Abstract
The isolation of a new factor, which can cause the in vitro association of 30S and 50S ribosomal subunits at low Mg++ concentration, is described. The association factor is eluted together with the dissociation protein when ribosomes of Bacillus stearothermophilus are washed with salt solutions of high concentration. The association activity is heat-stable, whereas dissociation factor is inactivated after 10 min at 80°C. This treatment allows the separation of both factors. Several properties rule out the possibility that uncharged, amino-acyl-, or peptidyl-tRNA are responsible for the association process described in this report. Digestion with trypsin shows that the association factor contains at least two components, one of which is a protein.
Keywords: ribosomal cycle, E. coli, dissociation factor, heat inactivation
Full text
PDF
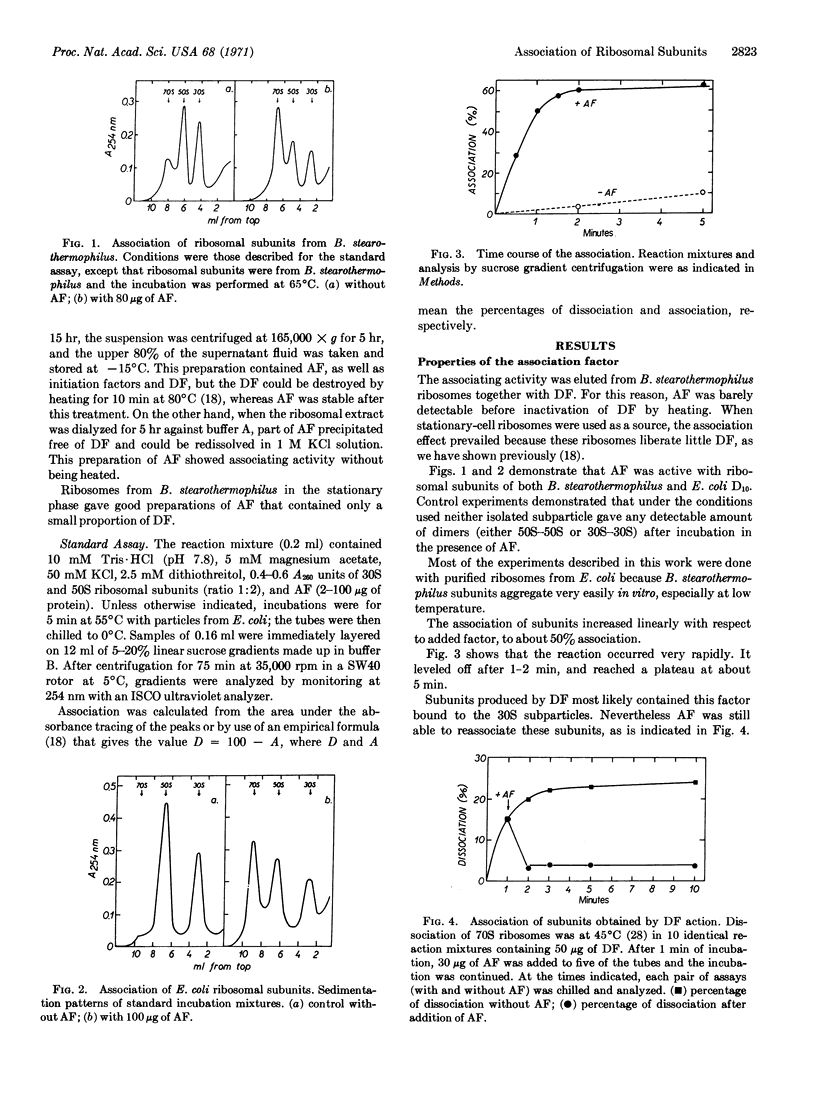
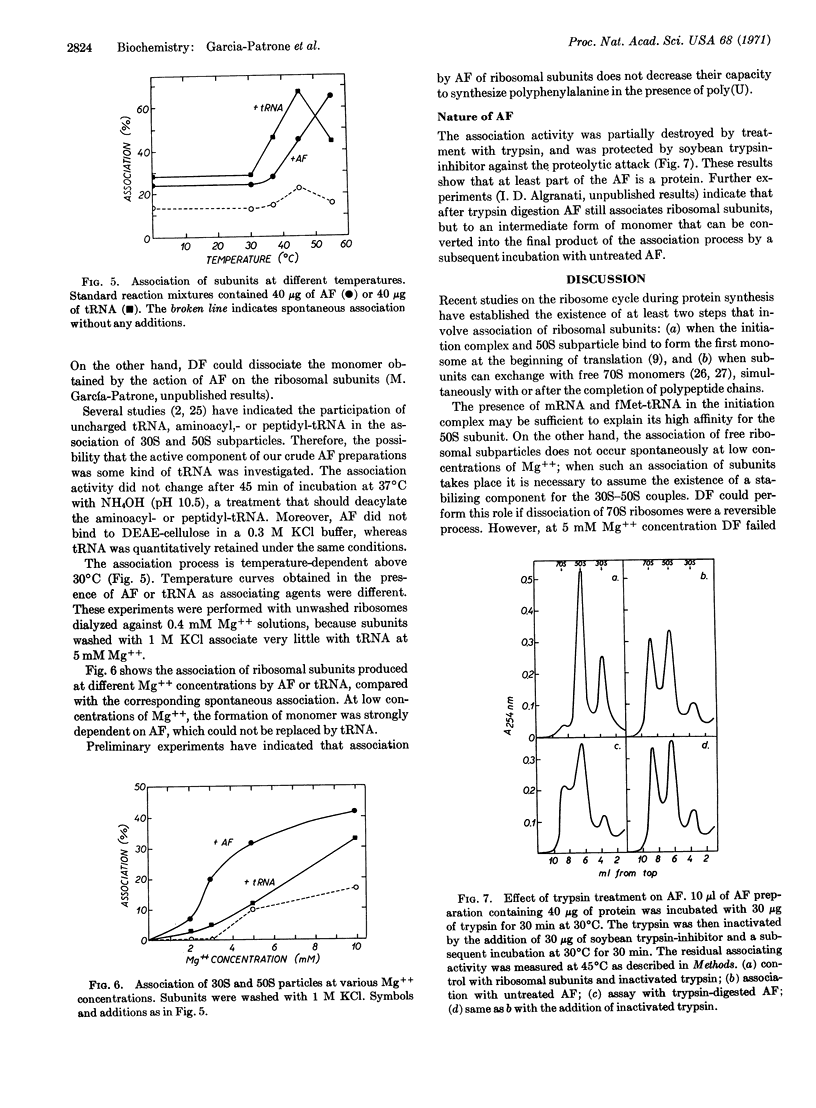

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algranati I. D., Gonzalez N. S., Bade E. G. Physiological role of 70S ribosomes in bacteria. Proc Natl Acad Sci U S A. 1969 Feb;62(2):574–580. doi: 10.1073/pnas.62.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algranati I. D. Occurrence of termination ribosomes as free 70 S particles. FEBS Lett. 1970 Oct 5;10(3):153–155. doi: 10.1016/0014-5793(70)80440-9. [DOI] [PubMed] [Google Scholar]
- Bade E. G., González N. S., Algranati I. S. Dissociation of 70S ribosomes: some properties of the dissociating factor from Bacillus stearothermophilus and Escherichia coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):654–660. doi: 10.1073/pnas.64.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsina N. V., Spirin A. S. Studies on the structure of ribosomes. IV. Participation of aminoacyl-transfer RNA and peptidyl-transfer RNA in the association of ribosomal subparticles. J Mol Biol. 1970 Aug 28;52(1):45–55. doi: 10.1016/0022-2836(70)90176-2. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Role of subunits in the ribosome cycle. Nature. 1971 May 21;231(5299):153–157. doi: 10.1038/231153a0. [DOI] [PubMed] [Google Scholar]
