Abstract
Aim
The aim was to examine the impact of fee-for-service pharmacist-led medication review on patient outcomes and quantify this according to the type of review undertaken, e.g. adherence support and clinical medication review.
Methods
Relevant published studies were identified from Medline, Embase and International Pharmaceutical Abstract databases (from inception to February 2011). Study inclusion criteria were fee-for-service medication review, presence of a control group and pre-specified patient outcomes. Outcomes were grouped into primary (changes in biomarkers, hospitalization, and mortality) and secondary outcomes (medication adherence, economic implications and quality of life). Meta-analyses for primary outcomes were conducted using random effects models and secondary outcomes were summarized using descriptive statistics.
Results
Of the 135 relevant articles located, 21 studies met the inclusion criteria for primary outcomes and 32 for secondary outcomes. Significant results favouring pharmacists' intervention were found for blood pressure (OR 3.50, 95% CI 1.58, 7.75, P = 0.002) and low density lipoprotein (OR 2.35, 95% CI 1.17, 4.72, P = 0.02). Outcomes on hospitalization (OR 0.69, 95% CI 0.39, 1.21, P = 0.19) and mortality (OR 1.50, 95% CI 0.65 to 3.46, P = 0.34) indicated no differences between the groups. On subgroup analysis, clinical medication review (OR 0.46, 95% CI 0.26, 0.83, P = 0.01) but not adherence support review (OR 0.88, 95% CI 0.59, 1.32, P = 0.54) reduced hospitalization.
Conclusions
The majority of the studies (57.9%) showed improvement in medication adherence. Fee-for-service pharmacist-led medication reviews showed positive benefits on patient outcomes. Interventions that include a clinical review had a significant impact on patient outcomes by attainment of target clinical biomarkers and reduced hospitalization.
Keywords: community pharmacy services, drug use review, hospitalization, medication review, medication therapy management, outcome assessment (health care)
Introduction
Medication review can be defined as a structured, critical examination of a patient's medications with the objectives of reaching an agreement with the patient about their treatment and optimizing the impact of medications on patient's health outcomes [1]. Pharmacist-led medication review services are available in several countries such as the United Kingdom (UK) (Medicines Use Review, MUR), United States of America (USA) (Medication Therapy Management, MTM), Australia (Home Medication Review, HMR), Canada (MedsCheck) and New Zealand (Medicines Use Review, MUR) [1–5].
Medication review can be classified into four types: (1) prescription review, (2) adherence support review, (3) clinical review and (4) clinical review with prescribing [1, 5]. (1) A prescription review aims to address the technical issues of a patient's prescription such as anomalies or changed items [1]. (2) An adherence support review, with the patient present, addresses a patient's medication-taking behaviour focusing on improving a patient's knowledge of medicines and adherence to them [1]. (3) A clinical medication review, with access to clinical notes and the patient present, is more comprehensive and addresses the patients' use of medication in the context of their clinical condition [6] (4) In some countries an extension of type 3 exists and includes the authority for prescribing [5]. These services, the last two particularly, must be conducted in close collaboration with physicians and other health professionals [2].
As previously mentioned several countries pay pharmacists for providing medication reviews for patients [1–4, 7]. These can be termed ‘fee-for-service’ medication reviews because pharmacists are remunerated by the government or a health provider for each item of service or under bulk funding or capitation models. To our knowledge no review has assessed the clinical benefits of fee-for-service medication reviews where pharmacists carry out these reviews in their usual practice settings (with time, staffing and resource constraints) and not in a highly controlled research environment [8]. Furthermore no research has been published to quantify the effect of medication review by its type. A pooled meta-analysis of all types will hide the individual influences of these services.
The current study therefore aimed to examine the impact of fee-for-service pharmacist-led medication review on patient outcomes and quantify these according to the type of review undertaken. The specific objectives were to evaluate and quantify (i) the primary outcomes of such services (such as hospitalization, mortality rate, clinical biomarkers or marker of disease progress) and (ii) any secondary outcomes (such as medication adherence, economic implications and quality of life).
Methods
Locating studies
Studies were located through a comprehensive literature search of electronic bibliographic databases of Medline (1946–February 2011), Embase (1947–February 2011) and International Pharmaceutical Abstracts (IPA) (1970–February 2011). The current study used a learning based search algorithm [9] to provide appropriate coverage of the databases.
Identifying relevant subject headings and locating articles
Details of the learning-based search algorithm have been described elsewhere [9]. An article by Planas et al. (2009) was used as the index article in the learning algorithm to find the relevant subject headings (SH). The relevant SH from the index articles: community pharmacy services.exp*, patient compliance.exp, quality of health care.exp, treatment outcome.exp, Medication Therapy Management.exp (for Medline), pharmacy.exp*, health care quality.exp, patient compliance.exp, treatment outcome.exp, Medication Therapy Management.exp (for Embase) and pharmacy community.sh*, pharmacy.sh, interventions.sh, compliance.sh, quality assurance.sh (for IPA), were combined to retrieve more articles. Regardless of the relevance of the article to the review topic, relevant SH from the article were used in combination with the Boolean operator ‘AND’, to increase specificity. Combinations of four (Medline and Embase) and five (IPA) SH were considered to produce an acceptable number of hits to review. All permutations were considered. A fixed subject heading, marked with * was always included in each combination. The next iteration of the search continued until no new relevant SH were found. At the end of the search, articles that were located from each combination were pooled using ‘OR’ to eliminate duplicates. SH used to retrieve the articles are listed in Appendix S1.
