Abstract
To assess centrally mediated analgesic mechanisms in clinical trials with pain patients, objective standardized methods such as electroencephalography (EEG) has many advantages. The aim of this review is to provide the reader with an overview of present findings in analgesics assessed with spontaneous EEG and evoked brain potentials (EPs) in humans. Furthermore, EEG methodologies will be discussed with respect to translation from animals to humans and future perspectives in predicting analgesic efficacy. We searched PubMed with MeSH terms ‘analgesics’, ‘electroencephalography’ and ‘evoked potentials’ for relevant articles. Combined with a search in their reference lists 15 articles on spontaneous EEG and 55 papers on EPs were identified. Overall, opioids produced increased activity in the delta band in the spontaneous EEG, but increases in higher frequency bands were also seen. The EP amplitudes decreased in the majority of studies. Anticonvulsants used as analgesics showed inconsistent results. The N-methyl-D-aspartate receptor antagonist ketamine showed an increase in the theta band in spontaneous EEG and decreases in EP amplitudes. Tricyclic antidepressants increased the activity in the delta, theta and beta bands in the spontaneous EEG while EPs were inconsistently affected. Weak analgesics were mainly investigated with EPs and a decrease in amplitudes was generally observed. This review reveals that both spontaneous EEG and EPs are widely used as biomarkers for analgesic drug effects. Methodological differences are common and a more uniform approach will further enhance the value of such biomarkers for drug development and prediction of treatment response in individual patients.
Keywords: analgesics, electroencephalography, evoked potentials, pain, pharmacologic actions
Introduction
Identification of the underlying analgesic mechanisms causing individual effectiveness of analgesics is a challenge in drug development. In many studies, the analgesic effect is assessed by means of subjective self-reporting which, however, does not identify the targeted underlying neural mechanisms in the central nervous system (CNS) and furthermore is confounded by psychological factors [1]. This may to some degree be encompassed using experimental models where the pain stimulation and assessment can be standardized. There have been a substantial number of publications in the field of human experimental pain models in recent decades, yet variable reliability of models remains an issue [2]. Hence, standardized objective methods to assess the analgesic CNS mechanisms in clinical trials are warranted.
One option is to use neurophysiological methods like electroencephalography (EEG), which reflects the electrical brain activity with high temporal resolution, and is related to structural and functional components of the pain experience and following pain attenuation after drug administration [3]. EEG may generally be recorded as the spontaneous electrical activity or as evoked brain potentials (EPs). Spontaneous EEG measures the neural activity during either rest or tonic painful stimulations of the subject and has been used to identify the pathophysiology of pain in chronic pain patients and alterations in the CNS during pharmacological intervention [4]. EPs reflects how the neural networks are synchronized and activated sequentially and in parallel as a response to an external phasic stimulus, which is useful in the study of altered nociceptive responses to acute pain during treatment with analgesics [5]. Taken together, spontaneous EEG and EPs provide complimentary information about the modulation of the CNS after drug administration. Both methods can give quantitative information about the central impact of drugs and are often termed pharmaco-EEG [6–9].
The electrical activity in the brain can be recorded by one or more electrodes on the scalp (surface EEG). It is also possible to record intracranially with electrodes within the brain (local field potential, also known as micro-, depth- or stereotactic EEG) or by subdural grids (electrocorticography) rather than by surface electrodes. The intracranial recording techniques enhance the signal-to-noise ratio and improve the spatial resolution for the area covered by the electrode or grid. Hence, intracranial recording methods may contribute to a deeper understanding of the neuronal activity of the brain [10]. However, due to the invasive nature of the recordings the methodology is not feasible in most human studies and consequently this review will focus solely on studies employing non-invasive surface EEG.
Pharmaco-EEG findings in clinical investigations are typically based on experimental pain models [5]. In such models, the investigator can control the experimentally induced pain (including the nature, localization, intensity, frequency and duration of the acute stimulus or the conditions related to the spontaneous EEG), and thereby provide reliable quantitative measures of the psychophysical and neurophysiological responses [11]. Consequently, the effort and enormous resources put into continued discovery of new biological targets in human pain may be optimized, as the underlying analgesic mechanisms can be identified earlier in the development process. Later the analgesics can be tested in patients exhibiting the corresponding pain mechanisms.
Furthermore, pharmaco-EEG models may translate from bench drug development to a clinical feasible bedside application. A substantial number of patients receive inadequate pain relief [12]. In contrast to the present trial and error principle in treatment, an EEG based application might help clinicians to optimize pain treatment by selection of the individual optimal analgesics, as it has been proposed in psychiatry [13]. This future perspective is further supported by the low cost and portable properties of the EEG recording devices.
The aim of this review is to provide the reader with an overview of present findings in spontaneous EEG and EPs for assessment of cerebral effects of analgesics in humans. Furthermore, pharmaco-EEG methodologies will be discussed with respect to translation from animals to humans and future perspectives in predicting analgesic efficacy.
Methods
Literature search
PubMed searches were performed for articles and abstracts published in English. There was no limit for the time of publication. Only studies in humans were taken into consideration for this review.
With regard to spontaneous EEG, MeSH and free-text terms for ‘analgesics’ were combined with ‘electroencephalography’. As the spontaneous EEG may be analysed in several different ways, a further inclusion criterion was to only include studies reporting alterations in the standard frequency bands delta, theta, alpha and beta.
For EPs MeSH and free-text terms for ‘analgesics’ were combined with ‘electroencephalography’ and ‘evoked potentials’. Only studies examining systemically administered analgesics were included. Studies with combinations of analgesics were only included if the analgesic in question was tested alone in one of the treatment arms. Studies with subjects undergoing general anaesthesia with a combination of anaesthetic agents were not included. The level of evidence was not graded due to the exploratory nature of many of the studies. The minimum sample size for the studies included in this review was eight.
Titles and abstracts were reviewed by the authors to identify studies dealing with the assessment of analgesic compounds and electroencephalographic recording. In addition to the structured literature search a manual search of references from articles included was also conducted. Thus a number of articles not identified by the original search were included in this review if all other requirements were met.
Electroencephalographical methodology
EEG is a two-dimensional (voltage vs. time) representation of the neural activity in the brain. When the recording electrodes are mounted on the surface of the scalp, the main contribution to the traces is the sum of excitatory and inhibitory postsynaptic activities. These postsynaptic activities are synchronized in a large population of neurons in various brain regions and transmitted to the surface by volume conduction [14]. The synchronization is regulated by complex homeostatic systems and results in rhythmic brain electrical activity with distinct oscillations depending on the anatomical region [15].
This electrical rhythmicity may be quantified by the number of oscillations per second and presented in the delta (1.5–6 Hz), theta (6–8.5 Hz), alpha (8.5–12.5 Hz) and beta (12.5–30 Hz) frequency bands. The alpha and beta bands can be subdivided into alpha-1 (8.5–10.5 Hz), alpha-2 (10.5–12.5 Hz), beta-1 (12.5–18.5 Hz), beta-2 (18.5–21.0 Hz) and beta-3 (21–30 Hz) as illustrated in Figure 1 [7]. This division of frequency bands is in concordance with the recommendations made by the International Pharmaco-EEG Group [8, 9]. However it should be noted that slight variations in the division of bands have been employed [16]
Figure 1.
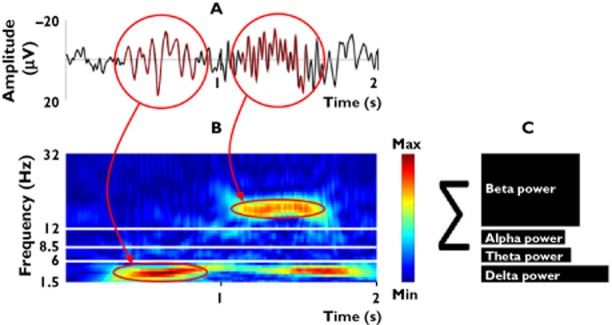
Spontaneous EEG representing the overall cortical neural processing is a mix of several brain oscillations. (A) Oscillations can be presented in a time domain. (B) The frequency distribution can be presented in a time–frequency domain. (C) Traditionally the oscillations are decomposed into specific frequency bands, with the slowest oscillations in the delta band (1.5–6 Hz) and the fastest oscillations into the beta band (12.5–30 Hz). Thus the frequency distribution can be presented in the frequency domain as the relative contributions of each frequency band to the overall power of the EEG
The rationale for the division of oscillations enables detailed interpretation of the brain regions being activated and how they are altered by analgesics [15, 17, 18]. A schematic illustration of the generation of oscillations is given in Figure 2. In a healthy adult person at rest, the main activity is generated by pacemaker neurons distributed throughout the thalamus and projected globally across the cortex. These oscillations are known as the alpha rhythm, and hence reflect the cortico-thalamic network, although local cortical connections also play an important role in the generation of alpha rhythms [15, 19]. The alpha rhythm can be altered by γ-aminobutyric acid (GABA) release. This hyperpolarizes the cell membranes of thalamic neurons causing a slowing of the rhythmicity into the theta range and additionally diminishes the sensory throughput to the cortex. Hence, the theta activity may represent the inhibitory action of GABAergic interneurons in the cortico-thalamic network. Furthermore, theta oscillations can be obtained by the cortex activating the glutamatergic pathways or by dopamine release. A further slowing of the oscillations into the delta rhythm reflects electrical activity generated by the cortex when no sensory input is processed. This intrinsic rhythm depends on the potassium fluxes at voltage dependent ion channels of cortical and thalamic neurons and hence represents cortico-thalamic dissociation [19]. In contrast, fast oscillations are generated from short-living interactions between interneurons and pyramidal cells reflecting cortico-cortical and thalamo-cortical transactions related to specific information processing. Increased beta band activity may be obtained by cholinergic and serotonergic mediation, which releases the thalamic cells from inhibition which facilitates information flow through the thalamus to the cortex [17].
Figure 2.
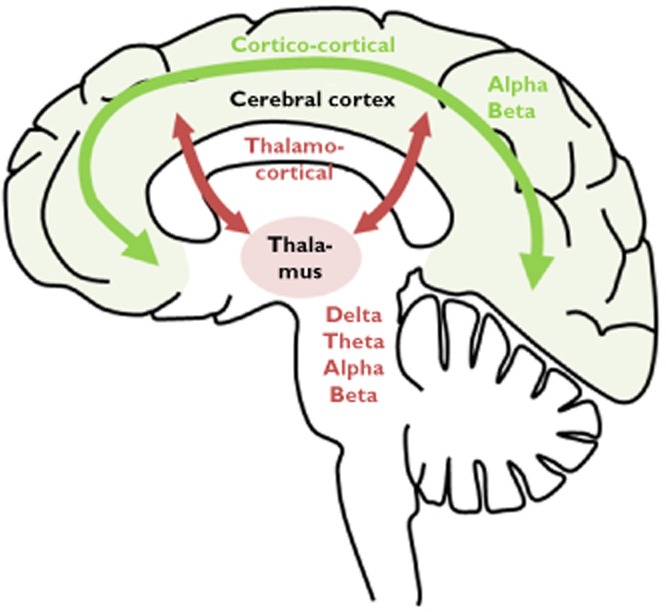
Interactions among brain regions hypothesized to constitute the homeostatic system that generates and regulates the electroencephalographic power spectrum
Two important phenomena should be noted during the design and interpretation of pharmaco-EEG studies. First, as a result of the homeostatic regulation, the EEG spectrum in healthy volunteers is reasonably stable with high specificity believed to reflect our common genetic heritage. Hence, due to this independence of cultural and ethnic factors, assessment of EEG findings is possible across origin and personal background [15]. Secondly, the EEG spectral distribution has been analysed for progression during maturation, which has clarified that a study population can only be compared with age matched subjects due to developmental properties of the EEG [17].
The spontaneous EEG is often analysed in the frequency domain by the Fast Fourier Transform (FFT), wavelet transform or matching pursuit [20, 21]. For the calculation of frequency spectra, typically a stretch of at least 1 min of EEG is selected with the assumption that the relevant brain state does not change significantly during this time interval (steady-state). The EEG is then either analysed for the entire artefact-free time interval or segmented into epochs of appropriate length (typically a number of samples being the power of 2 for mathematical optimization of the spectral decomposition algorithm). The frequency spectrum of all epochs is then calculated and epochs containing artefacts are removed from further analysis. The spectra of all accepted epochs are finally averaged to obtain a smooth spectrum that is amenable to analysis.
The information contained in the EEG signal can be calculated as an absolute measure of frequency distribution in each recording (absolute power) or as the relative distribution of frequencies (in %) normalized to baseline or placebo recordings. Thus the absolute power is sensitive to changes in the amount of total energy contained in the EEG signal, whereas relative power is sensitive to a change in the relative distribution of frequencies under different conditions.
In contrast to the frequency bands, the power spectrum may also be considered on a continuous scale which enables extraction of other characteristics of the EEG properties. One such characteristic is the peak frequency which identifies the exact frequency with the most power. Another characteristic is the median frequency, which is defined as the frequency that divides the power spectrum into 50% of the power being present at lower frequencies and the other 50% of power at higher frequencies. Likewise the spectral edge frequency 95% is the frequency at which the power spectrum is separated into 95% being at lower frequencies and 5% being present at higher frequencies [22].
In contrast to the spontaneous EEG, the EP presents the time-locked response to an external stimulus. As this response is highly influenced by the sequential activation of distinct brain centres, the morphology of the EP is different from the spontaneous EEG as illustrated in Figure 3 [23]. The EP is characterized by several peaks of both negative and positive polarity and may be quantified by the peak amplitudes and latencies. The amplitudes represent an estimate of synchronously activated neurons, while the latency represents the delay in activation due to cortico-cortical connections.
Figure 3.