- Dubnoff J. S., Maitra U. Isolation and properties of polypeptide chain initiation factor FII from Escherichia coli: evidence for a dual function. Proc Natl Acad Sci U S A. 1971 Feb;68(2):318–323. doi: 10.1073/pnas.68.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Patrone M., Perazzolo C. A., Baralle F., González N. S., Algranati I. D. Studies on dissociation factor of bacterial ribosomes: effect of antibiotics. Biochim Biophys Acta. 1971 Aug 26;246(2):291–299. doi: 10.1016/0005-2787(71)90139-0. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Bollen A., Herzog A. Ionic effects on the ribosomal quaternary structure. Eur J Biochem. 1970 Mar 1;13(1):132–136. doi: 10.1111/j.1432-1033.1970.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez N. S., Bade E. G., Algranati I. D. Effect of GTP on the dissociation of 70 S ribosomes. FEBS Lett. 1969 Aug;4(4):331–334. doi: 10.1016/0014-5793(69)80268-1. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nomura M. Initiation of protein synthesis: a critical test of the 30S subunit model. Nature. 1968 Jul 20;219(5151):232–235. doi: 10.1038/219232a0. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Turnock G. Stabilization of 70S ribosomes by spermidine. Nat New Biol. 1971 Jan 6;229(1):17–19. doi: 10.1038/newbio229017a0. [DOI] [PubMed] [Google Scholar]
- J Talens, Kalousek F., Bosch L. Dissociation of 70 S E. coli ribosomes induced by a ribosomal factor (DF). Electrophoretic studies of the ribosomal particles. FEBS Lett. 1970 Dec 23;12(1):4–8. doi: 10.1016/0014-5793(70)80580-4. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Dissociation of ribosomes on polypeptide chain termination and origin of single ribosomes. Nature. 1970 Nov 7;228(5271):534–537. doi: 10.1038/228534a0. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. Ribosomal subunit exchange during protein synthesis. Proc Natl Acad Sci U S A. 1968 Sep;61(1):106–113. doi: 10.1073/pnas.61.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T. Intermediate stage in the association and dissociation of Escherichia coli ribosomes and the combining properties of their subunits. Biochim Biophys Acta. 1969 May 20;182(1):135–146. doi: 10.1016/0005-2787(69)90528-0. [DOI] [PubMed] [Google Scholar]