Study selection
After the learning-based search algorithm had converged, articles were imported into EndNote and screened for pre-specified inclusion and exclusion criteria (Table 1). Since the present study focused on ‘fee-for-service’ medication reviews that occur in usual care settings (not in a highly controlled research environment), we considered both randomized controlled trials (RCTs) and non-randomized controlled trials (non-RCTs) should be included. Although RCTs are the best study design for such services, it is not convenient in all situations to conduct RCTs for fee-for-service medication reviews (for example if the service has been provided to patients before the study begins). Hence the present study also included non-RCT studies.
Table 1.
Inclusion and exclusion criteria for systematic review of fee-for-service medication review
| Inclusion criteria | |
| Study design | • Randomized controlled trial, quasi-experiment with control group, before and after study design or prospective/retrospective cohort with control group |
| Participant | • Adult participants defined by the individual study |
| Setting | • Intervention was conducted in the following setting: pharmacy, patients' home, community health centre, GP clinics |
| Intervention | • A medication review service: Medication review involved pharmacists providing pharmaceutical care and/or equivalent service to a patient. The current study considered a service as a medication review if the intervention included (a) and at least two of the following activities: |
| a) Review patient's medications for medication related issues | |
| b) Taking and documenting medication history | |
| c) Educating and counselling patients about medication and/or disease | |
| d) Providing a medication action plan | |
| e) Reaching an agreement with the patient about their medication treatment plan | |
| f) Monitoring drug treatment for effectiveness or adverse event | |
| g) Optimizing medication effectiveness and minimizing problems related to medication usage. | |
| • Pharmacists were reimbursed for the intervention. The intervention was considered as ‘fee-for service’ if: | |
| a) The country was known to provide funding for the particular service (e.g. Home Medication Review in Australia) | |
| b) The service was a demonstration/pilot service that received funding from interested parties (e.g. non-government agencies or university) | |
| c) The service received funding from independent parties such as patients, an employer or insurance company | |
| d) The service received funding through individual contracts with health agencies (e.g. District Health Board) | |
| Comparison | • The intervention must be compared with a control group that received usual care |
| Outcomes | • Quantitative: (1) Primary outcomes such as mortality, hospitalization and clinical biomarkers or marker of disease progress (2) Secondary outcomes such as medication adherence, economic and quality of life |
| For clinical biomarkers or marker of disease progress, data were included if the number of patients who achieved the target goal was defined or if mean and standard deviation (SD) or 95% confidence interval (95% CI) of the biomarker was reported. Only reported biomarkers that were assumed to be normally distributed were included in meta-analysis | |
| Exclusion criteria | |
| Study design | • Review articles, commentaries, editorial letters and studies without a control group |
| Participants | • Intervention conducted only on paediatric patients or patients unable to give consent (e.g. patients with dementia) |
| Setting | • Intervention in nursing home or assisted living facilities, academic setting, hospital, out-patient setting and call centre |
| Interventions | • Intervention that only involved a prescription review (e.g. type 1 medication review) |
| • Intervention that was not a medication review (e.g. smoking cessation clinic) | |
| • Pharmacist was only partly involved in delivering the medication review (combined interventions with other health professionals so that pharmacist's intervention cannot be distinctly quantified) | |
| • Intervention that was delivered only through phone calls or by pharmacy students | |
| • Intervention that was not reimbursed (e.g. a study funded through a University) or if: | |
| a) a fee was only provided for preparing and dispensing patient's medications | |
| Outcomes | • Qualitative outcomes (e.g. perceptions that were not quantified) |
| • Process outcomes (e.g. number of medication related problems identified or number of recommendations being accepted by doctors) |
Initial titles/abstracts screening was conducted by EH. The exclusion process using titles/abstracts by EH only occurred if the reason for exclusion was clear. If there was uncertainty the article was not excluded and each member of the research team (EH, JT, RB, SD) then reviewed the articles. All excluded ‘full text’ articles were reviewed by EH, JT, RB and SD independently to ensure the validity of the process and any disagreements on whether a study should be included/excluded were resolved through consensus. Since prescription review (type 1) is usually considered as part of pharmacists' routine in medication dispensing, this service is not considered in this review.
Data extraction
Data extracted from a full-text report using a data extraction form included study characteristics, participant characteristics, type of intervention/services and study outcomes. Medication reviews were categorized by type based on objective descriptions (see Table 2).
Table 2.
