Abstract
Background
The global rise in tobacco smoking using a waterpipe (hookah, narghile, shisha) has made understanding its health consequences imperative. One key to developing this understanding is identifying and quantifying carcinogens and other toxicants present in waterpipe smoke. To do so, the toxicant yield of machine-generated waterpipe smoke has been measured. However, the relevance of toxicant yields of machine-generated smoke to actual human exposure has not been established.
Methods
In this study, we examined whether CO and nicotine yields measured using a smoking machine programmed to replicate the puffing behavior of 31 human participants who smoked a waterpipe could reliably predict these participant’s blood-level exposure. In addition to CO and nicotine, yields of PAH, volatile aldehydes, NO, and “tar” were measured.
Results
We found that when used in this puff-replicating manner, smoking machine yields are highly correlated with blood-level exposure (Nicotine: r>0.76, p<0.001; CO: r>0.78, p<0.001). Total drawn smoke volume was the best predictor of toxicant yield and exposure, accounting for approximately 75–100% of the variability across participants in yields of NO, CO, volatile aldehydes and “tar”, and blood-level CO and normalized nicotine.
Conclusions
Machine-based methods can be devised in which smoke toxicant yields reliably track human exposure.
Impact
This finding indicates the basic feasibility of valid analytical laboratory evaluation of tobacco products for regulatory purposes.
Keywords: hookah, shisha, narghile, exposure, tobacco, smoking machine
INTRODUCTION
As elsewhere in the world, tobacco smoking using a waterpipe (hookah, narghile, shisha) is rapidly becoming an epidemic in the U.S., particularly among adolescents and young adults: 17% of a nationwide sample of 12th graders reported past-year waterpipe use [1] while waterpipe tobacco smoking is the second most popular form of tobacco use among U.S. university students [2]. With a tobacco waterpipe, burning charcoal is used to heat sweetened and flavored tobacco that is placed in the “head”. When users inhale through the waterpipe mouthpiece, hot charcoal combustion products are drawn through the tobacco, producing the mainstream smoke. The smoke is drawn through a water bubbler, and then travels through the hose to the user [3].
To assess potential hazards posed by this burgeoning tobacco use method, we have been studying waterpipe smoke toxicant content using laboratory smoking machines (e.g.[3,4]).These studies have demonstrated that machine-generated waterpipe smoke contains numerous toxicants implicated in smoking-related cardiovascular disease, cancer, lung disease, and addiction [4–6]. However, the relevance of smoking machine yields to waterpipe user toxicant exposure has not been established. Indeed, there is uncertainty about this relationship with cigarettes as well, despite decades of effort (e.g. [7–9]). This uncertainty has become particularly salient in the U.S. following passage of the Tobacco Control Act. The Act charged the U.S. Food and Drug Administration with regulating tobacco products and publishing product-specific test data on harmful smoke constituents. Importantly, the Act defines as a tobacco product “any product made or derived from tobacco that is intended for human consumption” and thus includes in its purview waterpipe tobacco. To inform this effort, in this study we examined whether the toxicant yield of machine-generated waterpipe smoke can predict human exposure to two important tobacco smoke toxicants: carbon monoxide (CO) and nicotine.
MATERIALS AND METHODS
The IRB-approved study involved recording digitally the puff topography of individual participants who smoked a waterpipe under controlled conditions in a clinical laboratory while their blood was sampled to assess CO exposure via carboxyhemoglobin (COHb) and nicotine exposure via blood nicotine concentration. Recruitment by printed media and word-of-mouth for the study resulted in 63 qualified individuals (2–5 self-reported waterpipe use sessions per month, aged between 18–50, healthy, <5 cigarettes/month) who consented to participate in the protocol. Of these, 22 did not pass initial screening and did not begin the study, and 4 were discontinued during the study. The resulting pool of 37 participants (three African-American, seven Asian, 20 Caucasian, one Hawaiian/Pacific Islander and six mixed/other ethnicity) included 8 women, and 29 men aged 20.5±2.1 years (mean±SEM), self-reported smoking waterpipe tobacco 2–5 times/month (3.8±1.0) for ≥six months (20.2±12.9). Individuals were free to schedule the smoking session for any time of day and were required to abstain from smoking from the previous night onward (verified by exhaled breath CO<10ppm). Participants were given a minimum of 45 min to puff freely from a waterpipe loaded with 10 g of their preferred flavor of tobacco while watching a video of their choice. Tobacco was sourced from www.hookahcompany.com. Additional details of the clinical laboratory work can be found elsewhere [10].