- Sabol S., Sillero M. A., Iwasaki K., Ochoa S. Purification and properties of initiation factor F3. Nature. 1970 Dec 26;228(5278):1269–1273. doi: 10.1038/2281269a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Chain initiation in primitive protein synthesis: a 60S intermediate in the formation of active 70S ribosomes. Nature. 1970 Jul 11;227(5254):128–133. doi: 10.1038/227128a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Conformational changes in ribosomes during protein synthesis. Proc Natl Acad Sci U S A. 1971 Apr;68(4):805–809. doi: 10.1073/pnas.68.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S. A model of the functioning ribosome: locking and unlocking of the ribosome subparticles. Cold Spring Harb Symp Quant Biol. 1969;34:197–207. doi: 10.1101/sqb.1969.034.01.026. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D., Beller R. J. The ribosome dissociation factor and the ribosome-polysome cycle. Cold Spring Harb Symp Quant Biol. 1969;34:223–230. doi: 10.1101/sqb.1969.034.01.028. [DOI] [PubMed] [Google Scholar]
- Subramanian A. R., Ron E. Z., Davis B. D. A factor required for ribosome dissociation in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Oct;61(2):761–767. doi: 10.1073/pnas.61.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Miyazawa F. Dissociation of ribosomes at high temperatures. J Mol Biol. 1966 Jun;17(2):537–540. doi: 10.1016/s0022-2836(66)80164-x. [DOI] [PubMed] [Google Scholar]
- van Diggelen O. P., Heinsius H. L., Kalousek F., Bosh L. Formation of 61 s and 70 s particles from ribosomal subunits of Escherichia coli. J Mol Biol. 1971 Jan 28;55(2):277–281. doi: 10.1016/0022-2836(71)90198-7. [DOI] [PubMed] [Google Scholar]
- van Duin J., VAN Dieijen G., Dieijen G., van Knippenberg P. H., Bosch L. Different species of 70S ribosomes of Escherichia coli and their dissociation into subunits. Eur J Biochem. 1970 Dec;17(3):433–440. doi: 10.1111/j.1432-1033.1970.tb01183.x. [DOI] [PubMed] [Google Scholar]


