Abstract
Iontophoresis is a method of non-invasive transdermal drug delivery based on the transfer of charged molecules using a low-intensity electric current. Both local and systemic administration are possible; however, the skin pharmacokinetics of iontophoretically delivered drugs is complex and difficult to anticipate. The unquestionable theoretical advantages of the technique make it attractive in several potential applications. After a brief review of the factors influencing iontophoresis, we detail the current applications of iontophoresis in therapeutics and the main potential applications under investigation, including systemic and topical drugs and focusing on the treatment of scleroderma-related ulcerations. Finally, we address the issue of safety, which could be a limitation to the routine clinical use of iontophoresis.
Keywords: Iontophoresis, skin, topical drug administration
Introduction
The excellent barrier properties of the stratum corneum (i.e. the outermost skin layer) make drug delivery into and across the skin challenging. Iontophoresis is a method for transdermal drug delivery based on the transfer of charged molecules using a low-intensity electric current. This original route of drug delivery is non-invasive and presents several advantages in comparison to the usual passive transdermal administration, such as faster release of the drug into the skin, the capacity to deliver macromolecules and better control of the delivered dose. Depending on the properties of the molecule, systemic administration can also be achieved without first-pass metabolism [1].
One of the first experiences of medication transfer by electricity may be attributed to Hermann Munk, as early as 1879. Indeed, after a 20–25 min exposure to an electrified strychnine solution, Munk observed spontaneous cramps in rabbits [2]. About 40 years later, Stéphane Leduc described methods to administer salicylic acid using an electric current to relieve pain and accelerate wound healing [3]. Since these early experiments, many drugs have been tested as candidates for iontophoresis. Recent technological advances have allowed the miniaturization of delivery systems, opening the way to clinical perspectives [4].
Two mechanisms are involved in iontophoretic transport. Electromigration (also referred to as electrorepulsion) is the movement of ions across a membrane (i.e. the skin) under the direct influence of an electric field. Negatively charged drugs are therefore repelled into the skin under the cathode, whereas the transfer of positively charged drugs occurs under the anode (Figure 1). The second mechanism is called electro-osmosis, which can be schematized as the volume flow induced by the current flow. As the isoelectric point (pI) of human skin is around 4–4.5, which is below its pH in physiological conditions, the skin will be charged negatively. Application of an electric field across the skin will therefore favour the movement of cations. Therefore, volume flow will be directed in the anode-to-cathode direction, facilitating the transport of positively charged drugs (Figure 1) [5]. Electro-osmosis also allows the diffusion of neutral molecules with anodal iontophoresis. The respective part of the transfer explained by electromigration or electro-osmosis depends mostly on the physicochemical properties of the molecules and the polarity of the applied current.
Figure 1.
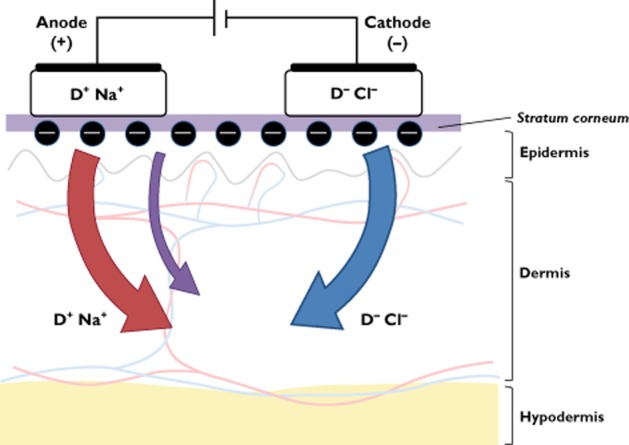
Schematic representation of iontophoretic transport. Positively charged drugs (D+) migrate under the anode, whereas negatively charges drugs (D–) migrate under the cathode. The red and blue arrows represent anodal and cathodal electromigration, respectively. The purple arrow represents electro-osmosis
Iontophoresis has also been studied to enable the extraction of molecules from the skin (the technique is often referred to as ‘reverse iontophoresis’). Although beyond the scope of this review, iontophoretic extraction has raised considerable interest, especially for glucose monitoring [6].
Factors influencing iontophoresis
Faraday's Law states that the number of moles of the transported ion depends on the time of application of the current and its intensity (i.e. the quantity of charge expressed in coulombs; amperes × seconds). The cross-sectional skin area in contact with the electrode should also be taken into account; current density, i.e. the intensity divided by cross-sectional skin area, is usually expressed in milliamperes per square centimetre [5]. There is a high variability in the current density used in humans, from 17 μA cm−2 [7] to 0.5 mA cm−2, which is usually considered to be the maximal current density, for safety reasons [8].
Drug concentration is one of the factors influencing iontophoretic transfer. However, while in some cases there is an almost linear relationship between concentration and flux, the flux often reaches a plateau as the concentration increases, which means that above a given concentration there is a saturation in the iontophoretic transport [5]. The quantity of charge, the skin area and the drug concentration are experimental variables that can be adjusted to modify the electrophoretic transfer.
Other parameters, such as the physicochemical properties of the molecule, cannot easily be influenced by the operator. The size, the partition ratio and, of course, the charge of the molecule are of primary importance [8]. In general, small and hydrophilic molecules are transported at a faster rate than larger, lipophilic molecules [8]. Nonetheless, this rule is not exclusive, because larger molecules, such as peptides, can be administered iontophoretically [9]. In the same way, iontophoresis of rather lipophilic drugs is possible (e.g. treprostinil log octanol/water partition coefficient is 4) [7].