Each puff topography record was then used to generate smoke using a human-mimic smoking machine programmed to reproduce the puffing behavior of each participant in detail resolved to 0.1 s [11]. The entire smoking session was replicated for each individual in the study whose puff topography record was valid (6 records could not be used due to technical errors with the topography instrument), and the resulting nitric oxide (NO), carbon monoxide (CO), nicotine, “tar”, volatile aldehydes (VA), and polyaromatic hydrocarbons (PAH) were quantified. Except for NO, all analytes were determined as previously reported [4–6,11], using GC-MS, HPLC-MS, Karl-Fisher titration, and electrochemical analyzers. NO was determined using a rapid-response EcoChem CLD 70S chemiluminescence analyzer. After passing through the filter assembly, a small fraction of the smoke drawn during each puff was diverted into the NO analyzer, and the resulting instantaneous NO volume concentration signal logged. NO yield was then computed as the average of the instantaneous NO concentration times the total drawn volume. Nicotine content of the raw products was also analyzed by GC-MS [4], and used to calculate normalized nicotine dose (NND) as . NND is a non-dimensional measure of blood level nicotine exposure relative to the total amount of nicotine available in the product. For each participant, blood volume was calculated based on height, weight, and sex using the formula of Nadler [12].
Pearson correlation coefficients (r) and probability values (p) were computed using the Vassarstat CORRWIN macro [13] running in MS Excel 2007.
RESULTS AND DISCUSSION
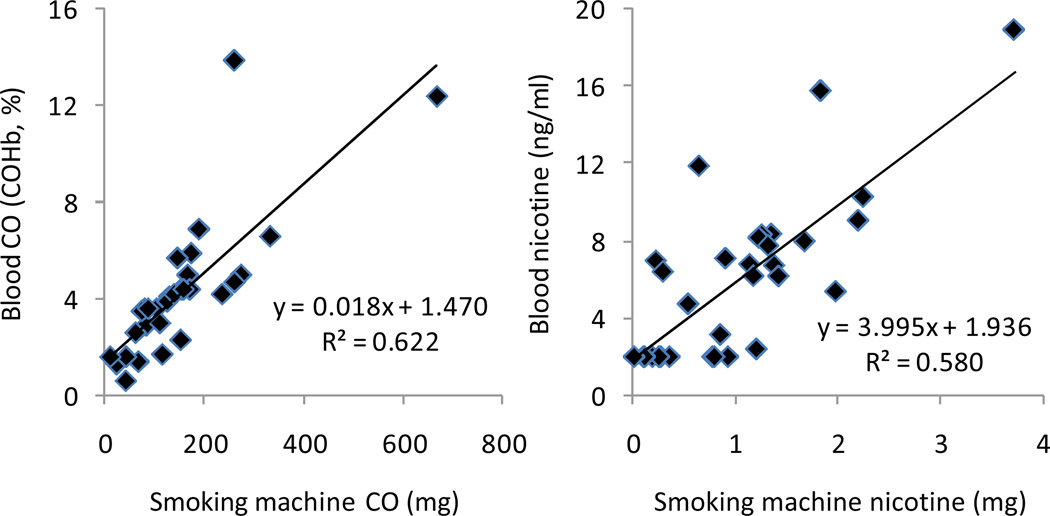
As shown in Figure 1, we found that CO and nicotine yields were highly correlated with COHb (r=0.789, p<0.0001) and plasma nicotine concentration (r=0.762, p<0.0001). Similar relationships were found when plasma concentrations were adjusted for participant blood volume. Thus at least for CO and nicotine, waterpipe smoke yield closely tracks user exposure when a machine is programmed to reproduce the individual smoking behavior of waterpipe tobacco smokers.
