbilt University, 2215 Garland Avenue, 515B Light Hall, Nashville, TN 37232, USA; Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA.
References
- Chelland CS, Moffatt RJ, Stamford BA. Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Dumitrescu L, Carty CL, Taylor K, Schumacher FR, Hindorff LA, Ambite JL, Anderson G, Best LG, Brown-Gentry K, Buzkova P, Carlson CS, Cochran B, Cole SA, Devereux RB, Duggan D, Eaton CB, Fornage M, Franceschini N, Haessler J, Howard BV, Johnson KC, Laston S, Kolonel LN, Lee ET, MacCluer JW, Manolio TA, Pendergrass SA, Quibrera M, Shohet RV, Wilkens LR, Haiman CA, Le ML, Buyske S, Kooperberg C, North KE, Crawford DC. Genetic determinants of lipid traits in diverse populations from the population architecture using genomics and epidemiology (PAGE) study. PLoS Genet. 2011;7:e1002138. doi: 10.1371/journal.pgen.1002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Morrison JM. QUANTO 1.1: a computer program for power and sample size calculations for genetic-epidemiology studies. 2006 http://hydra.usc.edu/gxe. [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, Pan H, Nettles DS, Beaty TH, Farrer LA, Kraft P, Marazita ML, Ordovas JM, Pato CN, Spitz MR, Wagener D, Williams M, Junkins HA, Harlan WR, Ramos EM, Haines J. The PhenX toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise TC, Ambite JL, Buyske S, Carlson CS, Cole SA, Crawford DC, Haiman CA, Heiss G, Kooperberg C, Marchand LL, Manolio TA, North KE, Peters U, Ritchie MD, Hindorff LA, Haines JL. The next PAGE in understanding complex traits: design for the analysis of population architecture using genetics and epidemiology (PAGE) study. Am J Epidemiol. 2011;174:849–859. doi: 10.1093/aje/kwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas JM, Robertson R, Cleirigh EN. Gene-gene and gene-environment interactions defining lipid-related traits. Curr Opin Lipidol. 2011;22:129–136. doi: 10.1097/MOL.0b013e32834477a9. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.