The pH of the solution is of primary importance, because it will determine the ionization of the compound. Indeed, according to the Henderson–Hasselbalch equation for weak acids, the ionic fraction increases with increasing pH of the solution. On the contrary, undissociated weak base increases with increasing pH. Therefore, one can calculate the percentage of ionization if the pKa of the compound (or its pI when there are several weakly acidic or basic groups) and the pH of the solution are known. Practically, the pH must be carefully set and controlled to ensure that the drug is ionized, while preserving skin integrity.
Finally, the integrity of the skin surface, its thickness and whether it is glabrous or not will influence iontophoretic transfer [5]. Although few data are available in vivo, traumatic skin injuries as well as diseases that affect barrier function or the hydratation state of the surface of the skin (e.g. atopic dermatitis or psoriasis) are expected to affect permeation of iontophoretically administered drugs. The thickness of the upper layer of the skin, the stratum corneum, is supposed to be a major obstacle to iontophoretic transport, which can, however, be overcome using physical or chemical enhancers [10]. Moreover, significant regional variations in local blood flow may influence drug clearance, high skin blood flow being associated with increased skin drug clearance. Indeed, nonglabrous skin contains a large proportion of arteriovenous anastomoses, making skin blood flow generally higher and particularly dependent on environmental conditions, especially temperature. For example, the iontophoresis of sodium nitroprusside on the finger pad (glabrous skin, with a thick stratum corneum and elevated skin blood flow) does not induce the vasodilatation observed on the forearm (where skin is nonglabrous and thinner, with low basal blood flow) [11].
Detailed expert reviews about the parameters influencing iontophoretic transport have been published previously [5, 8].
Pharmacokinetics
The skin pharmacokinetics of iontophoretically delivered drugs is complex and difficult to predict. Some drugs are supposed to be rapidly cleared from the dermis (depending on local blood flow) [11], whereas others concentrate in the skin layers for several days [12]. Therefore, depending on the properties of the molecule, iontophoresis may be used for local or systemic drug administration; this stresses the need for careful assessment of the pharmacokinetics of each iontophoretically administered drug.
In vitro studies are usually performed using classic (i.e. vertical) Franz cells or horizontal cells, which allow membrane permeation to be investigated, e.g. in porcine skin [13, 14]. In vivo evaluation of the pharmacokinetics of topically administered drugs is more complex. When possible, the measurement of concentrations in blood or urine is useful to assess systemic bioavailability and/or toxicity, although this is not always feasible; moreover, local concentrations provide useful information to characterize the kinetics of a drug better, although there is no generally accepted method [15].
Tape-stripping consists in the sequential application and removal of adhesive tape strips to collect thin layers of stratum corneum [16]. As this superficial layer is the principal barrier to the penetration of topical drugs, it has been suggested that drug levels in the stratum corneum can be correlated with those in the underlying skin layers [15, 17]. However, tape-stripping has not yet been properly validated. Another major issue when studying pharmacokinetics is that continuous quantification over the same skin area is not possible. This implies a different skin site for each time point, which makes the procedure more complicated as the number of required time points increases [15].
Microdialysis is a minimally invasive technique using a fibre inserted in the dermis or at the dermis–hypodermis interface. A short segment of the fibre is made of a semi-permeable membrane, through which molecules smaller than the cut-off value of the membrane diffuse between the extracellular fluid and the perfused physiological solution. This technique allows continuous sampling during topical drug delivery [15, 18, 19]. This sensitive tool has been used to assess local bioavailability of topically delivered drugs in humans [20–22], including iontophoretically administered compounds [23] (Figure 2).
Figure 2.
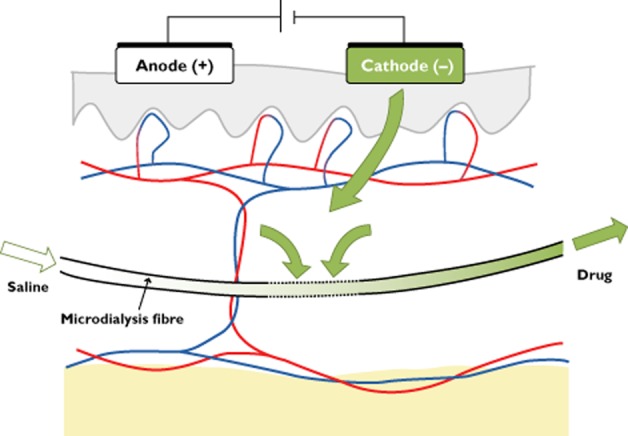
Assessment of the dermal diffusion of a drug administered through cathodal iontophoresis using microdialysis
Current applications and perspectives
Iontophoresis of acetylcholine and sodium nitroprusside, when combined with laser Doppler, have been used as markers of microvascular endothelium-dependent and -independent vasodilatation, respectively [24, 25]. Several decades before being used as reactivity tests, iontophoresis had known therapeutic applications, particularly in physical therapy and dermatology [1]. Indeed, tap water has been used in the treatment of palmar-plantar and axillary hyperhidrosis [26]. In the same way, the current itself may enhance wound healing [27], as exploited in electrotherapy (WoundEL®; DTF Medical, Saint-Etienne, France). In this section, however, we will only focus on iontophoretically delivered drugs approved by regulatory agencies or undergoing clinical development. Iontophoresis may be used as a route of administration for systemic drugs or as local therapy. Indeed, cutaneous iontophoretic delivery is faster than the usual topical routes and allows the administration of high concentrations in the target tissue while limiting systemic toxicity.