Figure 1.
We also found that toxicant yields and exposure were strongly correlated with CO yield, CO exposure, and drawn smoke volume (Table 1). These significant correlations suggest that any of these variables may have value as a convenient proxy measure for other smoke toxicants such as NO, PAH, and VA. Except for PAH yield, smoke volume was the best predictor (i.e. largest Pearson r) of toxicant yield and exposure, accounting for approximately 75–100% of the variability across participants in yields of NO, CO, VA, and “tar”, and exposure to CO and normalized nicotine dose.
Table 1.
Pearson’s r correlations of toxicant exposure and smoking machine yield obtained for CO yield, CO dose, and smoke volume. Dose = blood concentration × estimated blood volume. Normalized nicotine dose = nicotine dose/nicotine in tobacco product.
| CO yield | CO dose | Smoke volume | |
|---|---|---|---|
| Puff topography | |||
| Smoke volume | 0.92*** | 0.94*** | 1 |
| Blood exposure | |||
| COHb | 0.79*** | 0.98*** | 0.90*** |
| CO dose | 0.82*** | 1 | 0.94*** |
| Nicotine concentration | 0.37* | 0.44* | 0.41* |
| Nicotine dose | 0.36* | 0.43* | 0.41* |
| Normalized nicotine dose | 0.49* | 0.71*** | 0.79*** |
| Machine yields | |||
| "Tar" | 0.82*** | 0.64*** | 0.76*** |
| CO | 1 | 0.82*** | 0.92*** |
| NO | 0.97*** | 0.93*** | 0.98*** |
| Nicotine | 0.41* | 0.29(n.s.) | 0.36* |
| Nicotine (normalized) | 0.57* | 0.52** | 0.65*** |
| PAH | |||
| Fluoranthene | 0.65*** | 0.48*** | 0.59*** |
| Pyrene | 0.67*** | 0.51*** | 0.63*** |
| Benzo[a]anthracene | 0.72*** | 0.50*** | 0.60*** |
| Chrysene | 0.60*** | 0.48*** | 0.56*** |
| Benzo[b+k]fluoranthenes | 0.60*** | 0.43*** | 0.54*** |
| Benzo[a]pyrene | 0.56*** | 0.24*** | 0.33*** |
| Benzo[g,h,i]perylene | 0.58*** | 0.42*** | 0.42*** |
| Indeno[1,2,3-cd]pyrene | 0.57*** | 0.40*** | 0.42*** |
| Aldehydes | |||
| Formaldehyde | 0.62*** | 0.92*** | 0.84*** |
| Acetaldehyde | 0.91*** | 0.87*** | 0.94*** |
| Acetone | 0.85*** | 0.77*** | 0.87*** |
| Propionaldehyde | 0.82*** | 0.82*** | 0.86*** |
| Methacrolein | 0.71*** | 0.57*** | 0.65*** |
| Nicotine content in product | |||
| Nicotine in product | −0.069(n.s.) | 0.25(n.s.) | 0.23(n.s.) |
p<0.05,
p<0.01,
p<0.001,
n.s. = not significant.
It is notable in Table 1 that nicotine yields and exposure were the least well predicted parameters. However, when nicotine yield and exposure were normalized by the nicotine content of the raw tobacco product (NND = 15.2±3.0 µg/mg), the strengths of the correlations increased substantially and were similar in magnitude to those of other toxicant measures. Thus nicotine yield and exposure are related to both the quantity of nicotine in the tobacco as well as the quantity of smoke inhaled: the more the user inhales, and the more nicotine that is available in the tobacco, the greater the amount of nicotine to the user. That nicotine varied sufficiently across participants (and therefore tobacco products) to affect correlation with smoke volume, while other toxicants did not is consistent with a physical model in which some smoke toxicants originate from the tobacco preparation (e.g. nicotine, tobacco specific nitrosamines, “tar”; [3]), while others are produced by the burning charcoal (e.g. CO, PAH; [14]), or its interaction with tobacco humectants and flavorings (e.g., VA).