Objective descriptions of type of medication review by pharmacists
| Type* | Name of service | Possible intervention provided |
|---|---|---|
| 2 | Adherence review e.g. Medicines Use Review (MUR) | Addresses issues relating to a patients' medication taking behaviour, advice on medications use e.g. adverse effects, checking patients' technique and use of medication dosage forms e.g. inhalers, identify need for a change in dosage form. |
| 3 | Clinical medication review | Addresses issues relating to a patients' use of medication in the context of their clinical condition such as the appropriateness, effectiveness, cost-effectiveness and monitoring required to meet the patient's needs. |
| The intervention must be face to face with the patient and it could be with or without full patients' clinical notes. | ||
| 4 | Clinical medication review and prescribing | As in type 3 but pharmacist had the ability to prescribe or adjust the medication dose (either in a supplementary or fully independent role) |
For all types of medication review, the pharmacist should consider drug interactions, side effects, adherence to medications, lifestyle, non-medication interventions and unmet need.
Patient outcomes reported in each study were categorized into primary and secondary outcomes. Our categories on types of outcomes were based on how the outcomes reflect in patients' wellbeing. We defined primary outcomes as those that were considered as either directly affecting a patient's wellbeing or were a marker of disease progress such as mortality, hospitalization, changes in clinically important biomarkers, e.g. glycosylated haemoglobin, blood pressure, low density lipoprotein (LDL), opportunistic infection, asthma severity/control score. Secondary outcomes were those considered to indirectly reflect patient outcomes: medication adherence scores, quality of life and economic outcomes.
Appraisal of studies
The quality of the studies was assessed for potential bias in accordance with the Cochrane Collaboration guidelines [10, 11]. A summary of the quality assessment criteria used is provided in Appendix S2.
Data analysis
Meta-analyses were only conducted for primary outcomes as the definition and measurement of the outcomes, such as blood pressure and hospitalization, were more standardized and less varied than outcomes in the secondary group. Secondary outcomes were evaluated and summarized using descriptive statistics.
For the meta-analysis, outcomes were categorized into the number of successes or failures. Success was defined respectively as the number of patients who achieved the clinical biomarker goal defined in the studies, were not hospitalized or who survived. For studies that only reported mean and SD or 95% CI, the number of patients achieving the target goal was calculated assuming the data conformed to a normal distribution. Data were not assumed to be normally distributed, and were excluded from meta-analysis, if the number of samples was small (n < 30), a large amount of data was near zero or at a natural limit.
Summary effect size was pooled if there were at least four studies reporting the outcomes. Data were entered into the RevMan software (Ver 5.1, Cochrane library). For all analyses a random effects model was used to estimate the odds ratio (OR) in order to allow for study heterogeneity. Sensitivity analyses were performed to assess for studies that might be influential outliers on primary end points [12]. A one-study-removal method [13, 14] was used to examine the robustness of the results. A pre-specified subgroup analysis on the types of pharmacists' interventions (types 2–4, Table 2) was also conducted. For interest the present study also performed subgroup analysis on the types of study design (RCT vs. RCT and non-RCT). Publication bias was examined using funnel plots, the Begg-Mazumdar statistic (P < 0.05 for significance), classic fail-safe N and Duvel & Tweedie's Trim and Fill method.
Results
Details of the numbers of articles located and included/excluded for the systematic review are shown in Figure 1. Thirty-six articles were included in the systematic review and meta-analysis. Of these 21 studies reported primary outcomes of interest and 32 studies reported secondary outcomes.
Figure 1.
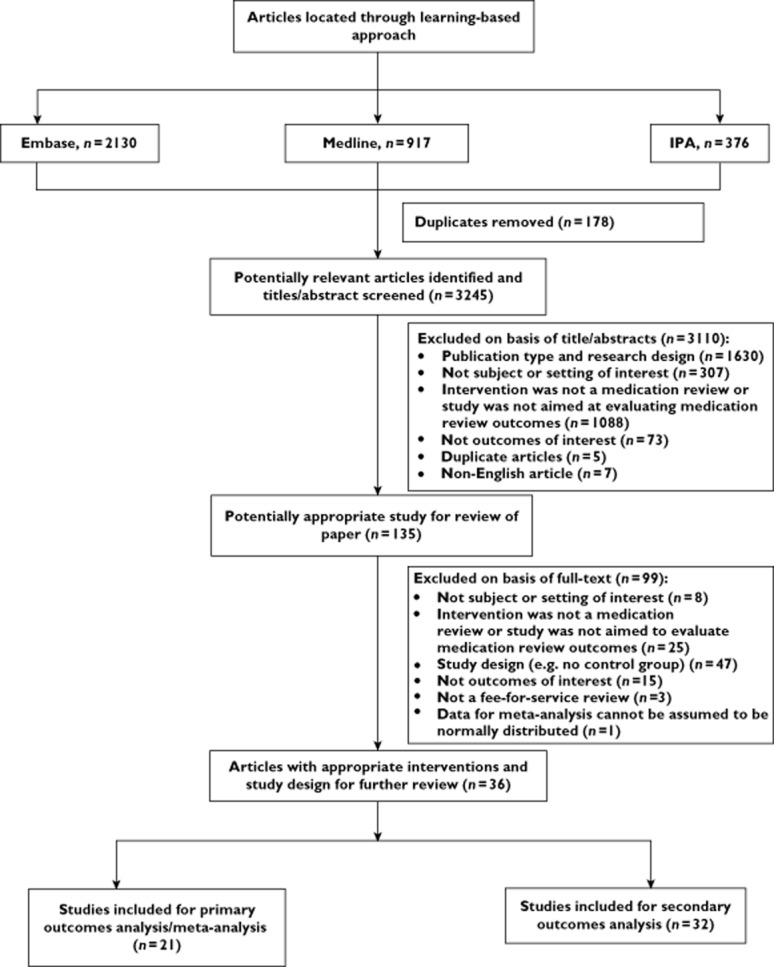
Systematic review inclusion and exclusion flowchart
Study characteristics
A summary of study characteristics for primary outcomes is presented in Table 3. Of the 36 studies, the majority of the medication reviews (n = 30) were disease-oriented interventions for patients with asthma (n = 7), diabetes (n = 3), hypertension (n = 3), hyperlipidaemia (n = 3), chronic diseases (n = 5) and other conditions (n = 9) such as stroke. Other services (n = 6) focused their interventions on the elderly (n = 4), patients newly discharged from hospital (n = 1) and patients with polypharmacy (n = 1).