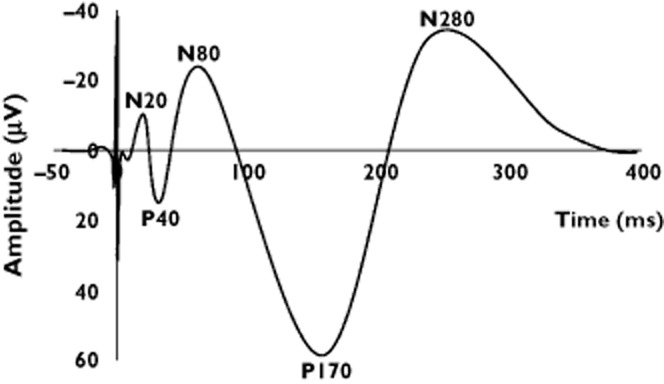
Evoked brain potential during painful electrical stimulation at the median nerve. The potential is an average of several repeated and identical stimulations. The cortical processing of the painful stimuli is traditionally assessed as amplitude and latency characteristics of both negative and positive peaks
Peaks can be classified by their latency as early, intermediate, late and ultra-late. Peaks occurring at 20–60 ms are generally thought to reflect somatosensory afferent input such as touch to the primary somatosensory cortex and are not specific to pain [24]. However, early peaks at high stimulus intensities may reflect concomitant nociceptor activation. Early peaks can be modified by analgesics and are thus used in many studies. For most studies in pain and analgesics changes in intermediate (60–120 ms) or late (120–350 ms) peaks are investigated [25, 26]. These reflect mainly afferent input from small myelinated (Aδ) fibres to the operculum and limbic system. While the intermediate peaks reflect how the afferent nociceptive signal activates supraspinal structures [24], late peaks around 300 ms may rather reflect discomfort or the emotional-motivational aspect of the painful experience [27]. Ultra-late peaks are difficult to record, but are thought to carry information from non-myelinated nociceptive C-fibre input [28]. Peaks are named according to their polarity (negative vs. positive) and their latency in milliseconds (e.g. N150, P240). Occasionally the negative and positive peaks are named in sequential order of their appearance with no regard to the latency (i.e. N1, P1, N2, P2 etc.).
Stimuli used for eliciting EPs must be short and rise quickly in intensity in order to be useful, and different modalities have been employed: electrical stimulation of tooth pulp, skin and viscera, thermal stimulation (laser) on skin and chemical stimulation by jets of carbon dioxide applied to the nasal mucosa.
To enhance the signal-to-noise ratio of EPs most studies employ across-trial averaging of the time-locked signal, with the limitation that any stimulus relevant information contained in the ongoing EEG is lost in the process. Employing joint time-frequency signal analysis methods to investigate single trial frequency content makes it possible to extract information on how EPs and spontaneous EEG co-vary [29].
Results
The search for studies with spontaneous EEG yielded 322 articles. Articles provided by the search were independently reviewed for eligibility by title and abstract and this resulted in a total of 47 relevant articles. The full texts of these articles, as well as six articles found in references, were reviewed and further articles were excluded. The sum of the structured literature search and the additional search was a total of 15 articles (Tables 1 and 2).
Table 1.
The effects of opioids on spontaneous EEG obtained in healthy volunteers and patients. In the design column the analysis method, the number of subjects (n), the duration of the EEG and lastly the recording electrodes used are shown (e.g. Px and Cx indicate the placement of the recording electrodes in the international 10–20 system). Subjects enrolled in the studies are healthy volunteers unless otherwise specified in the design column. In the results column the arrow indicates change of power in the respective frequency bands. Ways to divide the frequency band are delta (1.5–6 Hz), theta (6–8.5 Hz), alpha (8.5–12.5) and beta (12.5–30). The alpha and beta frequency bands can be subdivided into alpha-1 (8.5–10.5 Hz), alpha-2 (10,5–12,5 Hz), beta-1 (12,5–18,5 Hz), beta-2 (18,5–21,0 Hz) and beta-3 (21–30 Hz) [7]
| Drug (dose) | Study | Design | Results |
|---|---|---|---|
| Opioids | |||
| Alfentanil i.v. (1.5 mg min−1) | Egan et al. [31] | n = 10 Duration: NA F3-P3, F4-P4, C3-P3 and C2-P4 | delta ↑ |
| Fentanyl i.v. (1.5 mg 70 kg−1) | Greenwald et al. [25] | n = 8 heroin dependent patients 70 EEG recordings of 2.56 s F3, F4, , Fz, T3, T4, T5, T6, C3, C4, Cz, P3, P4, Pz,O1 and O2 | delta ↑ theta ↑ beta-1 ↑ beta-2 ↑ |
| Heroin i.v. (60–300 mg) | Stoermer et al. [44] | n = 9 heroin dependent patients 15 s EEG F3/C3/P3 and F4/C4/P4 | delta ↑ alpha ↑↓ |
| Meperidine p.o. (150 mg) | Bromm et al. [46] | n = 20 80 EEG recordings of 3 s Cz | delta ↑ theta ↑ alpha ↓ |
| Morphine i.v.† (0.14 mg kg−1 + 0.05 mg kg h−1) | Lötsch et al. [49] | n = 20 60 EEG recordings of 2048 ms Fz, C3, Cz, C4, Pz | delta ↑↓ alpha-1 ↑ beta-1↑ beta-2↑ |
| Morphine i.m. (10 mg) | Saletu et al. [48] | n = 20 5 min EEG F3, F4, P3, P4, O1, O2 | No changes |
| Morphine-6-glucuronide i.v.† (0.015 mg kg−1 + 0.0072 mg kg−1 h−1) (0.029 mg kg−1 + 0.014 mg kg−1 h−1) (0.044 mg kg−1 + 0.022 mg kg−1 h−1) | Lötsch et al. [49] | n = 20 60 EEG recordings of 2048 ms Fz , C3, Cz, C4, Pz | No changes |
| Pentazocine i.v. (30 mg) | Bromm et al. [54] | n = 20 80 recordings of 3 s Cz electrode | theta↓ alpha↓ beta↓ |
| Pentazocine i.v. (30 mg) | Bromm et al. [55] | n = 20 80 recordings of 3 s Cz electrode | delta ↑ theta ↑ alpha ↓ beta ↔ |
| Remifentanil i.v. (1–8 μg kg min−1) | Noh et al. [57] | n = 28 5 min EEG F3, F4, Cz, P3 and P4 | delta ↑ |
| Remifentanil i.v. (3 μg kg−1 min−1) | Egan et al. [31] | n = 10 Duration: NA F3-P3, F4-P4, C3-P3 and C2-P4 | delta ↑ |
| Tramadol p.o. (100 mg) (200 mg) | Thürauf et al. [62] | n = 20 30 recordings of 4096 ms F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 | Changes were seen in alpha-1,alpha-2 and beta-2 bands* Dose dependent changes alpha-2 ↓ |
It is not clearly specified in which direction the changes occurred.
Medicine was administered as a bolus plus an infusion. NA, Not available.
Table 2.
The effects of non-opioids on spontaneous EEG obtained in healthy volunteers and patients. In the design column the analysis method, the number of subjects (n), the duration of the EEG and lastly the recording electrodes used are shown (e.g. Px and Cx indicate the placement of the recording electrodes in the international 10–20 system). Subjects enrolled in the studies are healthy volunteers unless otherwise specified in the design column. In the result column the arrows indicates change of power in the respective frequency bands. Ways to divide the frequency band are delta (1.5–6 Hz), theta (6–8.5 Hz), alpha (8.5–12.5) and beta (12.5–30). The alpha and beta frequency bands can be subdivided into alpha-1 (8.5–10.5 Hz), alpha-2 (10,5–12,5 Hz), beta-1 (12,5–18,5 Hz), beta-2 (18,5–21,0 Hz) and beta-3 (21–30 Hz) [7]
| Drug (dose) | Study | Design | Results | |
|---|---|---|---|---|
| Anticonvulsants | ||||
| CM40907 orally (600 mg) (900 mg) (1200 mg) | Schaffler et al. [69] | n = 12 Duration: NA Cz , Oz | Absolute power 600 mg alpha-1 ↑ beta-2 ↓ 900 mg No changes reported 1200 mg beta-1 ↑ | Relative power 600 mg alpha-1 ↑ 1200 mg beta-1 ↑ |
| Pregabalin orally (75 mg–300 mg twice daily for 3 weeks) | Graversen et al. [73] | n = 28 chronic pancreatitis patients 2 min EEG 62 channels | delta ↔ theta ↑ alpha ↔ beta ↔ | |
| NMDA receptor antagonists | ||||
| Ketamine i.v. (1 mg kg−1) (5 mg kg−1) | Saletu et al. [48] | n = 20 5 min EEG F3, F4, P3, P4, O1, O2 | theta ↑ alpha ↓ Fast beta activity ↑ Slow beta waves ↓ | |
| Non-steroidal anti-inflammatory drugs | ||||
| Acetylsalicylic acid orally (1000 mg) | Schaffler et al. [69] | n = 12 Duration: NA Cz, Oz | Absolute power beta-2 ↓ | Relative power beta-2 ↓ |
| Acetylsalicylic acid orally (0,65 g) (1,95 g) | Fink & Irwin [78] | n = 18 15 min EEG Cz, O1 | 0,65 mg No changes 1,95 mg 2–5 Hz ↑ 8–9 Hz ↓ | |
| Azapropazone orally (300 mg) (600 mg) (1200 mg) | Lötsch et al. [84] | n = 20 30 EEG recordings of 4096 ms Cz,C3, C4, Fz, Pz | 300 mg theta ↓ alpha-1↓ 600 mg delta ↓ 1200 mg theta ↓ alpha-1↓ | |
| Miscellaneous | ||||
| Flupirtine i.v. (80 mg) | Bromm et al. [54] | n = 20 80 EEG recordings of 3 s Cz | theta ↑ beta ↑ alpha ↔ | |
| Flupirtine i.v. (80 mg) | Bromm et al. [55] | n = 20 80 EEG recordings of 3 s Cz | delta ↔ theta ↑ beta ↑ alpha ↔ | |
| Flupirtine orally (200 mg) | Kobal & Hummel [104] | n = 12 Duration: NA F3, Fz, F4, C3, Cz, C4, Pz, Fp2 | delta ↑ theta ↑ alpha ↑ beta ↑ | |
| Imipramine orally. (100 mg) | Bromm et al. [46] | n = 20 80 EEG recordings of 3 s Cz | delta ↑ theta ↑ alpha ↓ beta ↑ | |
NA, not available. Absolute power, absolute frequency distribution in each frequency band. Relative power, relative frequency distribution (in %) normalized to baseline or placebo recordings.
The search for studies with EPs yielded 168 articles. Further review for eligibility resulted in a total of 34 relevant articles. The full text of these articles was reviewed and further articles were excluded. An additional 27 articles were identified in references. These were reviewed in full text and 21 were included. The sum of the structured literature search and the additional search in references was a total of 55 articles (Tables 3, 4 and 5).
Table 3.
The effects of opioids on evoked potentials (EP) obtained in healthy volunteers. In the design column the analysis method, the number of subjects (n), stimulation method and the recording electrodes used are shown (e.g. Px and Cx indicate the placement of the recording electrodes in the international 10–20 system)
| Drug | Study | Design | EP amplitude | EP latency | Subjective pain |
|---|---|---|---|---|---|
| Opioids | |||||
| Alfentanil i.v. (15 μg kg−1) | Chapman et al. [34] | Electrical tooth pulp stimulation n = 10 Cz | ↓ | NA | ↓ |
| Alfentanil i.m. (30 μg kg−1) Naloxone | Arendt-Nielsen et al. [33] | Laser stimulation of the hand n = 6 Cz | ↓ Restored by naloxone | ↔ | ↓ |
| Alfentanil i.v. (5 μg kg−1) (10 μg kg−1) Naloxone | Freye et al. [32] | Electrical stimulation of median nerve n = 5 FpZ, C3 | ↓ Dose dependent, restored by naloxone | NA | ↑ Tolerance |
| Dihydrocodeine orally Controlled release (90 mg) | Hummel et al. [65] | Carbon dioxide jets on nasal mucosa n = 18 Fz, C3, Cz, C4, Pz | ↓ | NA | ↓ |
| Fentanyl i.v. (2 μg kg−1) | Chapman et al. [34] | Electrical tooth pulp stimulation n = 10 Cz | ↓ | NA | ↓ |
| Hydromorphone i.v. (10 μg kg−1) (20 μg kg−1) (40 μg kg−1) | Coda et al. [45] | Electrical tooth pulp stimulation n = 10 Cz | ↓ Dose dependent | NA | ↓ Dose dependent |
| Meperidine orally (150 mg) | Bromm et al. [46] | Electrical stimulation by intracutaneous electrode on fingertip n = 20 Cz | ↓ | NA | ↓ |
| Morphine-6-glucuronide i.v.* (0.015 mg kg−1 + 0.0072 mg kg−1 h−1) (0.029 mg kg−1 + 0.014 mg kg−1 h−1) (0.044 mg kg−1 + 0.022 mg kg−1 h−1) | Lötsch et al. [50] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↔ | ↔ | ↔ |
| Morphine i.v.* (0,14 mg kg−1 + 0.05 mg kg−1 h−1) | Lötsch et al. [50] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ | ↑ | ↓ |
| Morphine i.v. (10 mg) | Quante et al. [51] | Electrical stimulation by intracutaneous electrode on fingertip n = 7 Cz | ↓ | ↔ | ↓ |
| Morphine i.v. (142 μg kg−1) | Chapman et al. [34] | Electrical tooth pulp stimulation n = 10 Cz | ↓ | NA | ↓ |
| Morphine orally (30 mg) | Staahl et al. [52] | Electrical stimulation of the oesophagus n = 12 64 channel/ Cz | ↔ | ↔ | ↓ |
| Nalbuphine i.v. (100 μg kg−1) (500 μg kg−1) (1000 μg kg−1) Naloxone | Freye et al. [32] | Electrical stimulation of median nerve n = 15 Fpz, C3 | ↓ Not restored by naloxone | NA | ↑ Tolerance |
| Paracetamol + codeine orally (1 g + 60 mg) | Arendt-Nielsen et al. [38] | Laser stimulation of the hand n = 12 Cz | ↓ | ↔ | ↓ |
| Pentazocine i.v. (30 mg) | Bromm et al. [54] | Electrical stimulation by intracutaneous electrode on fingertip n = 20 Cz | ↓ | NA | ↓ |
| Remifentanil i.v. (0.1–0.6 μg kg min−1) | Schmidt et al. [58] | Electrical stimulation by intracutaneous electrode on fingertip Electrical stimulation of median nerve n = 9/10 128 channels | ↓ ↑ | NA | ↓ |
| Tramadol i.m. (100 mg) Naloxone | Truini et al. [63] | Laser stimulation of the hand n = 12 T3,T4, Cz | ↓ Partially restored by naloxone | NA | ↔ |
| Tramadol orally (100 mg) Tramadol orally, Controlled release (100 mg) (150 mg) | Hummel et al. [67] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ | NA | ↔ |
| Tramadol orally (100 mg) (200 mg) | Thürauf et al. [62] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ | ↔ | ↓ |
| Tramadol orally (50 mg) | Hummel et al. [65] | Carbon dioxide jets on nasal mucosa n = 18 HV Fz, C3, Cz, C4, Pz | ↓ | NA | ↓ |
| Tramadol orally (50 mg) | Lekic et al. [64] | Electrical tooth pulp stimulation n = 14/15 Cz | ↓ | NA | ↓ |
Medicine was administered as a bolus plus an infusion. NA, Not available.