Systemic drugs
Fentanyl
Iontophoretic delivery of fentanyl, an opioid analgesic, through a patient-controlled transdermal system (Ionsys®; Janssen, Beerse, Belgium) was approved in 2006 in Europe for the management of acute moderate to severe postoperative pain. Unlike lidocaine, fentanyl is not used topically but as a systemic drug, and iontophoresis allows on-demand administration [28]. In a large prospective, randomized, unblinded, controlled trial comparing iontophoresis of fentanyl with conventional patient-controlled analgesia with morphine, the investigators did not show any difference in efficacy [29], which was confirmed by analysis of pooled data from three trials [30]. However, although not statistically different, withdrawals because of inadequate analgesia were fewer in the intravenous patient-controlled analgesia morphine group than in the fentanyl iontophoresis group (10.3 and 15.2%, respectively; P = 0.07) [29].
However, technical issues concerning the device led to a suspension of marketing authorization by the European Medicines Agency in January 2009 (detailed below in the safety section).
Treatment of migraine
Among the drugs in the pipeline which could lead to new marketing applications soon, agonists of the 5-HT1 family receptors used as anti-migraine agents (i.e. triptans) have raised interest. Indeed, subcutaneous (s.c.) administration of sumatriptan leads to a rapid but transient effect, whereas oral or nasal administration suffers from poor bioavailability. The pharmacokinetics of iontophoretically delivered sumatriptan (4 mA for 1 h followed by 2 mA for 3 h) in healthy subjects showed comparable concentration over time areas under the curve (AUC) to the s.c. route [31, 32], but the maximal concentration was about 30% of that by the s.c. route, whereas time to maximum plasma concentration was 5.6- to 8.3-fold that of the s.c. route [32]. Given that it avoids patient exposure to a rapid increase and high plasma concentrations of sumatriptan in comparison to s.c. administration, iontophoresis may reduce typical triptan-related adverse events (i.e. chest tightness, chest heaviness, paresthesias and sedation/fatigue/malaise) [33]. A new drug application for an iontophoretic device containing sumatriptan (Zecuity®, formerly Zelrix®; NuPathe Inc., Conshohocken, PA, USA) has been approved by the US Food and Drug Administration (FDA) in January 2013 [34].
Other iontophoretically delivered 5-HT1 agonists, such as zolmitriptan or almotriptan, have been studied recently; to date, only preclinical data are available and suggest that these molecules are appropriate candidates for iontophoresis [14, 35].
Other potential applications
Iontophoresis has been suggested as a route of administration for nicotine in smoking cessation [36]. To our knowledge, however, there is no clinical study currently assessing the efficacy and safety of nicotine iontophoresis.
Recent investigations in animals highlighted the potential of iontophoresis of ranitidine [37] or phenobarbital [38] in paediatric patients. However, to our knowledge, there are no clinical data yet.
Finally, administration of proteins has raised interest in the past few years. Iontophoresis of insulin has been extensively studied since the mid-1980s, but a low penetration rate precluded its consideration for therapeutic use [9]. Recent improvements in penetration enhancement techniques and in formulation (i.e. nanovesicles) are encouraging [39]. Iontophoretic delivery of other proteins, such as calcitonin, luteinizing hormone-releasing hormone or vasopressin, has been also investigated in the late 1990s (expertly reviewed in reference [9]), but no clinical applications have emerged yet from these experiments.
Local treatment
Lidocaine/epinephrine
Lidocaine combined with epinephrine was approved by the FDA as a local anaesthetic in 2004 (Lidosite®; Vyteris Inc., Fair Lawn, NJ, USA). Combination with epinephrine aims at decreasing skin blood flow, thus reducing skin clearance of the drug and consequently increasing the dermal concentration [40] and prolonging the anaesthetic action of lidocaine in a dose-dependent manner [41]. The current density of Lidosite® is 0.35 mA cm−2, and it is applied for 10 min with a patch electrode pH of 4.5, at which both lidocaine and epinephrine are positively charged [42].
In a randomized, open-label, crossover study conducted in children undergoing repeated procedures requiring peripheral intraveous access, pain relief after 10 min of lidocaine/epinephrine iontophoresis (Iontocaine®; Abbott Laboratories, Abbott Park, IL, USA) was similar to that found after 60 min of local anaesthesia with lidocaine/prilocaine cream [43]. Two of 22 children discontinued iontophoresis before the complete dose was delivered because of intolerable tingling, itching and discomfort from the procedure [43]. In a randomized, double-blind study comparing iontophoresis of lidocaine/epinephrine with that of placebo, erythema at the site of iontophoresis was observed in about 50% of adults and in 60% of children, whatever the group. In most cases, it was mild, but still detectable after 24 h in about 6.5% of the adults [44]. Additional adverse events included mild oedema, itching and urticaria [44].
The pharmacokinetics of lidocaine iontophoresis have been assessed in animals and in humans. In a study using microdialysis in animal models, iontophoresis of lidocaine at 0.15 mA cm−2 resulted in a 40-fold increase in dermal lidocaine concentrations in comparison to passive drug delivery [45]. In children, lidocaine plasma concentrations were below the lower limit of detection (<5 ng ml−1; or below 10 ng ml−1 for one observation after three applications) [46].