Table 3.
Descriptions of studies and interventions
| Author, year | Country | Study design | Number of patients (mean age ± SD) | Patients' condition | Access to clinical notes* | Setting | Type of medication review | Primary outcome | Results# | Secondary outcome | Results# | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||||
| Park et al. 1996 [20] | USA | RCT | 27 (57.3) | 25 (63) | Hypertension | No | Pharmacy | CMR | Blood pressure goal | NM | Compliance QoL | I > C I > C‡ |
| Munroe et al. 1997 [21] | USA | Prospective cohort | 188 (67.2 ± 12.6) | 401 (63.3 ± 15.8) | Hypertension, diabetes, asthma, hypercholesterolaemia | Unclear | Pharmacy | CMR | – | – | Economics | I > C‡ |
| Begley et al. 1997 [22] | UK | RCT | 61 (84) | 66 (82) | Discharge from hospital, elderly | No | Patient's home | AR | – | – | Compliance | I > C |
| Carter et al. 1997 [23] | USA | RCT | 25 (67.3) | 26 (68.5) | Hypertension | Yes | Pharmacy, patient's home | CMR | Blood pressure goal | I = C | Economics QoL | C > I NM |
| Anonymous 2000 [24] | USA | Prospective cohort | NM | NM | Asthma, hypertension, diabetes, ischaemic heart disease | Unclear | Pharmacy | CMR | – | – | Economics | NM |
| Cordina et al. 2001 [25] | UK | RCT (Cluster) | 64 (41.3 ± 18.4) | 55 (45.9 ± 18.1) | Asthma | Yes | Pharmacy | CMR | Self reported symptoms¤ Hospitalization | I > C I > C | ComplianceQoL | I = C I > C‡ |
| Herborg et al. 2001 [26] | Denmark | Prospective cohort | 209 (38.8 ± 12.3) | 204 (42.4 ± 11.6) | Asthma | Unclear | Pharmacy | CMR | Hospitalization | I = C | QoL | I > C |
| Herborg et al. 2001 [27] | Denmark | Prospective cohort | 167 (38.8 ± 12.3) | 137 (42.4 ± 11.6) | Asthma | Unclear | Pharmacy | CMR | – | – | Compliance | I > C |
| Schulz et al. 2001 [28] | Germany | Prospective cohort | 101 (46.3 ± 11.4) | 63 (45.9 ± 12.5) | Asthma | Unclear | Pharmacy | AR | Asthma severity§ | I = C | QoL | I > C‡ |
| Finley et al. 2002 [29] | USA | Prospective cohort | 61 (59.9 ± 15.9) | 129 (61.1 ± 16.2) | Depression | No | GP clinic (Health centre), phone call | CMR with prescribing | – | – | Compliance | I > C |
| Fischer et al. 2002 [30] | USA | Prospective cohort | 231 (57) | 444 (58) | Heart and lung disease | Unclear | Pharmacy (community and health centre) | Unclear (between AR and CMR) | Mortality | I > C | Economics | I = C |
| Taylor et al. 2003 [31] | USA | RCT | 25 (64.4 ± 13.7) | 32 (66.7 ± 12.3) | Hypertension, diabetes, dyslipidemia, on warfarin | Yes | GP clinic | CMR | BP goal¤ LDL goal Hospitalization | I > C I > C I > C | Compliance QoL | I = C I = C |
| Sturgess et al. 2003 [32] | UK | RCT (Cluster) | 75 (73.1 ± 5.0) | 35 (74.2 ± 6.3) | Elderly | No | Pharmacy, patient's home | CMR | Hospitalization | I = C | Compliance QoL | I > C C > I‡ |
| Sellors et al. 2003 [33] | Canada | RCT (Cluster) | 379 (74 ± 6.1) | 409 (74 ± 6.0) | Elderly | Yes | GP clinic | CMR | Hospitalization | NM | Economics QoL | I = C I = C |
| Chabot et al. 2003 [34] | Canada | Prospective cohort | 35 (NM) | 56 (NM) | Hypertension | No | Pharmacy | CMR | BP goal (high income) | I > C‡ | Compliance (high income) | I > C‡ |
| Bouvy et al. 2003 [35] | Netherland | RCT | 48 (69.1 ± 10.2) | 43 (70 ± 11.2) | Heart failure on loop diuretics | No | Pharmacy | AR | Hospitalization(((heart failure)¤ Mortality | I = C I = C | Compliance QoL (generic) | I > C C > I‡ |
| Chrischilles et al. 2004 [36] | USA | Prospective cohort | 524 (54.1 ± 0.8) | 1687 (48.4 ± 0.5) | Polypharmacy | Unclear | Pharmacy | CMR | – | – | Economics | I = C |