Table 4.
The effects of opioids and non-opioids on evoked potentials (EP) obtained in patients. In the design column the analysis method, the number of subjects (n), stimulation method and the recording electrodes used are shown (e.g. Px and Cx indicate the placement of the recording electrodes in the international 10–20 system)
| Drug | Study | Design | EP amplitude | EP latency | Subjective pain |
|---|---|---|---|---|---|
| Alfentanil i.v. (125 μg kg−1) | Kalkman et al. [36] | Electrical stimulation of posterior tibial nerve n = 10 Surgical patients Fz(ref), Cz | ↓ | ↔ | NA |
| Fentanyl i.v. (50–60 μg kg−1) | Schubert et al. [40] | Electrical stimulation of median nerve n = 9 Surgical patients Fz, C3,C4 | ↓ | ↑ | NA |
| Fentanyl i.v. (25 μg kg−1) | McPherson et al. [42] | Electrical stimulation of median nerve n = 9 Surgical patients Fpz, C3, C4 | ↓ | ↑ | NA |
| Fentanyl i.v. (75 μg kg−1) | Hume et al. [41] | Electrical stimulation of median nerve n = 17 Surgical patients Fpz, P3,P4 | ↔ | ↔ | NA |
| Fentanyl i.v. (75 μg kg−1) | Kalkman et al. [36] | Electrical stimulation of posterior tibial nerve n = 10 Surgical patients Fz, Cz | ↓ | ↔ | NA |
| Pregabalin orally (300–600 mg twice daily for 3 weeks) | Olesen et al. [74] | Electrical stimulation of the oesophagus n = 26 Chronic pancreatitis patients Fz ,Cz, T7, T8 | ↔ | ↔ | ↓ |
| Sufentanil i.v. (5 μg kg−1) | Kalkman et al. [36] | Electrical stimulation of posterior tibial nerve n = 10 Surgical patients Fz, 2 cm posterior to Cz | ↓ | ↔ | NA |
| Sufentanil i.v. (5 μg kg−1) | Kimovec et al. [60] | Electrical stimulation of median nerve n = 15 Surgical patients Fz, 2 cm posterior to C3 and C4, Iz | ↓ | ↑ | NA |
NA, not available.
Table 5.
The effects of non-opioids on evoked potentials (EP) obtained in healthy volunteers. In the design column the analysis method, the number of subjects (n), stimulation method and the recording electrodes used are shown (e.g. Px and Cx indicate the placement of the recording electrodes in the international 10–20 system)
| Drug | Study | Design | EP amplitude | EP latency | Subjective pain |
|---|---|---|---|---|---|
| Anticonvulsants | |||||
| CM 40907 orally (600 mg) (900 mg) (1200 mg) | Schaffler et al [69] | Laser stimulation of the forearm n = 12 Cz | ↓ Dose dependent | ↔ | ↓ |
| Lamotrigine orally (300 mg) | Klamt et al. [71] | Carbon dioxide jets on nasal mucosa n = 18 Fz, Cz, Pz | ↔ | ↔ | ↔ |
| NMDA receptor antagonists | |||||
| Ketamine i.v. (0.25 mg kg−1) (0.5 mg kg−1) | Kochs et al. [76] | Electrical stimulation by intracutaneous electrode on fingertip n = 10 Cz | ↓ Dose dependent | NA | ↓ Dose dependent |
| Non-steroidal anti-inflammatory drugs | |||||
| Acetylsalicylic acid i.v. (1 g) | Kobal et al. [82] | Carbon dioxide jets on nasal mucosa n = 14 Fp2, F3, Fz, F4, C3, Cz, C4, Pz | ↓ | ↔ | ↔ |
| Acetylsalicylic acid orally (750 mg) Acetylsalicylic acid + lithium + quinine p.o. (750 mg + 126 mg + 4.5 mg) | Schaffler et al. [80] | Laser stimulation of the forearm n = 9 Cz | ↓ | ↔ | NA |
| Acetylsalicylic acid orally (1 g) | Bromm et al. [81] | Electrical stimulation by intracutaneous electrode on fingertip n = 32 Cz | ↓ | ↔ | ↓ |
| Acetylsalicylic acid orally (1 g) | Schaffler et al. [69] | Laser stimulation of the forearm n = 12 Cz | ↓ | ↔ | ↓ |
| Azapropazone i.v. (300 mg) (600 mg) (1200 mg) | Lötsch et al. [84] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ 1200 mg | NA | ↔ |
| Diclofenac orally (50 mg) | Bromm et al. [86] | Electrical stimulation by intracutaneous electrode on fingertip n = 38 Cz | ↓ | ↔ | ↓ |
| Diclofenac orally fast release (50 mg) (100 mg) Diclofenac orally (50 mg) | Lötsch et al. [87] | Carbon dioxide jets on nasal mucosa n = 21 Fz, C3, Cz, C4, Pz | ↓ Fast release formulation 100 mg | ↔ | ↔ |
| Diclofenac i.v. (75 mg) | Schaffler et al. [88] | Laser stimulation on the back n = 24 Cz | ↔ | NA | NA |
| Ibuprofen orally fast release (400 mg) (800 mg) Ibuprofen orally (400 mg) (800 mg) | Hummel et al. [92] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ | ↑ | ↓ |
| Ibuprofen oraally (400 mg) (800 mg) | Kobal et al. [90] | Carbon dioxide jets on nasal mucosa n = 14 Fz, Cz, Pz | ↓ Dose dependent | ↑ | ↔ |
| Ibuprofen orally (400 mg) (800 mg) | Lötsch et al. [91] | Carbon dioxide jets on nasal mucosa n = 18 F3 , Fz, F4, C3, Cz, C4, P3, Pz, P4 | ↔ | ↔ | ↔ |
| Ibuprofen orally (400 mg) Ibuprofen lysine orally (400 mg) | Seibel et al. [93] | Laser stimulation on the back n = 24 Cz | ↓ | NA | NA |
| Ketoprofen i.v. (50 mg) (100 mg) (150 mg) | Hummel et al. [39] | Carbon dioxide jets on nasal mucosa n = 18 Fz, C3, Cz, C4, Pz | ↑ Dose dependent | ↔ | ↑ |
| Miscellaneous | |||||
| Anpirtoline orally (60 mg) | Hummel et al. [66] | Carbon dioxide jets on nasal mucosa n = 16 F3, F4, P3, P4 | ↓ | ↔ | ↓ |
| Flupirtine i.v. (80 mg) | Bromm et al. [54] | Electrical stimulation by intracutaneous electrode on fingertip n = 20 Cz | ↓ | ↓ | |
| Flupirtine orally (50 mg) (100 mg) (200 mg) (300 mg) | Hummel et al. [105] | Carbon dioxide jets on nasal mucosa n = 20 F3, Fz, F4, C3, Cz, C4, Pz | ↓ Dose dependent | ↑ | ↓ Dose dependent |
| Imipramine orally (100 mg) | Hummel et al. [66] | Carbon dioxide jets on nasal mucosa n = 16 F3, F4, P3, P4 | ↔ | ↔ | ↓ |
| Imipramine orally (100 mg) | Sindrup et al. [107] | Laser stimulation of the hand n = 10 Cz | ↔ | NA | ↔ |
| Imipramine orally (100 mg) | Bromm et al. [46] | Electrical stimulation by intracutaneous electrode on fingertip n = 20 Cz | ↓ | NA | ↓ |
| Isoflurane inhaled End tidal concentration (0.08%) (0.16%) (0.24%) | Roth et al. [109] | Laser stimulation of the hand Electrical stimulation by intracutaneous electrode on fingertip n = 10 Cz | ↓ ↓ | ↔ | ↔ |
| Orphenadrine + diclofenac i.v. (30 mg + 75 mg) | Schaffler et al. [88] | Laser stimulation on the back n = 24 Cz | ↓ | NA | NA |
| Orphenadrine i.v. (30 mg) | Schaffler et al. [111] | Laser stimulation on the back n = 18 Cz | ↓ | NA | ↓ |
| Orphenadrine i.v. (30 mg) | Schaffler et al. [88] | Laser stimulation on the back n = 24 Cz | ↓ | NA | NA |
| Paracetamol orally (1 g) | Arendt-Nielsen et al. [38] | Laser stimulation of the hand n = 12 Cz | ↓ | ↔ | ↓ |
| Paracetamol orally (1 g) | Bromm et al. [97] | Electrical stimulation by intracutaneous electrode on fingertip n = 32 Cz | ↓ | ↔ | ↓ |
| Paracetamol orally (1 g) Paracetamol + caffeine orally (1 g + 0.130 g) | Renner et al. [98] | Carbon dioxide jets on nasal mucosa n = 24 Fz, C3, Cz, C4, Pz | ↓ | NA | ↔ |
| Propyphenazone orally (400 mg) (600 mg) Propyphenazone + caffeine p.o. (400 mg + 100 mg) (600 mg + 150 mg) | Kraetsch et al. [101] | Carbon dioxide jets on nasal mucosa n = 20 Fz, C3, Cz, C4, Pz | ↓ | ↔ | ↔ |
| ReN1869 orally (25 mg) (50 mg) ReN1869 orally (25 mg twice daily for 1 week) (50 mg twice daily for 1 week) | Schaffler et al. [113] | Laser stimulation on the back n = 21 Cz | ↓ | NA | ↔ |
NA, not available.
Studies dealing with spontaneous EEG in both healthy volunteers and patients are presented in Table 1 (opioids) and 4 (all other analgesics). Studies on EPs in healthy volunteers are presented in Table 3 (opioids) and 5 (all other analgesics). Studies on EPs in patients are presented in Table 4.
Opioids
Alfentanil
Alfentanil is a short acting synthetic μ-opioid receptor agonist, an analogue of fentanyl [30].
Spontaneous EEG
A study by Egan et al. revealed an increase in the delta band upon intravenous infusion of alfentanil and a downward shift in the 95% spectral edge [31].
Evoked potentials
In general alfentanil reduces the amplitude of peaks in the EP. Freye et al. demonstrated that alfentanil 5 and 10 μg kg−1 intravenously reduced the amplitude of the EP to high intensity electrical stimulation (early N peak at 20 ms) and increased pain tolerance in a dose dependent manner [32]. Similarly, alfentanil 30 μg kg−1 reduced the P2 peak at approximately 300 ms of the laser evoked potentials and increased thresholds to painful stimulation, but not the threshold to sensory stimulation [33]. To document an opioid specific effect it was shown that naloxone restored the EP amplitudes and pain thresholds [32, 33]. Alfentanil also reduced EP amplitude and pain rating to tooth pulp stimulation [34] and there was a dose dependent decrease in amplitudes to laser stimulation of the skin [35]. High dose alfentanil (125 μg kg−1) followed by an infusion for general anaesthesia also decreased the amplitude (P2-N2) of the EPs to 60–70% of baseline values [36].
Codeine and dihydrocodeine
Codeine and dihydrocodeine are weak μ-opioid receptor agonists with similarity in both structure and potency [37]. The effect on spontaneous EEG has not been investigated.
Evoked potentials
Codeine has been investigated in combination formulation with paracetamol where oral codeine 60 mg and paracetamol 1 g decreased LEP amplitudes by 26%, which was more than paracetamol alone. The combination formulation also provided the largest amount of subjective pain relief [38]. Hummel et al. observed that oral dihydrocodeine 90 mg sustained-release decreased EP amplitudes induced by jets of carbon dioxide to the nasal mucosa as well as pain ratings [39].
Fentanyl
Fentanyl is the oldest synthetic piperidine opioid agonist acting primarily on the μ-receptor [37].
Spontaneous EEG
Greenwald et al. investigated the effects of both self-administered and nurse administered fentanyl. However, as self-administration resulted in very variable dosing, this review merely reports the effect of nurse administered fentanyl. All participants were heroin addicts undergoing methadone treatment. EEG analysis yielded an increase in the power density of delta, theta, beta-1 and beta-2 ranges [25].
Evoked potentials
Like other fast-acting opioids fentanyl decreases the peaks in EPs, in particular when EPs are elicited by painful stimulation. Chapman et al. found a decrease in EP peak-to-peak amplitude (at 150–250 ms) and pain rating to electrical stimulation of tooth pulp after i.v. fentanyl [34].
Furthermore, fentanyl has been investigated in high doses where it decreased amplitude of the early peaks [40]. Kalkman et al. used a dose of fentanyl 75 μg kg−1 which decreased the N1-P2 and P2-N2 amplitudes to electrical EP [36]. However, in a study by Hume et al. there were no changes in EP amplitude to electrical stimulation after induction of anesthesia with 75 μg kg−1 fentanyl but only the N peak at 20 ms was considered for analysis [41]. Lower doses (25 μg kg−1) reduced amplitudes slightly at very early peaks but not at early peaks around 20 ms [42]. Due to the sedative effect of high dose opioids, these studies did not report subjective pain (Table 4).
Heroin
Heroin, also known as diamorphine, is a prodrug of morphine [43]. No studies investigating the effect on EPs were identified.