Current clinical investigations
Iontophoretic delivery of terbinafine has been proposed in the treatment of onychomycosis [47]. Preliminary data in humans compared terbinafine patches with (100 μA cm−2, the active electrode polarity being positive) or without current during 4 weeks (6–8 h overnight, every day, 5 days a week). Clinical improvement was observed in the iontophoresis group compared with control subjects from the second follow-up visit [48]. At the end of follow-up, mycological improvement (i.e. decrease in fungal elements) was also observed in patients from the iontophoresis group compared with control subjects [48]. The investigators hypothesized that deeper penetration of the drug into the nail bed under the influence of the electric field was responsible for these encouraging results [48]. Despite the prolonged treatment with elevated intensity, iontophoresis of terbinafine was well tolerated. Besides a tingling sensation, local irritation was reported by only two of 20 patients at the first application of the patch, and none of them stopped the therapy [48]. Nonetheless, iontophoresis was performed over a small area (1 cm2) on the toenail, which is less sensitive than plain skin.
Iontophoresis of antiviral agents was proposed in the treatment of herpes labialis as early as the mid-1980s. Indeed, the efficacy of topical cream is limited by low penetration of the drug into the basal epidermis. A single 10 min iontophoretic application of 5% acyclovir cream was superior to placebo in the time to healing [49]. Further research using a modified, self-administered, iontophoretic device (SoloVir®) was conducted in 2007 but, to our knowledge, the results have not been published (clinicaltrials.gov identifier: NCT00469300), and the development of SoloVir® was stopped [50].
Among other local therapies being investigated, a study comparing the efficacy and safety of iontophoretically administered azelaic acid twice weekly to topical cream twice daily in women with melasma is ongoing (clinicaltrials.gov identifier: NCT00848458).
Finally, iontophoresis of corticosteroids has been extensively studied, especially since the marketing of new devices (e.g. EyeGate® delivery system) which enable drug delivery to both the anterior and posterior segments of the human eye. Recent controlled studies suggest that dexamethasone administered through ophthalmic iontophoresis may be an effective treatment of dry eye [51], but this is beyond the scope of this review. On the skin, however, a pilot study reports the potential benefit of dexamethasone iontophoresis for temporomandibular joint involvement in juvenile idiopathic arthritis [52]. In contrast, dexamethasone iontophoresis was not effective in the treatment of mild to moderate carpal tunnel syndrome [53].
Besides tap water iontophoresis, which has been used for years in hyperhidrosis, Clostridium botulinum toxin type A (BTX-A) was successfully administered iontophoretically (100 IU diluted in 3 ml of saline; 1.1–2.2 mA cm−2 for 5 min) to two patients with severe palmar hyperhidrosis [54]. A small (n = 8), double-blind, randomized, placebo-controlled study performed by the same group showed reduced palmar sweating in the BTX-A group at 14 days post-treatment [55]. A preliminary randomized study comparing iontophoretically administered vs. intradermal injections of botulinum toxin treatment in patients with palmar hyperhidrosis recently showed that injections are more effective but more painful for the administration of BTX-A [56]. A larger trial is currently ongoing (clinicaltrials.gov identifier: NCT01262339) and will provide further data.
Perspectives in scleroderma-related ulcerations
Systemic sclerosis (scleroderma) is characterized by microvascular dysfunction leading to irreversible tissue ischaemia, associated with scarring, ulceration and sometimes gangrene. Iloprost, a prostacyclin (PGI2) analogue used intravenously, is the only drug approved for the treatment of existing digital ulcers [57]. However, the therapeutic effect of prostaglandin analogues is counterbalanced by potentially serious systemic adverse effects related to their potent vasodilator properties, such as severe headaches, flushing, tachycardia and systemic hypotension [57].
Iontophoresis of vasodilators has been proposed as a possible therapy for scleroderma-related ulcerations [58, 59]. Indeed, it could permit a significant drug concentration to be attained locally, while limiting systemic exposure, thus decreasing the risk of adverse drug events. Preliminary data on rats have revealed a sustained increase in skin blood flux after 20 min cathodal iontophoresis (85 μA cm−2) of two PGI2 analogues, treprostinil and iloprost [60]. These results were confirmed for treprostinil in a subsequent clinical study, showing a large and sustained increase in cutaneous blood flow after a 20 min cathodal iontophoresis (17 μA cm−2) of treprostinil in healthy volunteers [7]. In the extension of this work, the dermal and systemic pharmacokinetics of treprostinil iontophoresis is currently being investigated (clinicaltrials.gov identifier: NCT01554540).
Given that bosentan, an endothelin receptor antagonist, has also been shown to be effective in the prevention of digital ulcers in scleroderma [61, 62], iontophoresis of endothelin receptor antagonists was also tested, unsuccessfully, in animals and in humans [63].
Limitations
Safety
Cutaneous adverse events
Iontophoresis induces a sensation of tingling or itching, depending on the density of the applied current. Besides these uncomfortable but harmless effects, skin irritation is the most common local adverse effect of cutaneous iontophoresis. It occurs at both the anode and the cathode. Erythema is the most frequently described adverse effect, with a variable frequency according to the iontophoresis protocols [44, 64]. It was shown to occur consistently after 3 h iontophoresis at 250 μA cm−2, was rated as very slight (grade 1) and lasted up to 3 h after removal of iontophoresis [64]. Using the same protocol, oedema was observed in half of the patients, and both erythema and oedema were enhanced by non-occlusive pretreatment with a surfactant [64]. Skin irritation spontaneously and rapidly resolves, does not lead to permanent skin damage and does not disturbs the barrier function of the skin [64].