Taken together, the data show that waterpipe smoke that is generated by a machine programmed to mimic human behavior can be used to predict CO and nicotine exposure and that smoke volume or CO exposure may also be valuable in predicting exposure to other toxicants, especially when the contents of the smoked products are taken into account.
While this study used a machine that was programmed to reproduce multiple individuals’ puffing sequences, averaged smoking profiles may be more cost efficient and equally valuable. That is, we have previously found that CO, nicotine, and “tar” content of smoke sampled in real time from the waterpipes of individual users can be estimated reliably by programming a machine to smoke using puff parameters that represent the averaged puff number, volume, duration, and interpuff interval for those individuals [15]. Combined with the observation reported here that yield relates to exposure, it can be deduced that meaningful estimates of toxicant exposure can likely be obtained when a waterpipe tobacco product is machine-tested using an average puffing regimen that reflects the ordinary use of that product. Similar lessons may hold for other tobacco use methods, including cigarettes. However, the generalizability of the results may be limited to the population represented by the individuals whose topography records were averaged.
In summary, this study empirically demonstrates that machine-based methods can be devised in which smoke toxicant yields reliably track human exposure. This finding suggests the basic feasibility of laboratory evaluation of smoked tobacco products for regulatory purposes.
ACKNOWLEDGEMENTS
The authors thank the students and staff of VCU’s Clinical Behavioral Pharmacology Laboratory and AUB’s Aerosol Research Laboratory. The study sponsor had no role in the study design, data collection, data analysis and interpretation, or in the preparation of this report.
GRANT SUPPORT
This work was supported by U.S. Public Health Service Grant R01CA120142.
Footnotes
Conflicts of interest: None to report
REFERENCES
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings. Ann Arbor; Institute for Social Research, the University of Michigan; 2011. [Google Scholar]
- 2.Primack BA, Shensa A, Kim KH, Carroll M, Hoban M, Leino EV, et al. Hookah Tobacco Smoking Among US University Students. (in review) doi: 10.1093/ntr/nts076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem Tox. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 4.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, "tar", and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Tox. 2005;43:655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem Tox. 2008;46:1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem Tox. 2008;46:3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond D, Fong GT, Cummings KM, O'Connor RJ, Giovino GA, McNeill A. Cigarette yields and human exposure: A comparison of alternative testing regimens. Cancer Epidemiology Biomarkers and Prevention. 2006;15:1495–1501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–111. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- 9.Herning RI, Jones RT, Benowitz NL, Mines AH. How a cigarette is smoked determines blood nicotine levels. Clinical Pharmacology and Therapeutics. 1983;33:84–90. doi: 10.1038/clpt.1983.12. [DOI] [PubMed] [Google Scholar]
- 10.Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, et al. Acute effects of waterpipe tobacco smoking: A double-blind, placebo-control study. Drug and Alcohol Dependence. 2011;116:102–109. doi: 10.1016/j.drugalcdep.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shihadeh A, Azar S. A closed-loop control playback smoking machine for generating mainstream tobacco smoke. Aerosol Medicine. 2006;19:137–147. doi: 10.1089/jam.2006.19.137. [DOI] [PubMed] [Google Scholar]
- 12.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 13.Vassarstats statistical computation web site. [Accessed May 2011];Excel downloads: Linear Correlation & Regression utility. http://faculty.vassar.edu/lowry/VassarStats.html. [Google Scholar]
- 14.Monzer B, Sepetdjian E, Saliba NA, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Tox. 2008;46:2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhalation Toxicology. 2010;22:1101–1109. doi: 10.3109/08958378.2010.524265. [DOI] [PubMed] [Google Scholar]