Spontaneous EEG
Stoermer et al. investigated opioid-dependent patients in maintenance treatment with heroin. Participants were assigned to their individual maintenance dose or placebo. Heroin administration produced an increase in the delta band. Following administration an initial increase in the alpha power was observed 5 min after injection. However, the increase was followed by a distinct decrease [44].
Hydromorphone
Hydromorphone is a semi-synthetic morphine derivative acting primarily as a μ-opioid receptor agonist [37]. The effect on spontaneous EEG has not been investigated.
Evoked potentials
Hydromorphone in increasing doses showed dose dependent decreases in late EP amplitudes to electrical stimulation of tooth pulp (N-P at 150 and 250 ms) and pain report [45].
Meperidine
Mepiridine is μ-opioid receptor agonist with anticholinergic and local anaesthetic properties [37].
Spontaneous EEG
Upon administration of meperidine orally an increased delta and theta activity and decreased alpha-1 activity were seen. No changes were evident in the beta band [46].
Evoked potentials
Oral mepiridine reduced amplitudes of EPs evoked electrically by an intracutaneous electrode at 150 and 240 ms to 50% of baseline values as well as subjective pain [46].
Morphine and morphine-6-glucuronide
Morphine is a strong opioid that mainly activates the μ-opioid receptor. Morphine-6-glucuronide is an active metabolite of morphine that crosses the blood–brain barrier more slowly than morphine and has some analgesic effects [47].
Spontaneous EEG
In some of the studies there was a change in the EEG with a tendency to increase in the higher frequencies. A study by Saletu et al. found no significant alterations in the spontaneous EEG to 10 mg i.m. morphine [48]. In contrast to these findings, Lötsch et al. demonstrated that i.v. morphine produced effects on the spontaneous EEG seen as an increase in the alpha-1, beta-1 and beta-2 bands. Furthermore, some fluctuations were seen in the delta band, where a decrease observed shortly after administration, shifted to an increase over time. In addition, morphine produced an increase of the 95% spectral edge. In addition to morphine, three different doses of morphine-6-glucuronide were investigated (Table 1). All infusions were ongoing for 4 h. However, while neither dose produced any significant effects in the individual frequency bands, morphine-6-glucuronide did increase the 95% spectral edge significantly [49].
Evoked potentials
Like the faster acting, synthetic opioids, morphine reduced the amplitudes of peaks in the EP. In healthy volunteers, i.v. morphine in comparable doses (10–12 mg) decreased the amplitude of the late peaks of the EPs. The EP decreases were associated with a decrease in subjective pain ratings [34, 50, 51]. Lötsch et al. also investigated the effect of morphine-6-glucuronide in three different doses. Neither late EP amplitudes nor pain ratings changed, which was possibly due to the kinetics of the metabolite that enters the CNS more slowly than the mother compound [50].
After administering oral morphine 30 mg, Staahl et al. found no changes in the vertex amplitudes of EPs (P2 at 230 ms) elicited by oesophageal electrical stimulation, but observed a shift in the topography and a change of dipole sources. Furthermore, pain thresholds increased after morphine [52].
Nalbuphine
Nalbuphine is classified as an opioid agonist-antagonist. Nalbuphine has high μ-opioid receptor affinity but little μ-opioid receptor efficacy and a partial κ-receptor agonist activity [37]. The effect on spontaneous EEG has not been investigated.
Evoked potentials
Freye et al. observed that i.v. doses of 100, 500 and 1000 μg kg−1 nalbuphine decreased the EP amplitude (late N-peak at 100 ms) and increased the threshold to painful electrical stimulation. These changes were dose dependent and in contrast to findings for alfentanil they were not reversible by naloxone, an indication that other mechanisms than those related to the μ-receptor are involved. The authors suggest that these differences are due to different sites of action for μ- and κ-receptors, as well as differences in the modulation of predominantly the afferent nociceptive signal vs. modulation of the cognitive-emotional aspects of the painful experience [32].
Pentazocine
Pentazocine is a synthetically prepared mixed opioid agonist-antagonist [53].
Spontaneous EEG
Bromm et al. investigated the analgesic efficacy of intravenous pentazocine. This generated a decrease of EEG power in the theta, alpha and beta bands [54]. These changes may reflect an overall increase in EEG power as a second publication by the same authors evaluated the data with regard to relative power and a different picture was seen: an increase in the low frequency bands delta and theta, no changes in the beta band, whereas the decrease in the alpha band was consistent [55].
Evoked potentials
Two studies with 30 mg pentazocine demonstrated decreased amplitudes and pain ratings to electrical stimulation (N-P at 150 and 240 ms) and carbon dioxide jets (N1-P2 at 340 and 520 ms) [54, 55].
Remifentanil
Remifentanil is a short-acting fast-eliminated synthetic μ-opioid receptor agonist [56].
Spontaneous EEG
The effect of remifentanil on the spontaneous EEG has been investigated in two studies (Table 1). Both studies revealed an increase in the delta activity following i.v. infusion. Additionally, a downward shift in the 95% spectral edge was shown in both studies [31, 57]. In a study designed to investigate the cortical topography of changes in the EEG we found that infusion of remifentanil 0.1 μg kg–1 min−1 increased the delta and theta activity. The increase in delta activity was predominant in the frontal recordings (Malver et al., unpublished data).
Evoked potentials
The influence of remifentanil on painful or sensory EPs has primarily been investigated in settings of balanced anaesthesia, i.e. settings where sedatives or inhalational anaesthetics have been co-administered. Aiming to disentangle analgesia and sedation, Schmidt et al. investigated the separate effects of i.v. remifentanil and propofol on EPs to electrical stimulation by an electrode on the skin and stimulation by an electrode placed intracutaneously. Increasing doses remifentanil increased the amplitude (P-N at 50 and 150 ms), which correlated with pain report. On the other hand, when EPs were elicited by an intracutaneous electrode amplitudes decreased (late N-P at 150 and 260 ms). The late potentials evoked intracutaneously were also decreased by the hypnotic propofol and the discrimination between antinociceptive and sedative effects was only possible in the early peaks of the EP [58].
Sufentanil
Sufentanil is a synthetic μ-opioid receptor agonist analogue of fentanyl [59]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
Two studies found a decrease in early EP amplitudes after high-dose sufentanil (5 μg kg−1 and infusion 5 μg kg–1 h−1) [36, 60](Table 4).
Tramadol
Tramadol is a weak opioid with a mixed mode of action influencing the level of serotonin and norepinephrine in the brain [61].
Spontaneous EEG
Thürauf et al. studied two oral doses of tramadol (i.e. 100 mg and 200 mg). Both doses produced significant changes in the power density of alpha-1, alpha-2 and the beta-2 ranges, but there was no clear description on whether the power density increased or decreased. A dose dependent change was only found as decreased alpha-2 power [62].
Evoked potentials
Quite consistently EP amplitudes were decreased after tramadol treatment regardless of stimulation modality (Table 3). Tramadol i.m. decreased the amplitude of the EPs (N2, P2 at 200 and 280 ms) and the decrease was partially restored by naloxone, reflecting the mixed mode of action of tramadol on different receptors and pain mechanisms. However no significant changes in subjective pain were found [63]. Several studies have investigated tramadol administered orally. First, Lekic et al. reported decreased EP amplitude to electrical tooth pulp stimulation (N at 140 ms) after administration of tramadol 50 mg [64]. These findings were reproduced by another study where 50 mg tramadol decreased the amplitudes and pain intensity to jets of carbon dioxide in a design looking into circadian variation [65]. Other studies by the same authors found that tramadol 100, 150 and 200 mg in both conventional and sustained release formulations decreased the amplitudes (N1-P2 at approximately 315 and 500 ms) as well as pain ratings [62, 66, 67].
Non-opioids
Anticonvulsants
CM40907
CM 40907 is a new anticonvulsive compound working through enhancement of the GABAergic transmission with a potential as analgesic [68].
Spontaneous EEG
Schaffler et al. investigated the effect of different oral doses of CM40907 in healthy volunteers. Three different doses were used, 600, 900 and 1200 mg. Evaluation was performed with regard to both absolute and relative power. The 600 and 1200 mg dose induced an increase in absolute alpha-1 power. A similar picture was seen when data evaluation was performed as relative power. Effects were not reported with regard to the 900 mg dose [69].
Evoked potentials
CM 40907 was administered in oral doses of 600, 900, and 1200 mg and compared with acetylsalicylic acid for analgesic properties. A dose dependent decrease of late peaks of the EP to laser stimulation (P2 at 210–240 ms) was found along with a decrease in the pain report which was not dose dependent [69].
Lamotrigine
Lamotrigine is an anti-convulsive drug, a blocker of voltage-gated sodium channels. It is also used for treatment of pain [70]. No studies investigating the analgesic effect in spontaneous EEG were identified.
Evoked potentials
Oral lamotrigine 300 mg was studied to assess the possible analgesic effect, but it did not change EP amplitude (N1-P2 at approximately 275 and 425 ms) or pain ratings to intranasal carbon dioxide [71].
Pregabalin
Pregabalin is an anticonvulsive exerting its main effect by blocking the α2-δ subunits of voltage-dependent calcium channels. It has well established analgesic activity [72].
Spontaneous EEG
Graversen et al. identified changes in the EEG upon administration of pregabalin to patients with chronic pancreatitis. Treatment dose ranged from 300–600 mg daily. The treatment continued over three weeks. An increase in the theta band was found. No changes in delta, alpha and beta bands were apparent. In addition, changes in the EEG frequency distribution were correlated with changes in pain ratings [73].
Evoked potentials
Olesen et al. investigated oral pregabalin 300–600 mg day−1 in patients with chronic pancreatitis, and found no changes of late peaks in EPs to electrical stimulation of the rectosigmoid colon 3 weeks after initiation of therapy, although pain ratings to the stimuli were decreased [74](Table 4).
N-methyl-D-aspartate receptor antagonists
Ketamine
Ketamine is a rapid acting non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor [75].
Spontaneous EEG
Saletu et al. investigated the effect of two different doses of ketamine, 1 mg kg−1 and 5 mg kg−1. Administration of 1 mg kg−1 induced analgesia whereas the higher dose of 5 mg kg−1 induced anaesthesia. Ketamine produced an increase in EEG activity in the theta and fast beta bands and a decrease was evident in the alpha and the slow beta bands [48].
Evoked potentials
Like opioids, ketamine (0.25 and 0.5 mg i.v.) gave a dose dependent decrease in EP amplitudes (N-P at 150 and 250 ms) and pain following intracutaneous electrical stimulation [76].
Non-steroidal anti-inflammatory drugs and paracetamol
Acetylsalicylic acid
Acetylsalicylic acid (ASA) has analgesic, anti-inflammatory, anti-pyretic and anti-thrombotic properties. ASA acts on prostaglandin biosynthesis by inhibiting the cyclo-oxygenase (COX) enzyme irreversibly [77].
Spontaneous EEG
In a study by Fink & Irwin, two different oral doses of ASA (0.65 g and 1.95 g) were administered. No changes were seen upon administration of 0.65 g. However, the higher dose of 1.95 g generated a significant increase in the delta and theta frequency area, whereas a decrease was seen in the alpha range [78]. In another study administration of 1 g induced a decrease in the high frequency beta-2 activity [69].
Evoked potentials
ASA has been studied extensively and in general late peaks of the EP amplitudes are decreased. Amplitudes and pain ratings to tooth pulp stimulation decreased after oral ASA 0.5 g, ASA 1 g and ASA 1 g in combination with lithium and quinine. The amplitudes (N2 at approximately 240 ms) were affected in a dose dependent manner, whereas subjective pain ratings were decreased but not in a dose dependent manner [79]. Schaffler et al. compared oral ASA 750 mg with an ASA-lithium-quinine combination (750 mg ASA) and found decreases in amplitude (N1 at 160 ms) to laser stimulation, although the decrease was more pronounced for the combination preparation [80]. After oral ASA 1 g there was a decrease in EP amplitudes and pain to electrical and laser stimulations [69, 81]. Similarly Kobal et al. found decreased EP amplitudes (N1-P2, N1 at approximately 330–350 ms) to carbon dioxide jets after oral ASA 1 g, although pain reports did not change [82].
Azapropazone
Azapropazone is an analgesic with anti-inflammatory activity [83].
Spontaneous EEG
Lötsch et al. studied the effects of azapropazone in three doses. Azapropazone 300 mg and 1200 mg produced similar changes to the EEG. Decreases in the theta and the alpha bands were seen. No change was observed in the delta band. In contrast, the 600 mg dose provided a decrease in the delta band [84].
Evoked potentials
Intravenous azapropazone in doses of 300, 600 and 1200 mg in general decreased late EP amplitudes (N1-P2) to carbon dioxide jets, although only significant alterations were observed at a dose of 1200 mg. No significant changes in pain reports were found [84].
Diclofenac
Diclofenac sodium is a phenylacetic acid derivative working on both COX-1 and COX-2 isoenzymes [85]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
Bromm et al. investigated the analgesic efficacy of B-vitamin pre-treatment to oral diclofenac 50 mg. There was a decrease in EP amplitudes (N-P at 150 and 250 ms) and pain to electrical stimulation irrespective of B-vitamin treatment 100–120 min after medication [86]. In a comparative study of 50 and 100 mg fast release diclofenac and 50 mg diclofenac tablets [87] and only 100 mg fast release showed a significant decrease in amplitude (P1) to carbon dioxide jets. A dose of 50 mg fast-release showed a tendency toward a decrease in EP amplitude while no changes were seen after the 50 mg tablet. Pain ratings did not change in any medication. Schaffler et al. compared i.v. formulations of a combination of orphenadrine 30 mg and diclofenac 75 mg to each of the single active ingredients. The combination (as well as orphenadrine by itself) decreased the EP amplitude to laser stimulation (P2 at approximately 260 ms), whereas the decrease in EP amplitude induced by diclofenac 75 mg failed to reach a significant level. No subjective pain measures were reported in the study [88].
Ibuprofen
Ibuprofen is a non-steroidal anti-inflammatory analgesic with moderate inhibition of both COX 1 and COX 2 [89]. No studies investigating its effect on spontaneous EEG were identified.