Although rare, burns have been observed, mainly due to operator error and the incorrect choice of electrodes/formulation composition. Indeed, the electrochemistry at bare metal or graphite electrodes involves electrolysis of water, which induces changes in the pH of the skin by generating H+ and OH− at the positive and negative electrodes, respectively [65]. Variations in pH beyond the buffer capacities of the skin may lead to burns. High current density or prolonged application, as well as positioning of the electrodes over skin defects (which decrease skin resistance) increase the risk of burns. Burns are generally more serious under the cathode, due to involvement of OH− and rise in pH [1]. Indeed, an alkaline phase erodes the epidermis and reduces skin resistance, making skin erosion worse [65]. Therefore, an appropriate choice of buffer concentration in the formulation is needed to reduce the risk of burns. A better solution is to use Ag–AgCl rather than bare metal or carbon active electrodes, because they function at a lower potential and do not operate by water electrolysis.
Other cutaneous adverse effects have occasionally been described, such as galvanic urticaria [66, 67]. However, this was observed at high current intensities up to 24 mA, in the treatment of hyperhidrosis [67].
Several simple recommendations can decrease the risk of skin injury, such as avoiding pressure on the electrodes (not taping, binding or compressing either electrode) and ensuring that the electrode is uniformly wetted [68]. Indeed, most of the commercially available electrodes are made of small sponges in contact with the skin. After dampening the sponge with the drug solution, it conducts the current. Therefore, heterogeneity in sponge dampening locally increases current density and may lead to skin injury. In the same way, the adhesive seal should adhere uniformly to the skin to avoid leaks [68]. Moreover, cleansing the skin with alcohol and avoiding skin defects and contact between metal components and the skin are also recommended [1, 68]. Finally, current intensity should be <0.5 mA cm−2 [8].
Material defects
Material defects are the main cause of skin injuries, such as burns, usually resulting from a contact between metal components and the skin. For example, a partial-thickness burn has been reported in a paediatric patient, attributed to contact between the skin and a defect in the coating of the wires connecting the controller to the electrode patch [44].
Another consequence of material defects can be overdose, which is potentially harmful when drugs are delivered for a systemic action and have a narrow therapeutic index. The most striking example in the past few years was the iontophoretic delivery of fentanyl (Ionsys®); after receiving marketing authorization for the whole European Union in 2006, corrosion of a component within the system was found in one batch. Although no case of fentanyl overdose was reported, this defect could have resulted in fentanyl release without activation by the patient. This could have exposed patients to fentanyl overdose (the maximal theoretical dose was 3.2 mg), with a risk of severe respiratory depression [69]. Ionsys® has not been marketed in Europe since October 2008, and the marketing authorization holder did not apply for renewal of authorization [70]. Ionsys® has recently been acquired by another pharmaceutical company (Incline Therapeutics Inc., Redwood City CA, USA) currently developing new features into the system in order to obtain regulatory approval in the next few years.
Cost-effectiveness
In most cases, iontophoretic devices are more expensive than the usual topical formulations. Although there are limited data about the cost-effectiveness of iontophoresis, in some cases it has been shown to be in favour of iontophoresis compared with another formulation. For example, a cost-effectiveness analysis of various anaesthetic agents (including iontophoresis of lidocaine) to reduce the pain of peripheral intravenous cannulation in an emergency department setting suggests that lidocaine iontophoresis is more cost-effective than lidocaine/prilocaine cream (incremental cost-effectiveness ratios were 2.89 and 2982.04 vs. no anaesthetic, respectively), but not as cost-effective as a needle-free lidocaine jet injection device or the injection of buffered lidocaine [71].
To our knowledge, the cost-effectiveness of iontophoretically administered fentanyl has never been compared with that of the usual intravenous morphine patient-controlled analgesia, for which a reliable monetary figure has not been proposed [72].
Conclusion
Iontophoresis has many theoretical advantages, including non-invasiveness and avoidance of first-pass metabolism for systemic administration, as well as faster administration and better control of the delivered dose in comparison to the usual passive transdermal formulations. Despite many false starts in the development of the iontophoretic administration of drugs in the past few years, several applications show promise, especially as local therapies. Nonetheless, safety remains a central issue, closely related to the reliability of iontophoresis devices.
Acknowledgments
We thank Alison Foote for correction of English language usage.