| Krass et al. 2005 [37] | Australia | Prospective cohort | 106 (64 ± 9) | 82 (65 ± 10) | Type II diabetes | Unclear | Pharmacy | CMR | – | – | Compliance | I > C‡ |
| Paulos et al. 2005 [38] | Chile | RCT | 23 (64 ± 10) | 19 (66 ± 11) | Dyslipidaemia | Unclear | Pharmacy | CMR | Total cholesterol† | NM | QoL | NM |
| Shane-McWhorter et al. 2005 [39] | USA | Prospective cohort | 151 (69.1 ± 13.8) | 176 (54 ± 12.8) | Diabetes | Yes | GP clinic (Health centre) | CMR | Systolic blood pressure goal¤ LDL goal | I = C NM | – | – |
| Vrijens et al. 2006 [40] | Belgium | RCT | 194 (61.9 ± 19) | 198 (60.4 ± 10.2) | On atorvastatin | Yes | Pharmacy | AR | – | – | Compliance | I > C |
| Holland et al. 2007 [16] | UK | RCT | 136 (77.6 ± 9.0) | 144 (76.4 ± 9.5) | Heart failure | Yes | Patient's home | AR | HospitalizationMortality | I = C I = C | Compliance QoL | I = C I = C |
| Christensen et al. 2007 [41] | USA | Prospective before and after | 67 (67.7 ± 11.4) | 870 (66 ± 12.1) | Polypharmacy | Unclear | Pharmacy (community and health centre) | CMR | – | – | Economics | NM |
| Scott et al. 2007 [42] | UK | RCT | 883 (68.7 ± 9.2) | 472 (68.8 ± 9.1) | Coronary heart disease | Yes | Pharmacy | CMR | – | – | Economics | I > C‡ |
| Armour et al. 2007 [43] | Australia | RCT (Cluster) | 165 (47.5 ± 17.1) | 186 (50.4 ± 16.1) | Asthma | Unclear | Pharmacy and patient's home | CMR | Asthma severity | I > C | Compliance QoL | I > C I > C |
| Lenaghan et al. 2007 [44] | UK | RCT | 68 (84.5) | 66 (84.1) | Elderly | Yes | Patient's home | AR | Hospitalization Mortality | ¤ I = C I = C | QoL | I = C |
| Team 2007 [45] | UK | RCT | 883 (68.7 ± 9.2) | 472 (68.8 ± 9.1) | Coronary heart disease | Yes | Pharmacy | CMR | – | – | Compliance QoL | I = C I = C |
| Saini et al. 2008 [46] | Australia | Prospective cohort | 46 (50.8 ± 15.3) | 37 (50.4 ± 18.4) | Asthma | Unclear | Pharmacy and patient's home | CMR | Asthma severity† | I > C | Compliance QoL | I > C I = C |
| Mehuys et al. 2008 [47] | Belgium | RCT | 80 (36.3) | 70 (35.2) | Asthma | Unclear | Pharmacy | AR | Asthma control | I = C | Compliance QoL | I > C‡ I = C |
| Issets et al. 2008 [15] | USA | Prospective cohort | 285 (NM) | 126 (NM) | Had health claim on chronic disease | Unclear | GP clinic | CMR | Blood pressure goal¤ LDL goal | I > C I > C | – | – |
| Planas et al. 2009 [48] | USA | RCT | 25 (64.2 ± 10.5) | 15 (65.2 ± 14.1) | Diabetes with hypertension | Unclear | Pharmacy | CMR | Blood pressure goal | I > C | Compliance | I = C |
| Hugtenberg et al. 2009 [49] | Netherland | Prospective cohort | 336 (69.7 ± 15.0) | 379 (72.7 ± 11.2) | Discharge from hospital | No | Pharmacy, patient's home, phone call | AR | Mortality | I = C | – | – |
| Hirsch et al. 2009 [50] | USA | Prospective cohort | 1353 (46) | 5665 (46.7) | HIV/AIDS | No | Pharmacy | CMR | Opportunistic infection | I = C | Compliance Economics | I > C C > I |
| Roughead et al. 2009 [51] | Australia | Retrospective cohort | 273 (81.6 ± 4.8) | 5444 (81.6 ± 4.8) | Heart failure, elderly | Yes | Patient's home | CMR | Hospitalization | I > C | – | – |
| Hohmann et al. 2009 [52] | Germany | Prospective cohort | 73 (68.2 ± 9.7) | 157 (68.1 ± 10.8) | Transient ischaemic attack, ischaemic stroke | No | Pharmacy | Unclear (between AR and CMR) | – | – | QoL | I > C‡ |
| Villeneuve et al. 2010 [53] | Canada | RCT (Cluster) | 101 (59.3 ± 9.6) | 110 (62.2 ± 12.0) | On statin with inadequate control | Yes | Pharmacy | CMR with prescribing | LDL target | I = C | Compliance | I = C |
Include summary/ discharge notes from doctors,
Results based on significant finding reported in individual study, QoL = Quality of Life.