Evoked potentials
In general ibuprofen decreases EP amplitudes (Table 5). Oral ibuprofen 400 and 800 mg decreased EP amplitudes to carbon dioxide jets by 20% and 30% (P1-N1 at approximately 230 and 300 ms) [90]. Pain intensity tended to decrease at the higher dose. Lötsch et al. did a similar study and compared oral ibuprofen 400 and 800 mg, and found contradictory results as no changes in amplitude (N1-P2) were observed for either dose, yet placebo decreased the EP amplitudes [91]. Pain reports also showed no significant changes. In another study oral ibuprofen 400 and 800 mg and oral ibuprofen 400 and 800 mg fast release were compared [92]. EP amplitudes (P1-N1, peak N1 at approximately 290 ms) to carbon dioxide jets decreased 20–25% in a dose dependent manner in both formulations. Comparing oral ibuprofen 400 mg with oral ibuprofen 400 mg in the form of a lysine salt it was found that the lysine salt of ibuprofen decreased the EP amplitude (N1-P2) whereas plain ibuprofen did not significantly alter the EP morphology to laser stimulation [93]. No subjective pain measures were reported in the study.
Ketoprofen
Ketoprofen exhibits analgesic, anti-inflammatory and anti-pyretic activity [94]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
After i.v. ketoprofen 50, 100 and 150 mg, Hummel et al. found EP amplitudes (N1-P2) and pain ratings to carbon dioxide jets to increase in a dose dependent manner which is in contrast to previous findings [39].
Paracetamol
Paracetamol is one of the most frequently used analgesics and antipyretics. It is not completely clear how it works. It may inhibit prostaglandin synthesis [95] and recent findings even suggest an indirect activation of the cannabinoid receptor CB1 as its mode of action [96]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
Several studies have investigated oral paracetamol dosed at 1 g. A decreased amplitude (N2 at approximately 240 ms) and pain response to electrical tooth pulp stimulation was found [79]. Correspondingly, other studies showed decreases in late EP amplitudes and increased pain threshold [38, 97] (Table 5). Renner et al. compared oral administration of paracetamol 1 g with combined paracetamol 1 g and caffeine 130 mg as well as 130 mg caffeine alone, and saw a decrease in EP amplitude (P2) to carbon dioxide jets after both paracetamol and the combination formulation. Pain intensity to the stimuli eliciting the EPs did not change [98].
Phenazone and propyphenazone
Phenazone is an antipyretic analgesic with little anti-inflammatory properties [99]. Propyphenazone is a derivate of phenazone. It acts as a reversible COX inhibitor [100]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
Oral phenazone 1 g gave a 19% decrease in EP amplitudes (N-P at 150 and 250 ms) as well as decreased pain intensity to intracutaneous electrical stimulation [97]. Kraetsch et al. studied oral propyphenazone 400 and 600 mg as well as 400 and 600 mg in combination formulations with caffeine. EP amplitudes (N1) to carbon dioxide jets decreased according to dose with an enhancing effect of caffeine. Propyphenazone 400 mg plus caffeine decreased the amplitude more than propyphenazone 600 mg and propyphenazone 400 mg did not produce a significant effect on the EP. However, pain intensity was not changed [101].
Miscellaneous
Anpirlotine
Anpirtoline is an agonist at the 5-hydroxytryptamine 1B receptor with an analgesic potential [102]. No studies investigating the effect on spontaneous EEG were identified.
Evoked potentials
The analgesic effect of oral anpirlotine 60 mg was compared with tramadol and imipramine and was found to decrease both pain and EP amplitudes (N1-P2) to carbon dioxide jets [66].
Flupirtine
Flupirtine has a mixed mode of action, including opening of different potassium channels, causing neuronal hyperpolarization [103].
Spontaneous EEG
Two studies have investigated the effect of flupirtine on the spontaneous EEG [54, 104]. Intravenous flupirtine 80 mg revealed an increase in absolute theta and beta power [54]. A second publication [55] evaluated the data with regard to relative power and the result was the same. In addition Kobal & Hummel et al. tested the effects of 200 mg oral flupirtine and demonstrated an increase in all frequency bands [104].
Evoked potentials
In a study by Bromm et al., flupirtine 80 mg decreased pain and amplitudes (N-P at 150 and 240 ms) after carbon dioxide jets [54]. Flupirtine 50, 100, 200, 300 mg orally was also compared and there were decreased EP amplitudes (N1-P2 at 325 and 530 ms) for all doses. Decreases were not dose dependent, while pain report was decreased in a linear, dose dependent manner for all doses [105].
Imipramine
Imipramine is a tricyclic antidepressant widely used in the treatment of neuropathic pain [106].
Spontaneous EEG
Oral imipramine 100 mg was found to have an effect on the spontaneous EEG. The delta, theta, beta-1 and beta-2 activity increased, whereas the alpha-1 and alpha-2 activity decreased [46].
Evoked potentials
Oral imipramine 100 mg decreased amplitudes to intracutaneous electrical stimulation (N-P at 150 and 240 ms) of around 32% and decreased pain report of 23% [46]. In another study of oral imipramine 100 mg however, there were no changes in EP amplitude (N1-P2) following carbon dioxide jets, but a decrease in pain report [66]. On the other hand, Sindrup et al. did not detect any differences in amplitudes or pain thresholds to laser stimulation after oral imipramine 100 mg [107].
Isoflurane
Isoflurane, a fluorinated ether, is used primarily as an inhalation anaesthetic [108].
Evoked potentials
Roth et al. investigated the possible analgesic effects of subanaesthetic doses of inhaled isoflurane (0.08, 0.16, 0.24 volume % in exhaled air) and found a decrease of amplitudes (P1-N1 and N1-P2) to laser and electrical stimulation of the skin in the higher concentrations (0.16 and 0.24 vol %), although pain reports were unaffected [109].
Orphenadrine
Orphenadrine is a monomethylated derivative of diphenhydramine and has been used both as a muscle relaxant as well as an analgesic (alone or as a constituent of combination products) [110].
Evoked potentials
Intravenous orphenadrine 30 mg has been investigated alone and in combination with diclofenac [88, 111]. Schaffler et al. compared a combination of orphenadrine 30 mg and diclofenac 75 mg with each of the single active ingredients. The combination (as well as orphenadrine by itself) decreased the EP amplitude (P2 at approximately 260 ms) to laser stimulation [88]. Correspondingly the earlier study found decreased amplitudes of the late peaks (N1, P2) [111]. No subjective pain ratings were reported.
ReN1869
ReN1869 is selective histamine H1-receptor antagonist. The drug was developed for analgesic purposes, but was never marketed [112].
Evoked potentials
ReN1869 has been investigated with regards to analgesic properties. A single oral dose of 25 or 50 mg or oral administration of 25 or 50 mg twice daily for 1 week was found to decrease LEP amplitudes (N1-P2) in a dose dependent manner, with the most pronounced changes seen after 1 week [111, 113]. No significant effect was seen in subjective pain ratings.
Discussion
Summary of findings
For this narrative review we identified 15 articles where the effect of various analgesics was assessed by spontaneous EEG and 55 articles where the effect was assessed by EPs, often in comparison with changes in subjective pain intensity. Differences in methodology were quite prominent, especially regarding the stimulation paradigms for eliciting EPs.
Opioids generally induced a slowing of the spontaneous EEG (an increase in the delta band), and a decrease of the late component amplitude in EPs evoked by painful stimuli, whereas non-painful somatosensory stimuli were unaffected by opioids.
Analgesic effects of anticonvulsants, the NMDA receptor antagonist ketamine and a tricyclic antidepressant (imipramine) were investigated in very few studies.
EPs have been used quite extensively to investigate weak analgesics including non-steroidal anti-inflammatory analgesics and a decrease in amplitudes was seen fairly consistently.
Latencies of the EPs are infrequently reported and when reported no coherent pattern was seen.
Overall the EEG and, in particular, the changes in EP amplitudes were quite consistent with the clinical effect, i.e. the pain relief provided. Several studies demonstrated dose dependent changes in EP amplitudes.
Considerations relating to methodology
Both EPs and spontaneous EEG have been investigated as potential biomarkers for analgesic drug effects. However, the knowledge gained from this field is, to some extent, limited by the fact that a wide array of methodologies is used in different studies. Differences in methodology are obviously an obstacle when data are compared across studies. An example of methodologies that provides incomparable data is the use of absolute or relative power in spectral analysis. This was seen in two studies of Bromm et al., where the same data were used for spectral analysis yielding different findings [54, 55]. Although not widely used in pharmaco-EEG studies of analgesics, it should be noted that standardized methods have been developed to provide an assessment of absolute and relative power simultaneously. One such method is cordance which is associated with cortical perfusion and metabolism and has been used to predict clinical efficacy of pharmacological compounds in psychiatric patients [114–116]. Furthermore, cordance has been applied to pharmacokinetic/pharmacodynamic models as a surrogate measure of the dynamics in clinical and research applications. In short, cordance is calculated for each electrode by extracting the mean absolute and relative power for the electrode, and dividing the values by the mean of all neighbouring channels in each frequency band. These two normalized scores are then summarized and spatially normalized across all electrode sites using Z-scores [117].
A few of the studies identified for this review used the spectral edge frequency 95% as an additional measure of the spontaneous EEG [31, 57]. The spectral edge frequency 95% has been investigated as a possible measure of anaesthetic depth during general anaesthesia [22]. However changes in the low frequencies are poorly reflected by this measure and it is rarely used in studies concerning analgesics.
Other methodologies disabling direct comparison of results between studies deal with placement of electrodes and selection of the reference electrode as well as recording with open or closed eyes. To account for these conflicting methodologies and to ensure comparable results in the future, the International Pharmaco-EEG Group has defined standardized procedures during recording and processing of EEG data, which it is recommended to follow. These concise guidelines clearly outline requirements for EEG recording equipment, calibration procedure, electrode number and positions, environmental conditions, recording conditions and data processing [9].
In the studies included in this review a number of stimulation modalities for evoking EPs are presented. It can be argued that in order to provide meaningful information about analgesic efficacy, the stimulus eliciting the evoked potential should be painful [24]. This implies that studies activating nociceptive Aδ- and C-fibres yield more specific information of pain than studies activating Aβ fibres [118]. Electrical stimulation activates afferent nerves unselectively and while low intensities will predominantly activate Aβ-fibres, higher intensities will also activate Aδ- and C-fibres [26]. To ensure a more selective activaton of Aδ- and C-fibres Bromm & Meier introduced the intracutaneous somatosensory evoked potential. In this methodology a small hole is drilled in the stratum corneum of the skin and the electrode is inserted in the immediate vicinity of the Aδ- and C-fibre nociceptors [119]. Subjectively, this induces a more localized, sharp and painful sensation. In a recent study we showed that brain sources generating the EPs were more nociceptive-like for the intracutaneous electrode as compared with surface electrodes [120]. Yet it can still be argued that intracutaneous electrical stimulation will likely stimulate an unselective spectrum of nerve fibres in a similar fashion to transcutaneous electrical stimulation [26]. By stimulating tissues devoid of mechano- and thermoreceptors such as tooth pulp, the bias of unselective activation of nerve afferents has been addressed resulting in a more reliable measure of pain [121].
Laser-evoked potentials are well established for assessing function of the pain relevant Aδ-fibre pathways in patients with neuropathic pain [122] and several studies have been published on the assessment of analgesic efficacy by laser stimulation and EPs. In 1985 Kobal introduced the chemosomatosensory event-related potentials model for assessing pain and analgesic efficacy by activating nociceptors, but not mechano or proproioceptors. The model applies short pulses of carbon dioxide in varying concentrations to the nasal mucosa [123].
As clinical pain is not confined to skin and teeth, a model of eliciting cortical potentials in deep tissue such as the viscera would be valuable. This has the advantage of more selective stimulation of Aδ-fibres as there are no Aβ fibres in the gut. However stimulating the upper or lower gastrointestinal tract with an electrode on the mucosa is challenging as the probe for stimulation is often inserted blindly and the exact position in relation to the intestinal wall may vary in between stimulations [124–126]. Only a few studies have used deep stimulation in pharmaco-EEG [52, 74].
While the amplitude and latency of peaks in the EP waveform are conceptually independent, they may possibly confound each other [127]. However in the studies identified for this review latencies are infrequently reported and no coherent pattern is seen when they are reported. With regard to laser evoked potentials it has been suggested that such discrepancies reflect the lack of standardization across laboratories [128] and similar issues most likely affect other stimulation modalities.
Drawbacks and strengths with spontaneous EEG in comparison with EPs
Chronic pain often results in depression of behaviour and mood [129]. As restoration of this pain depressed behaviour is the main goal of pharmacological treatment, it would make sense to have a biomarker that can reveal this. Traditionally, human biomarkers for pain have been pain evoked measures, e.g. the individual ratings on visual or numerical rating scales to a painful stimulus or an EP. In preclinical research it is widely established that pain evoked measures often fail to predict the clinical response of analgesics [130]. Accordingly, one can argue that it is unlikely that pain evoked measures, even when applied in humans, will be predictive of successful restoration of pain depressed behaviour. It is, however, likely that spontaneous EEG reflects the overall altered neural activity in the central nervous system including depression of behaviour and mood seen in pain patients, which may predict the clinical outcome of analgesic treatment. Certainly, this has been the case when treating patients with major depression and schizophrenia [13, 131]. Furthermore this approach is supported by two recent studies in fibromyalgia and chronic pancreatitis, where parameters found in the spontaneous EEG predicted reductions in the brief pain inventory i.e. a parameter closely related to restoration of pain depressed behaviour [73, 132].
On a practical consideration it should be said that EPs normally require application of several stimuli (e.g. 20–200 dependent on method) and for patients it can be strenuous to keep still and relaxed for the time it takes to apply the stimuli. In comparison, recording of spontaneous EEG is feasible even in clinical settings.
It was previously believed the spontaneous EEG is a simple measure of sedation. However, a preclinical study in 1986 by Dimpfel et al. clearly showed that opioids, benzodiazepines and the potassium channel modulator flupirtine had completely different effects on the EEG of freely moving rats. As flupirtine is a drug with quite sedative actions it was remarkable to see that the EEG activity increased after administration of this compound [133].