Conflict of Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Costello CT, Jeske AH. Iontophoresis: applications in transdermal medication delivery. Phys Ther. 1995;75:554–563. doi: 10.1093/ptj/75.6.554. [DOI] [PubMed] [Google Scholar]
- 2.Helmstadter A. The history of electrically-assisted transdermal drug delivery (‘iontophoresis’) Pharmazie. 2001;56:583–587. [PubMed] [Google Scholar]
- 3.Leduc S. Iiie Congrès International De Physiothérapie (20 Mars-2 Avril 1910) Paris: Masson & Cie; 1910. Ionisation destructive. Ses indications, ses résultats immédiats ou éloignés; pp. 164–174. [Google Scholar]
- 4.Kasha PC, Banga AK. A review of patent literature for iontophoretic delivery and devices. Recent Pat Drug Deliv Formul. 2008;2:41–50. doi: 10.2174/187221108783331438. [DOI] [PubMed] [Google Scholar]
- 5.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Leboulanger B, Guy RH, Delgado-Charro MB. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol Meas. 2004;25:R35–50. doi: 10.1088/0967-3334/25/3/r01. [DOI] [PubMed] [Google Scholar]
- 7.Blaise S, Roustit M, Hellmann M, Millet C, Cracowski JL. Cathodal Iontophoresis of Treprostinil Induces a Sustained Increase in Cutaneous Blood Flux in Healthy Volunteers. J Clin Pharmacol. 2013;53:58–66. doi: 10.1177/0091270011434352. [DOI] [PubMed] [Google Scholar]
- 8.Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis – an approach for controlled drug delivery: a review. Curr Drug Deliv. 2007;4:1–10. doi: 10.2174/1567201810704010001. [DOI] [PubMed] [Google Scholar]
- 9.Gratieri T, Kalaria D, Kalia YN. Non-invasive iontophoretic delivery of peptides and proteins across the skin. Expert Opin Drug Deliv. 2011;8:645–663. doi: 10.1517/17425247.2011.566265. [DOI] [PubMed] [Google Scholar]
- 10.Artusi M, Nicoli S, Colombo P, Bettini R, Sacchi A, Santi P. Effect of chemical enhancers and iontophoresis on thiocolchicoside permeation across rabbit and human skin in vitro. J Pharm Sci. 2004;93:2431–2438. doi: 10.1002/jps.20152. [DOI] [PubMed] [Google Scholar]
- 11.Roustit M, Blaise S, Cracowski JL. Sodium nitroprusside iontophoresis on the finger pad does not consistently increase skin blood flow in healthy controls and patients with systemic sclerosis. Microvasc Res. 2009;77:260–264. doi: 10.1016/j.mvr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Siddoju S, Sachdeva V, Friden PM, Yu YY, Banga AK. Acyclovir skin depot characterization following in vivo iontophoretic delivery. Skin Res Technol. 2011;17:234–244. doi: 10.1111/j.1600-0846.2010.00490.x. [DOI] [PubMed] [Google Scholar]
- 13.Takasuga S, Yamamoto R, Mafune S, Sutoh C, Kominami K, Yoshida Y, Ito M, Kinoshita M. In-vitro and in-vivo transdermal iontophoretic delivery of tramadol, a centrally acting analgesic. J Pharm Pharmacol. 2011;63:1437–1445. doi: 10.1111/j.2042-7158.2011.01355.x. [DOI] [PubMed] [Google Scholar]
- 14.Calatayud-Pascual MA, Balaguer-Fernandez C, Serna-Jimenez CE, Del Rio-Sancho S, Femenia-Font A, Merino V, Lopez-Castellano A. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharm. 2011;416:189–194. doi: 10.1016/j.ijpharm.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Herkenne C, Alberti I, Naik A, Kalia YN, Mathy FX, Preat V, Guy RH. In vivo methods for the assessment of topical drug bioavailability. Pharm Res. 2008;25:87–103. doi: 10.1007/s11095-007-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benson H, Watkinson A. Topical and Transdermal Drug Delivery : Principles and Practice. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 17.Rougier A, Dupuis D, Lotte C, Roguet R, Schaefer H. In vivo correlation between stratum corneum reservoir function and percutaneous absorption. J Invest Dermatol. 1983;81:275–278. doi: 10.1111/1523-1747.ep12518298. [DOI] [PubMed] [Google Scholar]
- 18.Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev. 2002;54(Suppl. 1):S99–121. doi: 10.1016/s0169-409x(02)00117-5. [DOI] [PubMed] [Google Scholar]
- 19.Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol. 2010;23:225–243. doi: 10.1159/000314698. [DOI] [PubMed] [Google Scholar]
- 20.Au WL, Skinner MF, Benfeldt E, Verbeeck RK, Kanfer I. Application of dermal microdialysis for the determination of bioavailability of clobetasol propionate applied to the skin of human subjects. Skin Pharmacol Physiol. 2012;25:17–24. doi: 10.1159/000330489. [DOI] [PubMed] [Google Scholar]
- 21.Brunner M, Davies D, Martin W, Leuratti C, Lackner E, Muller M. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects. Br J Clin Pharmacol. 2011;71:852–859. doi: 10.1111/j.1365-2125.2011.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tegeder I, Muth-Selbach U, Lotsch J, Rusing G, Oelkers R, Brune K, Meller S, Kelm GR, Sorgel F, Geisslinger G. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen administration. Clin Pharmacol Ther. 1999;65:357–368. doi: 10.1016/S0009-9236(99)70128-1. [DOI] [PubMed] [Google Scholar]
- 23.Stagni G, O'Donnell D, Liu YJ, Kellogg DL, Morgan T, Shepherd AM. Intradermal microdialysis: kinetics of iontophoretically delivered propranolol in forearm dermis. J Control Release. 2000;63:331–339. doi: 10.1016/s0168-3659(99)00214-x. [DOI] [PubMed] [Google Scholar]
- 24.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109–116. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19:47–64. doi: 10.1111/j.1549-8719.2011.00129.x. [DOI] [PubMed] [Google Scholar]