=Not included in meta-analysis, =include in combined primary outcome meta-analysis. I > C = intervention is significantly better than control, I = C = no significant difference between intervention and control, NM = significant value for comparison between groups was not mentioned clearly/conducted or information was not available, C > I = control is significantly better than intervention.
Significant in certain domains/outcomes. AR, Adherence review; CMR Clinical medication review.
Meta-analyses for primary outcomes were conducted for 21 studies (see Figure 1). Of the 21 studies, 13 (61.9%) were RCTs, seven (33.3%) were of prospective cohort and one (4.76%) was of retrospective cohort design. The majority (n = 8, 38.19%) of the studies were conducted in the USA, followed by in the UK (n = 4, 19%) and Canada (n = 3, 14.3%). Other studies were performed in the Netherlands (n = 2, 9.5%), Australia (n = 2, 9.5%), Belgium (n = 1, 4.8%) and Denmark (n = 1, 4.8%). Interventions were conducted mostly in a community pharmacy (n = 9, 42.9%), five studies (23.8%) in multiple settings (at the pharmacy and the patient's home), four (19%) at GP clinics/surgeries or at community health centres and three (14.3%) at the patient's home. The study quality assessment table is presented in Appendix S3.
Types of medication review were assigned to each study included in the meta-analysis. Five studies (23.8%) were classified as an adherence support review (type 2) and 13 studies (61.9%) were clinical medication reviews (type 3). Of the latter, half (n = 7, 33.3%) were conducted with some source of clinical information. Clinical medication review and prescribing (type 4) was described in two studies and one study was judged to be between types 2 and 3.
Primary outcomes: meta-analysis
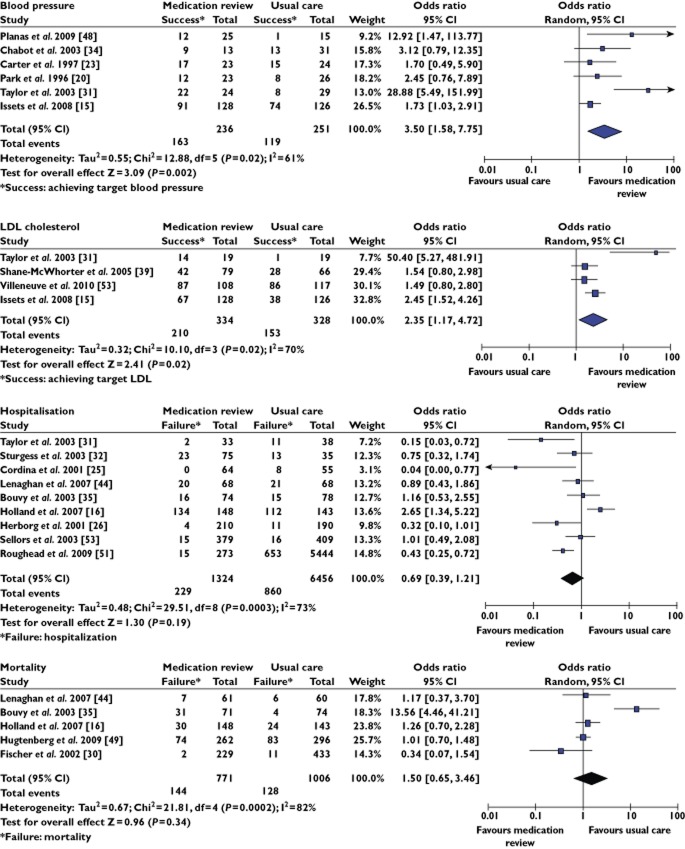
Meta-analyses were conducted for the following outcomes: blood pressure (n = 6), low density lipoprotein (LDL) (n = 4), hospitalization (n = 9) and mortality (n = 5) (see Figure 2). Pharmacist intervention was found to improve significantly the attainment of target biomarkers for blood pressure (OR 3.50, 95% CI 1.58, 7.75, P = 0.002) and LDL (OR 2.35, 95% CI 1.17, 4.72, P = 0.02) statistically. However there was no statistically significant difference found between pharmacists' interventions and usual care for hospitalization (OR 0.69, 95% CI 0.39, 1.21, P = 0.19) or mortality (OR 1.50, 95% CI 0.65, 3.46, P = 0.34).
Figure 2.