It is recommended and common to control for vigilance in spontaneous EEG experiments to ensure valid data acquisition. This is done by asking subjects perform simple performance tasks [9]. Recently there has been an increasing interest in the importance of cognition and attention on pain related EEG studies. Lorenz et al. investigated six chronic pain patients before and after initiation of morphine treatment. They proposed the theory that stable or even enhanced laser evoked peaks at 300 ms and auditory evoked potentials reflect improved perception and concentration when morphine treatment removes persistent pain as a disruptive-perceptual stressor. Amplitudes at 400 ms to laser evoked potentials were significantly reduced reflecting pain relief during morphine treatment [134, 135]. This reflects modulation of the bottom-up capture of attention by pharmacologically induced pain relief.
The importance of variations in attention to pain (top-down control) has been studied by varying the stimulus paradigms (regarding intensity, location and stimulus intervals) as well as concomitant performance tasks. The importance of cognitive-emotional processes on pain related EP experiments, both bottom-up and top-down has been well reviewed [136]. To our knowledge there has not yet been published any studies dealing with both attentional and pharmacological modulation of the pain related EEG. However it is paramount that the advances in the field of attention to pain are taken into consideration when pain related EP studies are designed.
As opioids are powerful analgesics in the clinic, EPs should equally be highly affected by opioids to be a good biomarker with clinical relevance. However, high doses of synthetic opioids administered in several studies did not obtain consistent results. Hume et al. found no alteration in the EP waveform during fentanyl anaesthesia [41], whereas other studies found decreases in the EP amplitude [40, 42, 60] (Table 4). It should be noted that these studies had a different perspective than assessing analgesic efficacy: They investigated the usefulness of EPs in intra-operative monitoring of neural integrity during opioid anaesthesia. i.e. if the EP changes or disappears during surgery is this a consequence of the drugs providing anaesthesia or due to neural damage. Thus all studies conclude that high dose opioid anaesthesia only induces negligible changes in the EPs and that this quality renders the EP useful in intra-operative monitoring of potential damage to the nervous system during surgery. The stimulation intensity in these studies was kept just slightly above the threshold to elicit a motor twitch in the stimulated extremity or a recordable EP signal. Consequently, these studies clearly evoked a response from an Aβ-fibre and as μ-opioid receptor agonists mainly affect responses from Aδ and C-fibres this could explain the minor change in response in the cortical activation [137].
In contrast, studies applying stimulus intensities in the painful range (i.e. activating Aβ- plus C- and Aδ-fibres) have demonstrated effects of opioid analgesics as seen by Freye et al. who employed painful electrical EPs and found dose dependent decreases in EP amplitudes to both κ-agonists, nalbuphine and alfentanil. This was performed by stimulating with a current twice the intensity of the motor threshold [32]. Furthermore, Schmidt et al. observed dose dependent decreases of EP amplitude and pain relief after remifentanil when assessed by intracutaneous electrical stimulation at twice the intensity of the individual pain threshold [58].
The model of chemically induced phasic pain by jets of carbon dioxide on the nasal mucosa consistently shows decreased EP amplitudes and pain intensity during opioid administration. However, results for studies in non-opioid analgesics are more contradictory as some studies fail to show a decrease in pain intensity [82, 87] (Table 5). Kobal et al. drew attention to the fact that the effect of anti-inflammatory drugs on acute non-inflammatory pain is minor [82], which may well explain the lack of effect on the phasic pain in particular.
Translatability of preclinical EEG studies
In the past decades only few novel analgesics have entered the market to be of use for pain patients. Of these analgesics only one new target has been discovered, namely the gabapentinoids [138]. There are several reasons for this lack of success in drug development and one of them is that the preclinical pain models do not predict clinical efficacy [139–141].
As drug development and testing is based on animal studies, a major limitation is the differences between species. Hence, the pain mechanisms are targeted differently from pharmacological intervention between species and consequently analgesics may target pain mechanisms in animals but not humans. Biomarkers obtained from EEG investigations in animals are likely to contribute to an improved translation between species, and can complement the behavioural measures that are currently most often used [142]. By increasing the use of preclinical EEG measures for decision making it could ensure a better selection of compounds that should be tested in humans.
Another obstacle in drug development is the lack of knowledge on the relationship between human pharmacokinetics and pharmacodynamics when designing the first phase II study. This often results in administration of too low or too high doses. When wrong doses are applied in clinical trials this can produce either an unacceptable level of adverse events or a lack of efficacy. This may result in the termination of the development of the current drug [143]. Enriched phase I studies applying biomarkers from either spontaneous EEG or EPs can, with the application of PKPD modelling, give quantitative knowledge about target engagement and expression of pharmacology [141].
Besides the presence of species differences, preclinical models predominantly investigate evoked pain, whereas the clinical trial endpoints are often a questionnaire. Questionnaire endpoints reflect a sum of different components, where the predominant determinant should be spontaneous and on-going pain, but with large bias from level of side-effects, level of depression, social situation etc [144]. The sum of co-morbidities and reduced function in patients is often referred to as the so-called pain depressed behaviour. Theoretically the spontaneous EEG could reflect the pain depressed behaviour in both rodents and humans. In line with this it has been seen that spontaneous EEG in psychiatric diseases has served as a biomarker predicting treatment outcomes and it is possible that this could also be seen in patients with pain [145, 146]. Indeed it has been found in two studies that the spontaneous EEG did predict treatment outcome in patients with chronic pancreatitis and fibromyalgia [73, 132].
Future perspectives for pharmaco-EEG
A future clinical application of pharmaco-EEG could be directed towards development of methods to predict the effect of analgesics. EEG shortly after initiation of a given therapy could predict the long term chances of treatment success. Such methods may require application of some of the methodologies proven valuable in predicting the response to treatment in psychiatric patients [13, 116, 131]. The methods include source localization (i.e. from what areas in the brain are the observed changes being driven) and connectivity analysis (how are different areas of the brain sequentially activated) followed by classification by statistical learning. Predicting the likely clinical outcome would aid in choosing the right drug for the right patient and thus reduce the risk of side effects from inefficient treatment. Furthermore such advanced analysis could be used to understand the functional state of the central nervous system during pain and the following analgesic mechanisms of drugs.
To establish such a prediction model for analgesics, it may be necessary to record EEG signals reflecting the specific pain processing in the individual patient. This could be achieved during a tonic experimental pain stimulus or by evoking the clinical pain in a controlled condition such as, for example, rotating a limb to standardized pain intensity in patients with osteoarthritis. In contrast to the resting EEG, this pain-predominant EEG would eliminate the random cognitive processing and hence improve the pain specific signal-to-noise ratio. Additionally, the EP analysis may be further improved by decomposition of the traces before source localization and enhancement to analyse single-sweep characteristics at both group and individual basis [147, 148–150]. Such an approach would enable analysis of how EPs and event-related spontaneous EEG co-vary. Taken together, multivariate pattern analysis of combined findings from resting EEG, tonic pain evoked EEG and EPs may lead to new approaches for personalized medicine to tailor individual pain treatment and optimize drug consumption.
From this review it can be seen that the recording of spontaneous EEG yields information about the changes induced by analgesic drugs in the brain. The recording of EPs may yield valuable information on the afferent nociceptive signal entering the central nervous system and how this information may be modulated by analgesics.
While methodological differences are frequent, both spontaneous EEG and EPs appear sensitive to changes induced by analgesics. Improved methods as well as a more standardized approach may well provide a valuable tool with the potential to provide a specific fingerprint of how analgesics work in the central nervous system. This may again be used in development of new drugs with an analgesic potential, as well as in investigations of the analgesic potential of compounds in use for other indications. Finally pharmaco-EEG may emerge as a biomarker for prediction of treatment response to analgesics in the individual patient.
Acknowledgments
The research at Aalborg University Hospital leading to this review has received funding from the Danish Agency for Science, Technology and Innovation: Det Strategiske Forskningsråd; grant No.10–092786.
Competing Interests
There are no competing interests to declare.
References
- 1.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 2.Olesen AE, Andresen T, Staahl C, Drewes AM. Human experimental pain models for assessing the therapeutic efficacy of analgesic drugs. Pharmacol Rev. 2012;64:722–779. doi: 10.1124/pr.111.005447. [DOI] [PubMed] [Google Scholar]
- 3.Saab C. Visualizing the complex brain dynamics of chronic pain. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9378-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Prichep LS, John ER, Howard B, Merkin H, Hiesiger EM. Evaluation of the pain matrix using EEG source localization: a feasibility study. Pain Med. 2011;12:1241–1248. doi: 10.1111/j.1526-4637.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- 5.Chizh BA, Priestley T, Rowbotham M, Schaffler K. Predicting therapeutic efficacy – experimental pain in human subjects. Brain Res Rev. 2009;60:243–254. doi: 10.1016/j.brainresrev.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Dumermuth G, Ferber G, Herrmann WM, Hinrichs H, Kunkel H International pharmaco-EEG group (IPEG) Committee on standardization of data acquisition and analysis in pharmaco-EEG investigations. Neuropsychobiology. 1987;17:213–218. doi: 10.1159/000118367. [DOI] [PubMed] [Google Scholar]
- 7.Knott VJ. Quantitative EEG methods and measures in human psychopharmacological research. Hum Psychopharmacol. 2000;15:479–498. doi: 10.1002/1099-1077(200010)15:7<479::AID-HUP206>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Versavel M, Leonard JP, Herrmann WM. Standard operating procedure for the registration and computer-supported evaluation of pharmaco-EEG data. ‘EEG in Phase I’ of the Collegium Internationale Psychiatriae Scalarum (CIPS) Neuropsychobiology. 1995;32:166–170. doi: 10.1159/000119230. [DOI] [PubMed] [Google Scholar]
- 9.Jobert M, Wilson FJ, Ruigt GS, Brunovsky M, Prichep LS, Drinkenburg WH IPEG Pharmaco-EEG Guidelines Committee. Guidelines for the recording and evaluation of pharmaco-EEG data in man: the International Pharmaco-EEG Society (IPEG) Neuropsychobiology. 2012;66:201–220. doi: 10.1159/000343478. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents – EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95:97–111. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 12.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377:2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 13.Khodayari-Rostamabad A, Reilly JP, Hasey G, Debruin H. Using pre-treatment EEG data to predict response to SSRI treatment for MDD. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:6103–6106. doi: 10.1109/IEMBS.2010.5627823. [DOI] [PubMed] [Google Scholar]
- 14.Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2ed edn. New York: Oxford University Press; 2006. [Google Scholar]
- 15.Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999;11:190–208. doi: 10.1176/jnp.11.2.190. [DOI] [PubMed] [Google Scholar]
- 16.Nuwer MR, Lehmann D, da Silva FL, Matsuoka S, Sutherling W, Vibert JF. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:15–20. [PubMed] [Google Scholar]
- 17.John ER, Prichep LS. The anesthetic cascade: a theory of how anesthesia suppresses consciousness. Anesthesiology. 2005;102:447–471. doi: 10.1097/00000542-200502000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Constant I, Sabourdin N. The EEG signal: a window on the cortical brain activity. Paediatr Anaesth. 2012;22:539–552. doi: 10.1111/j.1460-9592.2012.03883.x. [DOI] [PubMed] [Google Scholar]
- 19.Olejniczak P. Neurophysiologic basis of EEG. J Clin Neurophysiol. 2006;23:186–189. doi: 10.1097/01.wnp.0000220079.61973.6c. [DOI] [PubMed] [Google Scholar]
- 20.Durka PJ. From wavelets to adaptive approximations: time-frequency parametrization of EEG. Biomed Eng Online. 2003;2:1. doi: 10.1186/1475-925X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samar VJ, Bopardikar A, Rao R, Swartz K. Wavelet analysis of neuroelectric waveforms: a conceptual tutorial. Brain Lang. 1999;66:7–60. doi: 10.1006/brln.1998.2024. [DOI] [PubMed] [Google Scholar]