- 26.Eisenach JH, Atkinson JL, Fealey RD. Hyperhidrosis: evolving therapies for a well-established phenomenon. Mayo Clin Proc. 2005;80:657–666. doi: 10.4065/80.5.657. [DOI] [PubMed] [Google Scholar]
- 27.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 28.Chelly JE, Grass J, Houseman TW, Minkowitz H, Pue A. The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: a multicenter, placebo-controlled trial. Anesth Analg. 2004;98:427–433. doi: 10.1213/01.ANE.0000093314.13848.7E. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Viscusi ER, Reynolds L, Chung F, Atkinson LE, Khanna S. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA. 2004;291:1333–1341. doi: 10.1001/jama.291.11.1333. [DOI] [PubMed] [Google Scholar]
- 30.Viscusi ER, Siccardi M, Damaraju CV, Hewitt DJ, Kershaw P. The safety and efficacy of fentanyl iontophoretic transdermal system compared with morphine intravenous patient-controlled analgesia for postoperative pain management: an analysis of pooled data from three randomized, active-controlled clinical studies. Anesth Analg. 2007;105:1428–1436. doi: 10.1213/01.ane.0000281913.28623.fd. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Siegel SJ, O'Neill C, Dube LM, Kaldeway P, Morris R, Jackson D, Sebree T. A unique iontophoretic patch for optimal transdermal delivery of sumatriptan. Pharm Res. 2007;24:1919–1926. doi: 10.1007/s11095-007-9317-1. [DOI] [PubMed] [Google Scholar]
- 32.Pierce M, Marbury T, O'Neill C, Siegel S, Du W, Sebree T. Zelrix: a novel transdermal formulation of sumatriptan. Headache. 2009;49:817–825. doi: 10.1111/j.1526-4610.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport AM, Freitag F, Pearlman SH. Innovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929–940. doi: 10.2165/11317540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.NuPathe. NuPathe's Zecuity approved by the FDA for the acute treatment of migraine. Available at http://ir.nupathe.com/press-releases/nupathe-s-zecuity-approved-by-the-fda-for-the-acut-nasdaq-path-975802 (last accessed 25 April 2013)
- 35.Patel SR, Zhong H, Sharma A, Kalia YN. Controlled non-invasive transdermal iontophoretic delivery of zolmitriptan hydrochloride in vitro and in vivo. Eur J Pharm Biopharm. 2009;72:304–309. doi: 10.1016/j.ejpb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Escobar-Chavez JJ, Merino V, Lopez-Cervantes M, Rodriguez-Cruz IM, Quintanar-Guerrero D, Ganem-Quintanar A. The use of iontophoresis in the administration of nicotine and new non-nicotine drugs through the skin for smoking cessation. Curr Drug Discov Technol. 2009;6:171–185. doi: 10.2174/157016309789054924. [DOI] [PubMed] [Google Scholar]
- 37.Djabri A, Guy RH, Delgado-Charro MB. Transdermal iontophoresis of ranitidine: an opportunity in paediatric drug therapy. Int J Pharm. 2012;435:27–32. doi: 10.1016/j.ijpharm.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Djabri A, Guy RH, Delgado-Charro MB. Passive and iontophoretic transdermal delivery of phenobarbital: implications in paediatric therapy. Int J Pharm. 2012;435:76–82. doi: 10.1016/j.ijpharm.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J, Shi T, Zhao Y, Xu H, Yang X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release. 2009;139:63–72. doi: 10.1016/j.jconrel.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Riviere JE, Monteiro-Riviere NA, Inman AO. Determination of lidocaine concentrations in skin after transdermal iontophoresis: effects of vasoactive drugs. Pharm Res. 1992;9:211–214. doi: 10.1023/a:1018985323001. [DOI] [PubMed] [Google Scholar]
- 41.Wakita R, Oono Y, Oogami S, Hayashi S, Umino M. The relation between epinephrine concentration and the anesthetic effect of lidocaine iontophoresis. Pain Pract. 2009;9:115–121. doi: 10.1111/j.1533-2500.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 42.Lidosite®. 2004. Full Prescribing Information. Vyteris Inc.
- 43.Galinkin JL, Rose JB, Harris K, Watcha MF. Lidocaine iontophoresis versus eutectic mixture of local anesthetics (EMLA) for IV placement in children. Anesth Analg. 2002;94:1484–1488. doi: 10.1097/00000539-200206000-00020. table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Zempsky WT, Sullivan J, Paulson DM, Hoath SB. Evaluation of a low-dose lidocaine iontophoresis system for topical anesthesia in adults and children: a randomized, controlled trial. Clin Ther. 2004;26:1110–1119. doi: 10.1016/s0149-2918(04)90183-x. [DOI] [PubMed] [Google Scholar]
- 45.Holovics HJ, Anderson CR, Levine BS, Hui HW, Lunte CE. Investigation of drug delivery by iontophoresis in a surgical wound utilizing microdialysis. Pharm Res. 2008;25:1762–1770. doi: 10.1007/s11095-007-9490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearns GL, Heacook J, Daly SJ, Singh H, Alander SW, Qu S. Percutaneous lidocaine administration via a new iontophoresis system in children: tolerability and absence of systemic bioavailability. Pediatrics. 2003;112:578–582. doi: 10.1542/peds.112.3.578. [DOI] [PubMed] [Google Scholar]
- 47.Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, Friden PM, Murthy SN. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98:4130–4140. doi: 10.1002/jps.21711. [DOI] [PubMed] [Google Scholar]
- 48.Amichai B, Nitzan B, Mosckovitz R, Shemer A. Iontophoretic delivery of terbinafine in onychomycosis: a preliminary study. Br J Dermatol. 2010;162:46–50. doi: 10.1111/j.1365-2133.2009.09414.x. [DOI] [PubMed] [Google Scholar]