Forest plot for blood pressure, LDL, hospitalization and mortality outcomes. OR is >1 when medication review increased the number of patients achieving the target BP. OR is >1 when medication review increased the number of patients achieving the target LDL. OR is <1 when medication review reduced hospitalization. OR is <1 when medication review reduced mortality
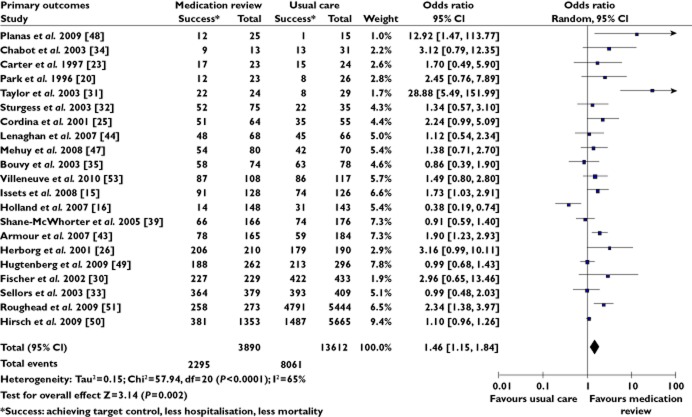
Meta-analysis was also conducted on combined primary outcomes and only one outcome per study was included in the analysis. The primary outcome from the study was selected, or if there were multiple primary outcomes, then the outcome that had the largest number of participating patients was selected. In this analysis (Figure 3), patients who received fee-for–service medication reviews were found to achieve target clinical outcomes (e.g. biomarker target, less hospitalization, less mortality) more commonly than the patients in the usual care group (OR 1.46, 95% CI 1.15, 1.84, P = 0.002).
Figure 3.
Forest plot of combined primary outcomes. OR is >1 when medication review decreased hospitalization or increased attainment of target control
Subgroup analysis
Subgroup analysis according to type of interventions (e.g. adherence support review, clinical medication review), found a statistically significant reduction in the hospitalization rate in patients receiving a clinical medication review compared with patients having usual care (OR 0.46, 95% CI 0.26, 0.83, P = 0.01) (Figure 4). This was not seen for adherence support reviews when considered separately (OR 0.88, 95% CI 0.59, 1.32, P = 0.54). A similar pattern, favouring clinical medication review (OR 1.83, 95% CI 1.35, 2.49, P = 0.0001), was also seen in combined primary outcomes (see Appendix S4).
Figure 4.
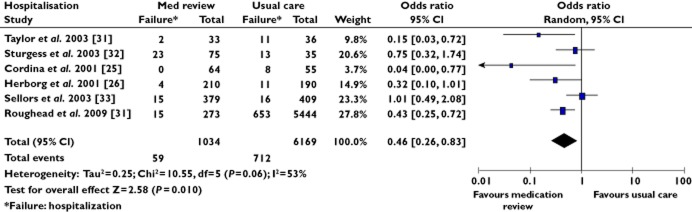
Forest plot of hospitalization outcome for studies with clinical medication review. OR is <1 when medication review reduced hospitalization
The only difference that was apparent when non-RCTs were removed from the analysis was for clinical medication review as the intervention, against the outcome of hospitalization (see Appendix 4). For this outcome there were four RCTs and two non-RCTs. In the analysis of RCTs only, we find the same OR as in the combined (RCT and non-RCT) analysis. However the confidence interval increased and now included 1 (RCT only: OR 0.45, 95% CI 0.17, 1.23, P = 0.12) compared with (RCT and non-RCT: OR 0.46, 95% CI 0.26, 0.83, P = 0.01). The details of the subgroup analyses and publication bias assessment are available in Appendix S4.
Sensitivity analysis
Removal of any one study did not change the meta-analysis findings on blood pressure, mortality and the combined primary outcomes. Only the meta-analyses for LDL (no longer significant when Isset et al. [15] was removed, OR 2.68, 95% CI 0.88, 8.18, P = 0.08) and hospitalization (significant favouring the intervention group when Holland et al. [16] was removed, OR 0.60, 95% CI 0.37, 0.95, P = 0.03) were affected by removing one study.
Secondary outcomes
Studies reporting secondary outcomes (n = 32) are summarized in Appendix S5. Nineteen studies reported improved adherence/compliance to medications as an outcome of their research. Of these, 12 provided clinical medication reviews (type 3), five studies were an adherence review (type 2) and two studies were a clinical medication review with prescribing (type 4). The majority of the studies (n = 11, 57.9%), eight clinical medication reviews and three adherence reviews, reported a significant improvement in adherence to medication as a result of pharmacists' interventions. Patients' quality of life was measured in 17 studies and nine studies measured the economic outcomes of the study. The findings for secondary outcomes are summarized in Table 4.
Table 4.
Summary of reported findings of secondary outcomes
| Secondary outcomes | Number of trials | Favour medication review | Favour usual care | No significant differences between the groups | Study reported both *significant and non-significant difference |
|---|---|---|---|---|---|
| Adherence | 19 | 11 | – | 6 | 2 |
| Economics | 9 | ||||
| Total medical costs | 4 | 1 | 2 | 1 | – |
| Total medication costs | 6 | 1 | 2 | 3 | – |
| Total cost for healthcare services | 3 | – | 1 | 2 | – |
| Quality of life | 16 | ||||
| All domains | 3 | – | 8 | – | |
| Some domains† | 3 | 2 | – | – |
Outcomes were measured using two different tools, for example self-reported adherence and medication refill. Analysis with one tool may show a significant finding but the other one may not.
e.g. vitality (SF-36), energy/fatigue (HSQ) and mental summary (SF-36).
Discussion
This is the first study that has investigated the outcomes of a fee-for-service medication review. We excluded services provided at research and academic sites and/or were part of a trial. Studies on unfunded services that existed only as a part of a trial, while providing high quality information, may not reflect the reality of clinical practice. Pharmacists providing fee-for-service medication reviews will be subject to a number of limitations because of their responsibilities for many other aspects of the running of the pharmacy [8].