- 22.Tonner PH, Bein B. Classic electroencephalographic parameters: median frequency, spectral edge frequency etc. Best Pract Res Clin Anaesthesiol. 2006;20:147–159. doi: 10.1016/j.bpa.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Fabiani M, Gratton G, Coles MGH. Event-related brain potentials – methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Bertson GG, editors. Handbook of Psychophysiology. 2ed edn. Cambridge: Cambridge University Press; 2000. pp. 55–83. [Google Scholar]
- 24.Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. 1998;107:227–253. doi: 10.1016/s0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–221. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- 26.Handwerker HO, Kobal G. Psychophysiology of experimentally induced pain. Physiol Rev. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- 27.Zaslansky R, Sprecher E, Katz Y, Rozenberg B, Hemli JA, Yarnitsky D. Pain-evoked potentials: what do they really measure? Electroencephalogr Clin Neurophysiol. 1996;100:384–391. [PubMed] [Google Scholar]
- 28.Mouraux A, Plaghki L. Are laser-evoked brain potentials modulated by attending to first or second pain? Pain. 2007;129:321–331. doi: 10.1016/j.pain.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Larijani GE, Goldberg ME. Alfentanil hydrochloride: a new short-acting narcotic analgesic for surgical procedures. Clin Pharm. 1987;6:275–282. [PubMed] [Google Scholar]
- 31.Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84:821–833. doi: 10.1097/00000542-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Freye E, Buhl R, Ciaramelli F. Opioids with different affinity for subreceptors induce different effects on early and late sensory evoked potentials (SEP) in man. NIDA Res Monogr. 1986;75:551–554. [PubMed] [Google Scholar]
- 33.Arendt-Nielsen L, Oberg B, Bjerring P. Analgesic efficacy of i.m. alfentanil. Br J Anaesth. 1990;65:164–168. doi: 10.1093/bja/65.2.164. [DOI] [PubMed] [Google Scholar]
- 34.Chapman CR, Hill HF, Saeger L, Gavrin J. Profiles of opioid analgesia in humans after intravenous bolus administration: alfentanil, fentanyl and morphine compared on experimental pain. Pain. 1990;43:47–55. doi: 10.1016/0304-3959(90)90049-J. [DOI] [PubMed] [Google Scholar]
- 35.Petersen-Felix S, Arendt-Nielsen L, Bak P, Fischer M, Zbinden AM. Psychophysical and electrophysiological responses to experimental pain may be influenced by sedation: comparison of the effects of a hypnotic (propofol) and an analgesic (alfentanil) Br J Anaesth. 1996;77:165–171. doi: 10.1093/bja/77.2.165. [DOI] [PubMed] [Google Scholar]
- 36.Kalkman CJ, Leyssius AT, Bovill JG. Influence of high-dose opioid anesthesia on posterior tibial nerve somatosensory cortical evoked potentials: effects of fentanyl, sufentanil, and alfentanil. J Cardiothorac Anesth. 1988;2:758–764. doi: 10.1016/0888-6296(88)90099-3. [DOI] [PubMed] [Google Scholar]
- 37.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–153. [PubMed] [Google Scholar]
- 38.Arendt-Nielsen L, Nielsen JC, Bjerring P. Double-blind, placebo controlled comparison of paracetamol and paracetamol plus codeine – a quantitative evaluation by laser induced pain. Eur J Clin Pharmacol. 1991;40:241–247. doi: 10.1007/BF00315203. [DOI] [PubMed] [Google Scholar]
- 39.Hummel T, Huber H, Menzel S, Kobal G. Tonic versus phasic pain: dose-related effects of ketoprofen. Eur J Clin Pharmacol. 1995;49:7–14. doi: 10.1007/BF00192351. [DOI] [PubMed] [Google Scholar]
- 40.Schubert A, Drummond JC, Peterson DO, Saidman LJ. The effect of high-dose fentanyl on human median nerve somatosensory-evoked responses. Can J Anaesth. 1987;34:35–40. doi: 10.1007/BF03007679. [DOI] [PubMed] [Google Scholar]
- 41.Hume AL, Durkin MA. Central and spinal somatosensory conduction times during hypothermic cardiopulmonary bypass and some observations on the effects of fentanyl and isoflurane anesthesia. Electroencephalogr Clin Neurophysiol. 1986;65:46–58. doi: 10.1016/0168-5597(86)90036-5. [DOI] [PubMed] [Google Scholar]
- 42.McPherson RW, Sell B, Traystman RJ. Effects of thiopental, fentanyl, and etomidate on upper extremity somatosensory evoked potentials in humans. Anesthesiology. 1986;65:584–589. doi: 10.1097/00000542-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Sawynok J. The therapeutic use of heroin: a review of the pharmacological literature. Can J Physiol Pharmacol. 1986;64:1–6. doi: 10.1139/y86-001. [DOI] [PubMed] [Google Scholar]
- 44.Stoermer R, Drewe J, Dursteler-Mac Farland KM, Hock C, Mueller-Spahn F, Ladewig D, Stohler R, Mager R. Safety of injectable opioid maintenance treatment for heroin dependence. Biol Psychiatry. 2003;54:854–861. doi: 10.1016/s0006-3223(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 45.Coda B, Tanaka A, Jacobson RC, Donaldson G, Chapman CR. Hydromorphone analgesia after intravenous bolus administration. Pain. 1997;71:41–48. doi: 10.1016/s0304-3959(97)03336-8. [DOI] [PubMed] [Google Scholar]
- 46.Bromm B, Meier W, Scharein E. Imipramine reduces experimental pain. Pain. 1986;25:245–257. doi: 10.1016/0304-3959(86)90100-4. [DOI] [PubMed] [Google Scholar]
- 47.Paul D, Standifer KM, Inturrisi CE, Pasternak GW. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther. 1989;251:477–483. [PubMed] [Google Scholar]
- 48.Saletu B, Saletu M, Brown M, Stern J, Sletten I, Ulett G. Hypno-analgesia and acupuncture analgesia: a neurophysiological reality? Neuropsychobiology. 1975;1:218–242. doi: 10.1159/000117497. [DOI] [PubMed] [Google Scholar]
- 49.Lotsch J, Kobal G, Geisslinger G. No contribution of morphine-6-glucuronide to clinical morphine effects after short-term administration. Clin Neuropharmacol. 1998;21:351–354. [PubMed] [Google Scholar]
- 50.Lotsch J, Kobal G, Stockmann A, Brune K, Geisslinger G. Lack of analgesic activity of morphine-6-glucuronide after short-term intravenous administration in healthy volunteers. Anesthesiology. 1997;87:1348–1358. doi: 10.1097/00000542-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Quante M, Scharein E, Zimmermann R, Langer-Brauburger B, Bromm B. Dissociation of morphine analgesia and sedation evaluated by EEG measures in healthy volunteers. Arzneimittelforschung. 2004;54:143–151. doi: 10.1055/s-0031-1296951. [DOI] [PubMed] [Google Scholar]
- 52.Staahl C, Krarup AL, Olesen AE, Brock C, Graversen C, Drewes AM. Is electrical brain activity a reliable biomarker for opioid analgesia in the gut? Basic Clin Pharmacol Toxicol. 2011;109:321–327. doi: 10.1111/j.1742-7843.2011.00727.x. [DOI] [PubMed] [Google Scholar]
- 53.Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs. 1991;41:326–344. doi: 10.2165/00003495-199141030-00002. [DOI] [PubMed] [Google Scholar]
- 54.Bromm B, Ganzel R, Herrmann WM, Meier W, Scharein E. Pentazocine and flupirtine effects on spontaneous and evoked EEG activity. Neuropsychobiology. 1986;16:152–156. doi: 10.1159/000118317. [DOI] [PubMed] [Google Scholar]
- 55.Bromm B, Ganzel R, Herrmann WM, Meier W, Scharein E. The analgesic efficacy of flupirtine in comparison to pentazocine and placebo assessed by EEG and subjective pain ratings. Postgrad Med J. 1987;63(Suppl. 3):109–112. [PubMed] [Google Scholar]
- 56.Servin FS, Billard V. Remifentanil and other opioids. Handb Exp Pharmacol. 2008;182:283–311. doi: 10.1007/978-3-540-74806-9_14. [DOI] [PubMed] [Google Scholar]
- 57.Noh GJ, Kim KM, Jeong YB, Jeong SW, Yoon HS, Jeong SM, Kang SH, Linares O, Kern SE. Electroencephalographic approximate entropy changes in healthy volunteers during remifentanil infusion. Anesthesiology. 2006;104:921–932. doi: 10.1097/00000542-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt GN, Scharein E, Siegel M, Muller J, Debener S, Nitzschke R, Engel A, Bischoff P. Identification of sensory blockade by somatosensory and pain-induced evoked potentials. Anesthesiology. 2007;106:707–714. doi: 10.1097/01.anes.0000264774.09910.c6. [DOI] [PubMed] [Google Scholar]
- 59.Monk JP, Beresford R, Sufentanil WA. A review of its pharmacological properties and therapeutic use. Drugs. 1988;36:286–313. doi: 10.2165/00003495-198836030-00003. [DOI] [PubMed] [Google Scholar]
- 60.Kimovec MA, Koht A, Sloan TB. Effects of sufentanil on median nerve somatosensory evoked potentials. Br J Anaesth. 1990;65:169–172. doi: 10.1093/bja/65.2.169. [DOI] [PubMed] [Google Scholar]
- 61.Reeves RR, Burke RS. Tramadol: basic pharmacology and emerging concepts. Drugs Today (Barc) 2008;44:827–836. doi: 10.1358/dot.2008.44.11.1289441. [DOI] [PubMed] [Google Scholar]
- 62.Thurauf N, Fleischer WK, Liefhold J, Schmid O, Kobal G. Dose dependent time course of the analgesic effect of a sustained-release preparation of tramadol on experimental phasic and tonic pain. Br J Clin Pharmacol. 1996;41:115–123. doi: 10.1111/j.1365-2125.1996.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 63.Truini A, Panuccio G, Galeotti F, Maluccio MR, Sartucci F, Avoli M, Cruccu G. Laser-evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. Eur J Pain. 2010;14:222–225. doi: 10.1016/j.ejpain.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lekic D, Cenic D. Pain and tooth pulp evoked potentials. Clin Electroencephalogr. 1992;23:37–46. doi: 10.1177/155005949202300109. [DOI] [PubMed] [Google Scholar]
- 65.Hummel T, Kraetsch HG, Lotsch J, Hepper M, Liefhold J, Kobal G. Analgesic effects of dihydrocodeine and tramadol when administered either in the morning or evening. Chronobiol Int. 1995;12:62–72. doi: 10.3109/07420529509064501. [DOI] [PubMed] [Google Scholar]
- 66.Hummel T, Hummel C, Friedel I, Pauli E, Kobal G. A comparison of the antinociceptive effects of imipramine, tramadol and anpirtoline. Br J Clin Pharmacol. 1994;37:325–333. doi: 10.1111/j.1365-2125.1994.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hummel T, Roscher S, Pauli E, Frank M, Liefhold J, Fleischer W, Kobal G. Assessment of analgesia in man: tramadol controlled release formula vs. tramadol standard formulation. Eur J Clin Pharmacol. 1996;51:31–38. doi: 10.1007/s002280050156. [DOI] [PubMed] [Google Scholar]
- 68.Chambon JP, Brochard J, Hallot A, Heaulme M, Brodin R, Roncucci R, Biziere K. CM 40907: a structurally novel anticonvulsant in mice, rats and baboons. J Pharmacol Exp Ther. 1985;233:836–844. [PubMed] [Google Scholar]
- 69.Schaffler K, Wauschkuhn CH, Gierend M. Analgesic potency of a new anticonvulsant drug versus acetylsalicylic acid via laser somatosensory evoked potentials. Randomized placebo-controlled double-blind (5-way) crossover study. Arzneimittelforschung. 1991;41:427–435. [PubMed] [Google Scholar]
- 70.Tarnawa I, Bolcskei H, Kocsis P. Blockers of voltage-gated sodium channels for the treatment of central nervous system diseases. Recent Pat CNS Drug Discov. 2007;2:57–78. doi: 10.2174/157488907779561754. [DOI] [PubMed] [Google Scholar]
- 71.Klamt JG, Posner J. Effects of lamotrigine on pain-induced chemo-somatosensory evoked potentials. Anaesthesia. 1999;54:774–777. doi: 10.1046/j.1365-2044.1999.00959.x. [DOI] [PubMed] [Google Scholar]
- 72.Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl. 6):13–18. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
- 73.Graversen C, Olesen SS, Olesen AE, Steimle K, Farina D, Wilder-Smith OH, Bouwense SA, van Goor H, Drewes AM. The analgesic effect of pregabalin in patients with chronic pain is reflected by changes in pharmaco-EEG spectral indices. Br J Clin Pharmacol. 2012;73:363–372. doi: 10.1111/j.1365-2125.2011.04104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olesen SS, Graversen C, Olesen AE, Frokjaer JB, Wilder-Smith O, van Goor H, Valeriani M, Drewes AM. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment Pharmacol Ther. 2011;34:878–887. doi: 10.1111/j.1365-2036.2011.04802.x. [DOI] [PubMed] [Google Scholar]
- 75.Persson J. Wherefore ketamine? Curr Opin Anaesthesiol. 2010;23:455–460. doi: 10.1097/ACO.0b013e32833b49b3. [DOI] [PubMed] [Google Scholar]
- 76.Kochs E, Scharein E, Mollenberg O, Bromm B, Schulte am Esch J. Analgesic efficacy of low-dose ketamine. Somatosensory-evoked responses in relation to subjective pain ratings. Anesthesiology. 1996;85:304–314. doi: 10.1097/00000542-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 77.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 78.Fink M, Irwin P. Central nervous system effects of aspirin. Clin Pharmacol Ther. 1982;32:362–365. doi: 10.1038/clpt.1982.172. [DOI] [PubMed] [Google Scholar]
- 79.Rohdewald P, Derendorf H, Drehsen G, Elger CE, Knoll O. Changes in cortical evoked potentials as correlates of the efficacy of weak analgesics. Pain. 1982;12:329–341. doi: 10.1016/0304-3959(82)90178-6. [DOI] [PubMed] [Google Scholar]
- 80.Schaffler K, Reeh PW, Zentzis K, Hamperl W. Analgesic effect of acetylsalicylic acid (ASA) versus a lithium-ASA combination: an evoked potential study employing radiant heat stimulation with a CO2 laser. Pharmacopsychiatry. 1987;20:217–221. doi: 10.1055/s-2007-1017107. [DOI] [PubMed] [Google Scholar]
- 81.Bromm B, Rundshagen I, Scharein E. Central analgesic effects of acetylsalicylic acid in healthy men. Arzneimittelforschung. 1991;41:1123–1129. [PubMed] [Google Scholar]
- 82.Kobal G, Hummel C, Nuernberg B, Brune K. Effects of pentazocine and acetylsalicylic acid on pain-rating, pain-related evoked potentials and vigilance in relationship to pharmacokinetic parameters. Agents Actions. 1990;29:342–359. doi: 10.1007/BF01966467. [DOI] [PubMed] [Google Scholar]
- 83.Jones CJ. The pharmacology and pharmacokinetics of azapropazone – a review. Curr Med Res Opin. 1976;4:3–16. doi: 10.1185/03007997609109277. [DOI] [PubMed] [Google Scholar]
- 84.Lotsch J, Mohammadian P, Hummel T, Florin S, Brune K, Geisslinger G, Kobal G. Effects of azapropazone on pain-related brain activity in human subjects. Br J Clin Pharmacol. 1995;40:545–552. doi: 10.1111/j.1365-2125.1995.tb05799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20:24–48. doi: 10.2165/00003495-198020010-00002. [DOI] [PubMed] [Google Scholar]