- 49.Morrel EM, Spruance SL, Goldberg DI. Topical iontophoretic administration of acyclovir for the episodic treatment of herpes labialis: a randomized, double-blind, placebo-controlled, clinic-initiated trial. Clin Infect Dis. 2006;43:460–467. doi: 10.1086/505872. [DOI] [PubMed] [Google Scholar]
- 50.Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011;12:431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patane MA, Cohen A, From S, Torkildsen G, Welch D, Ousler GW., 3rd Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5:633–643. doi: 10.2147/OPTH.S19349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mina R, Melson P, Powell S, Rao M, Hinze C, Passo M, Graham TB, Brunner HI. Effectiveness of dexamethasone iontophoresis for temporomandibular joint involvement in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:1511–1516. doi: 10.1002/acr.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amirjani N, Ashworth NL, Watt MJ, Gordon T, Chan KM. Corticosteroid iontophoresis to treat carpal tunnel syndrome: a double-blind randomized controlled trial. Muscle Nerve. 2009;39:627–633. doi: 10.1002/mus.21300. [DOI] [PubMed] [Google Scholar]
- 54.Kavanagh GM, Oh C, Shams K. BOTOX delivery by iontophoresis. Br J Dermatol. 2004;151:1093–1095. doi: 10.1111/j.1365-2133.2004.06250.x. [DOI] [PubMed] [Google Scholar]
- 55.Kavanagh GM, Shams K. Botulinum toxin type A by iontophoresis for primary palmar hyperhidrosis. J Am Acad Dermatol. 2006;55:S115–117. doi: 10.1016/j.jaad.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Montaser-Kouhsari L, Zartab H, Fanian F, Noorian N, Sadr B, Nassiri-Kashani M, Firooz A. Comparison of intradermal injection with iontophoresis of abobotulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2012.739679. doi: 10.3109/09546634.2012.739679. [DOI] [PubMed] [Google Scholar]
- 57.Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol. 1992;19:1407–1414. [PubMed] [Google Scholar]
- 58.Murray AK, Herrick AL, Gorodkin RE, Moore TL, King TA. Possible therapeutic use of vasodilator iontophoresis. Microvasc Res. 2005;69:89–94. doi: 10.1016/j.mvr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Murray AK, Moore TL, King TA, Herrick AL. Vasodilator iontophoresis a possible new therapy for digital ischaemia in systemic sclerosis? Rheumatology (Oxford) 2008;47:76–79. doi: 10.1093/rheumatology/kem314. [DOI] [PubMed] [Google Scholar]
- 60.Blaise S, Roustit M, Millet C, Ribuot C, Boutonnat J, Cracowski JL. Cathodal iontophoresis of treprostinil and iloprost induces a sustained increase in cutaneous flux in rats. Br J Pharmacol. 2011;162:557–565. doi: 10.1111/j.1476-5381.2010.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, Rich E, Carpentier P, Molitor J, Seibold JR, Hsu V, Guillevin L, Chatterjee S, Peter HH, Coppock J, Herrick A, Merkel PA, Simms R, Denton CP, Furst D, Nguyen N, Gaitonde M, Black C. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–3993. doi: 10.1002/art.20676. [DOI] [PubMed] [Google Scholar]
- 62.Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P, Wigley FM, Black CM, Fessler BJ, Merkel PA, Pope JE, Sweiss NJ, Doyle MK, Hellmich B, Medsger TA, Jr, Morganti A, Kramer F, Korn JH, Seibold JR. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70:32–38. doi: 10.1136/ard.2010.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roustit M, Blaise S, Arnaud C, Hellmann M, Millet C, Godin-Ribuot D, Dufournet B, Boutonnat J, Ribuot C, Cracowski JL. Iontophoresis of endothelin receptor antagonists in rats and men. PLoS ONE. 2012;7:e40792. doi: 10.1371/journal.pone.0040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li GL, Van Steeg TJ, Putter H, Van Der Spek J, Pavel S, Danhof M, Bouwstra JA. Cutaneous side-effects of transdermal iontophoresis with and without surfactant pretreatment: a single-blinded, randomized controlled trial. Br J Dermatol. 2005;153:404–412. doi: 10.1111/j.1365-2133.2005.06741.x. [DOI] [PubMed] [Google Scholar]
- 65.ECRI Institute. Lesions and shocks during iontophoresis. Health Devices. 1997;26:123–125. [PubMed] [Google Scholar]
- 66.Meffert JJ. Galvanic urticaria. Cutis. 1999;63:327–328. [PubMed] [Google Scholar]
- 67.Lacy KE, Kennedy CT. Galvanic urticaria. Clin Exp Dermatol. 2006;31:739–740. doi: 10.1111/j.1365-2230.2006.02177.x. [DOI] [PubMed] [Google Scholar]
- 68.Warden GD. Electrical safety in iontophoresis. Rehab Manag. 2007;20:22–23. [PubMed] [Google Scholar]
- 69.Prescrire. Fentanyl transdermal system: market withdrawal. Exposes patients to a risk of overdose. The market withdrawal, due to a manufacturing defect, is more than welcome. Prescrire Int. 2009;18:110. [PubMed] [Google Scholar]
- 70.European Medicines Agency. Ionsys (fentanyl) – Non-renewal of the marketing authorisation in the European Union, 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2011/01/WC500101495.pdf (last accessed 27 March 2012)
- 71.Pershad J, Steinberg SC, Waters TM. Cost-effectiveness analysis of anesthetic agents during peripheral intravenous cannulation in the pediatric emergency department. Arch Pediatr Adolesc Med. 2008;162:952–961. doi: 10.1001/archpedi.162.10.952. [DOI] [PubMed] [Google Scholar]
- 72.Viscusi ER, Schechter LN. Patient-controlled analgesia: finding a balance between cost and comfort. Am J Health Syst Pharm. 2006;63:S3–13. doi: 10.2146/ajhp060011. quiz S15–6. [DOI] [PubMed] [Google Scholar]