- 86.Bromm K, Herrmann WM, Schulz H. Do the B-vitamins exhibit antinociceptive efficacy in men? Results of a placebo-controlled repeated-measures double-blind study. Neuropsychobiology. 1995;31:156–165. doi: 10.1159/000119186. [DOI] [PubMed] [Google Scholar]
- 87.Lotsch J, Kettenmann B, Renner B, Drover D, Brune K, Geisslinger G, Kobal G. Population pharmacokinetics of fast release oral diclofenac in healthy volunteers: relation to pharmacodynamics in an experimental pain model. Pharm Res. 2000;17:77–84. doi: 10.1023/a:1007574710140. [DOI] [PubMed] [Google Scholar]
- 88.Schaffler K, Reitmeir P, Gschanes A, Eggenreich U. Comparison of the analgesic effects of a fixed-dose combination of orphenadrine and diclofenac (Neodolpasse) with its single active ingredients diclofenac and orphenadrine: a placebo-controlled study using laser-induced somatosensory-evoked potentials from capsaicin-induced hyperalgesic human skin. Drugs R D. 2005;6:189–199. doi: 10.2165/00126839-200506040-00001. [DOI] [PubMed] [Google Scholar]
- 89.Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 90.Kobal G, Hummel C, Gruber M, Geisslinger G, Hummel T. Dose-related effects of ibuprofen on pain-related potentials. Br J Clin Pharmacol. 1994;37:445–452. doi: 10.1111/j.1365-2125.1994.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lotsch J, Hummel T, Kraetsch H, Kobal G. The negative mucosal potential: separating central and peripheral effects of NSAIDs in man. Eur J Clin Pharmacol. 1997;52:359–364. doi: 10.1007/s002280050301. [DOI] [PubMed] [Google Scholar]
- 92.Hummel T, Cramer O, Mohammadian P, Geisslinger G, Pauli E, Kobal G. Comparison of the antinociception produced by two oral formulations of ibuprofen: ibuprofen effervescent vs ibuprofen tablets. Eur J Clin Pharmacol. 1997;52:107–114. doi: 10.1007/s002280050258. [DOI] [PubMed] [Google Scholar]
- 93.Seibel K, Schaffler K, Reeh P, Reitmeir P. Comparison of two different preparations of ibuprofen with regard to the time course of their analgesic effect. A randomised, placebo-controlled, double-blind cross-over study using laser somatosensory evoked potentials obtained from UV-irritated skin in healthy volunteers. Arzneimittelforschung. 2004;54:444–451. doi: 10.1055/s-0031-1296997. [DOI] [PubMed] [Google Scholar]
- 94.Sarzi-Puttini P, Atzeni F, Lanata L, Bagnasco M, Colombo M, Fischer F, D'Imporzano M. Pain and ketoprofen: what is its role in clinical practice? Reumatismo. 2010;62:172–188. doi: 10.4081/reumatismo.2010.172. [DOI] [PubMed] [Google Scholar]
- 95.Hinz B, Brune K. Paracetamol and cyclooxygenase inhibition: is there a cause for concern? Ann Rheum Dis. 2012;71:20–25. doi: 10.1136/ard.2011.200087. [DOI] [PubMed] [Google Scholar]
- 96.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bromm B, Forth W, Richter E, Scharein E. Effects of acetaminophen and antipyrine on non-inflammatory pain and EEG activity. Pain. 1992;50:213–221. doi: 10.1016/0304-3959(92)90165-8. [DOI] [PubMed] [Google Scholar]
- 98.Renner B, Clarke G, Grattan T, Beisel A, Mueller C, Werner U, Kobal G, Brune K. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J Clin Pharmacol. 2007;47:715–726. doi: 10.1177/0091270007299762. [DOI] [PubMed] [Google Scholar]
- 99.Brune K. Next generation of everyday analgesics. Am J Ther. 2002;9:215–223. doi: 10.1097/00045391-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Brogden RN. Pyrazolone derivatives. Drugs. 1986;32(Suppl. 4):60–70. doi: 10.2165/00003495-198600324-00006. [DOI] [PubMed] [Google Scholar]
- 101.Kraetsch HG, Hummel T, Lotsch J, Kussat R, Kobal G. Analgesic effects of propyphenazone in comparison to its combination with caffeine. Eur J Clin Pharmacol. 1996;49:377–382. doi: 10.1007/BF00203781. [DOI] [PubMed] [Google Scholar]
- 102.Schlicker E, Werner U, Hamon M, Gozlan H, Nickel B, Szelenyi I, Gothert M. Anpirtoline, a novel, highly potent 5-HT1B receptor agonist with antinociceptive/antidepressant-like actions in rodents. Br J Pharmacol. 1992;105:732–738. doi: 10.1111/j.1476-5381.1992.tb09047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Devulder J. Flupirtine in pain management: pharmacological properties and clinical use. CNS Drugs. 2010;24:867–881. doi: 10.2165/11536230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Kobal G, Hummel T. Effects of flupirtine on the pain-related evoked potential and the spontaneous EEG. Agents Actions. 1988;23:117–119. doi: 10.1007/BF01967210. [DOI] [PubMed] [Google Scholar]
- 105.Hummel T, Friedmann T, Pauli E, Niebch G, Borbe HO, Kobal G. Dose-related analgesic effects of flupirtine. Br J Clin Pharmacol. 1991;32:69–76. doi: 10.1111/j.1365-2125.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 107.Sindrup SH, Nielsen JC, Bjerring P, Arendt-Nielsen L. Imipramine does not affect argon-laser-induced pin-prick pain thresholds and laser-evoked cerebral potentials. Eur J Pain. 1998;2:127–132. doi: 10.1016/s1090-3801(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 108.Curley MA, Molengraft JA. Providing comfort to critically ill pediatric patients: isoflurane. Crit Care Nurs Clin North Am. 1995;7:267–274. [PubMed] [Google Scholar]
- 109.Roth D, Petersen-Felix S, Bak P, Arendt-Nielsen L, Fischer M, Bjerring P, Zbinden AM. Analgesic effect in humans of subanaesthetic isoflurane concentrations evaluated by evoked potentials. Br J Anaesth. 1996;76:38–42. doi: 10.1093/bja/76.1.38. [DOI] [PubMed] [Google Scholar]
- 110.Hunskaar S, Donnell D. Clinical and pharmacological review of the efficacy of orphenadrine and its combination with paracetamol in painful conditions. J Int Med Res. 1991;19:71–87. doi: 10.1177/030006059101900201. [DOI] [PubMed] [Google Scholar]
- 111.Schaffler K, Reitmeir P. Analgesic effects of low-dose intravenous orphenadrine in the state of capsaicin hyperalgesia. A randomised, placebo-controlled, double-blind cross-over study using laser somatosensory evoked potentials obtained from capsaicin-irritated skin in healthy volunteers. Arzneimittelforschung. 2004;54:673–679. doi: 10.1055/s-0031-1297020. [DOI] [PubMed] [Google Scholar]
- 112.Skrumsager BK, Ingwersen SH, Gerrits M. Safety and pharmacokinetics of ReN1869: a first human dose study in healthy subjects after single-dose administration. J Clin Pharmacol. 2003;43:66–73. doi: 10.1177/0091270002239708. [DOI] [PubMed] [Google Scholar]
- 113.Schaffler K, Seibel K, Thomsen M, Edwards M. Effect of the new H1-antagonist ReN1869 on capsaicin-induced hyperalgesia in human skin. Human phase-I trial using somatosensory evoked potentials induced by a CO2 laser. Arzneimittelforschung. 2004;54:187–191. doi: 10.1055/s-0031-1296957. [DOI] [PubMed] [Google Scholar]
- 114.Leuchter AF, Cook IA, Lufkin RB, Dunkin J, Newton TF, Cummings JL, Mackey JK, Walter DO. Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994;1:208–219. doi: 10.1006/nimg.1994.1006. [DOI] [PubMed] [Google Scholar]
- 115.Rabinoff M, Kitchen CM, Cook IA, Leuchter AF. Evaluation of quantitative EEG by classification and regression trees to characterize responders to antidepressant and placebo treatment. Open Med Inform J. 2011;5:1–8. doi: 10.2174/1874431101105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leuchter AF, Cook IA, Gilmer WS, Marangell LB, Burgoyne KS, Howland RH, Trivedi MH, Zisook S, Jain R, Fava M, Iosifescu D, Greenwald S. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res. 2009;169:132–138. doi: 10.1016/j.psychres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Bewernitz M, Derendorf H. Electroencephalogram-based pharmacodynamic measures: a review. Int J Clin Pharmacol Ther. 2012;50:162–184. doi: 10.5414/CP201484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Treede RD, Lorenz J, Baumgartner U. Clinical usefulness of laser-evoked potentials. Neurophysiol Clin. 2003;33:303–314. doi: 10.1016/j.neucli.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 119.Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol. 1984;6:405–410. [PubMed] [Google Scholar]
- 120.Lelic D, Morch CD, Hennings K, Andersen OK, Drewes AM. Differences in perception and brain activation following stimulation by large versus small area cutaneous surface electrodes. Eur J Pain. 2012;16:827–837. doi: 10.1002/j.1532-2149.2011.00063.x. [DOI] [PubMed] [Google Scholar]
- 121.Martin RW, Chapman CR. Dental dolorimetry for human pain research: methods and apparatus. Pain. 1979;6:349–364. doi: 10.1016/0304-3959(79)90053-8. [DOI] [PubMed] [Google Scholar]
- 122.Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, Haanpaa M, Jensen TS, Serra J, Treede RD. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018. doi: 10.1111/j.1468-1331.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 123.Kobal G. Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain. 1985;22:151–163. doi: 10.1016/0304-3959(85)90175-7. [DOI] [PubMed] [Google Scholar]
- 124.Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115–1130. doi: 10.1080/00365520310004399. [DOI] [PubMed] [Google Scholar]
- 125.Rossel P, Drewes AM, Petersen P, Nielsen J, Arendt-Nielsen L. Pain produced by electric stimulation of the rectum in patients with irritable bowel syndrome: further evidence of visceral hyperalgesia. Scand J Gastroenterol. 1999;34:1001–1006. doi: 10.1080/003655299750025101. [DOI] [PubMed] [Google Scholar]
- 126.Drewes AM, Sami SA, Dimcevski G, Nielsen KD, Funch-Jensen P, Valeriani M, Arendt-Nielsen L. Cerebral processing of painful oesophageal stimulation: a study based on independent component analysis of the EEG. Gut. 2006;55:619–629. doi: 10.1136/gut.2005.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luck SJ. Ten Simple Rules for Designing ERP Experiments. In: Handy TC, editor. Event Related Potentials. A Methods Handbook. Cambridge: MIT Press; 2004. pp. 17–32. [Google Scholar]
- 128.Chen ACN, Arendt-Nielsen L, Plaghki L. Laser-evoked potentials in human pain: I. Use and possible misuse. Pain Forum. 1998;7:174–184. [Google Scholar]
- 129.Cho CH, Jung SW, Park JY, Song KS, Yu KI. Is shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance? J Shoulder Elbow Surg. 2013;22:222–228. doi: 10.1016/j.jse.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 130.Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol. 2010;617:79–91. doi: 10.1007/978-1-60327-323-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khodayari-Rostamabad A, Hasey GM, Maccrimmon DJ, Reilly JP, de Bruin H. A pilot study to determine whether machine learning methodologies using pre-treatment electroencephalography can predict the symptomatic response to clozapine therapy. Clin Neurophysiol. 2010;121:1998–2006. doi: 10.1016/j.clinph.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Hunter AM, Leuchter AF, Cook IA, Abrams M, Siegman BE, Furst DE, Chappell AS. Brain functional changes and duloxetine treatment response in fibromyalgia: a pilot study. Pain Med. 2009;10:730–738. doi: 10.1111/j.1526-4637.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 133.Dimpfel W, Spuler M, Nickel B. Radioelectroencephalography (Tele-Stereo-EEG) in the rat as a pharmacological model to differentiate the central action of flupirtine from that of opiates, diazepam and phenobarbital. Neuropsychobiology. 1986;16:163–168. doi: 10.1159/000118319. [DOI] [PubMed] [Google Scholar]
- 134.Lorenz J, Beck H, Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997;73:369–375. doi: 10.1016/S0304-3959(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 135.Lorenz J, Beck H, Bromm B. Differential changes of laser evoked potentials, late auditory evoked potentials and P300 under morphine in chronic pain patients. Electroencephalogr Clin Neurophysiol. 1997;104:514–521. doi: 10.1016/s0168-5597(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 136.Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiol Clin. 2012;42:325–336. doi: 10.1016/j.neucli.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 138.Chizh BA, Greenspan JD, Casey KL, Nemenov MI, Treede RD. Identifying biological markers of activity in human nociceptive pathways to facilitate analgesic drug development. Pain. 2008;140:249–253. doi: 10.1016/j.pain.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 140.Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SD. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 142.Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochem Pharmacol. 2011;81:1408–1421. doi: 10.1016/j.bcp.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Lowe PJ, Hijazi Y, Luttringer O, Yin H, Sarangapani R, Howard D. On the anticipation of the human dose in first-in-man trials from preclinical and prior clinical information in early drug development. Xenobiotica. 2007;37:1331–1354. doi: 10.1080/00498250701648008. [DOI] [PubMed] [Google Scholar]
- 144.Shankland WE., 2nd Factors that affect pain behavior. Cranio. 2011;29:144–154. doi: 10.1179/crn.2011.023. [DOI] [PubMed] [Google Scholar]
- 145.Coburn KL, Lauterbach EC, Boutros NN, Black KJ, Arciniegas DB, Coffey CE. The value of quantitative electroencephalography in clinical psychiatry: a report by the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2006;18:460–500. doi: 10.1176/jnp.2006.18.4.460. [DOI] [PubMed] [Google Scholar]
- 146.Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 147.Liberati D, DiCorrado S, Mandelli S. Topographic mapping of single sweep evoked potentials in the brain. IEEE Trans Biomed Eng. 1992;39:943–951. doi: 10.1109/10.256428. [DOI] [PubMed] [Google Scholar]
- 148.Yordanova J, Kolev V. Single-sweep analysis of the theta frequency band during an auditory oddball task. Psychophysiology. 1998;35:116–126. [PubMed] [Google Scholar]
- 149.Gram M, Graversen C, Mørch CD, Andresen T, Drewes AM. Wavelet analysis of single-sweep pharmaco-EEG. International Pharmaco-EEG Conference New York. 2012 IPEG 17th Biennial Conference 2012 Abstract Book: 65. [Google Scholar]
- 150.Graversen C, Olesen AE, Farina D, Drewes AM. The analgesic effect of morphine is reflected by changes in single-sweep evoked brain potentials. International Pharmaco-EEG Conference New York. 2012 IPEG 17th Biennial Conference 2012 Abstract Book: 66. [Google Scholar]