Our meta-analyses found that fee-for-service medication reviews by pharmacists performed as part of their daily practice resulted in some significant positive impacts on patients' clinical outcomes, primarily driven by the attainment of biomarker targets. Specifically they increased the number of patients achieving targets for blood pressure and LDL, but no clear effect was found on hospitalization or mortality rates. The outcome relating to blood pressure was found to have the lowest precision (OR 3.50, 95% CI 1.58, 7.75, P = 0.002) which we believe to be due to the small total sample size of the studies included. However, when all outcomes were pooled, the meta-analysis showed a statistically significant increase in the number of patients achieving the target clinical outcomes, reduced hospitalization and reduced mortality.
The magnitude of benefit seen in the present study is consistent with earlier meta-analyses on blood pressure [14, 17] and LDL outcomes [14, 18]. A meta-analysis of pharmacists' direct patient care interventions conducted in the USA found that systolic and diastolic blood pressure decreased by −7.8 mmHg (SD = 1.5, 95% CI −9.7, −5.8) and −2.9 mm Hg (SD = 0.7, 95% CI −3.8, −2.0), respectively [14]. In the same study, the mean difference of LDL reduction between pharmacists' interventions and the control group was −0.16 mmol l−1 (SD = 0.12, 95% CI −0.16, −0.17). Their study, however, did not differentiate the different types of medication review considered in our meta-analysis. It included all services with educational, behavioural and technical interventions, performed in all settings such as retail, in-patient, institutional and emergency department and was not focused on fee-for-service medication review in community settings [14]. Similar findings of a significant reduction in patients' systolic and diastolic blood pressure were also reported in a study focused on hypertension management [17]. Interventions included pharmacists' providing medication management, drug therapy monitoring and/or patient education and counselling at medical clinics and community pharmacies [17]. The magnitude of blood pressure reduction was −10.7 ± 11.6 mmHg in the intervention group and −3.2 mmHg ± 12.2 in the control group (P = 0.047) [17]. Interventions by pharmacists were also reported to lower patients' LDL concentration by −0.28 mmol l−1 (95% CI −0.44, −0.12 mmol l−1) more than patients in the standard care group [18]. The scope of findings in these studies [17, 18], however, were limited to hypertension and dyslipidaemia and did not encompass evidence in other situations such as patients with asthma, with multiple medications or recently discharged from hospital.
We also evaluated the influence of different types of medication review on patients' outcomes which has not been considered previously. Our subgroup analysis showed that clinical medication review had positive impacts on blood pressure (OR 3.50, 95% CI 1.58, 7.75, P = 0.002) and LDL (OR 3.20, 95% CI 1.14, 8.98, P = 0.03). Furthermore, clinical medication review improved patient outcomes on hospitalization (OR 0.46, 95% CI 0.26, 0.83, P = 0.01) and this was not seen in an earlier systematic review with elderly patients, that did not separate the types of medication review [19]. A subgroup analysis on mortality for clinical medication review was not conducted as only one study reported this outcome. The odds ratio of achieving successful events in combined primary outcomes was increased, from 1.46 (95% CI 1.15, 1.84, P = 0.002) to 1.83 (95% CI 1.35, 2.49, P = 0.0001), when only studies with clinical medication review services were pooled. Adherence reviews did not show any statistically significant effects on hospitalization (OR 1.42, 95% CI 0.73, 2.78, P = 0.08), mortality (OR 1.92, 95% CI 0.79, 4.70, P = 0.15) or combined primary outcomes (OR 0.88, 95% CI 0.59, 1.32, P = 0.54).
These findings could be explained by the different focus of the types of medication review services compared. Clinical medication review (type 3) is perceived to be more comprehensive than an adherence review (type 2) as it promotes adherence in medication that has been assessed for its appropriateness, effectiveness and safety. An adherence review is founded on the assumption that the medication regimen is already optimal and focuses only on patient's problems with day-to-day use of medications.
Our study found that both clinical medication review (n = 8) and adherence review (n = 3) improved adherence to medications. We cannot confirm the impact of medication review on patients' quality of life as 50% (n = 8) of the studies showed no significant difference between the intervention and control group. Similarly, at least 85% of the studies in the systematic reviews by Chisholm-Burns et al. [14] and Machando et al. [17] reported non-significant differences in quality of life between the intervention and control group. However when the results on the general health dimension were pooled in a meta-analysis, the quality of life outcome was significant favouring pharmacists' intervention [14]. Whether the results can be generalized to pharmacists' services performed in the usual clinical setting, however, is unknown [14].
One limitation of the present study is that the analysis of ‘combined primary outcomes’ were conducted by combining different types of outcomes. Combining different outcomes is generally not suitable in meta-analyses as it may result in bias due to heterogeneity. However, since the goal of this study was to explore potential effects of different types of medication review services, the outcomes were pooled together to increase the power to see differences should a difference exist. To provide a basis for comparison outcomes were expressed as a change from baseline. Based on the exploratory nature and normalization of outcomes the decision to pool across outcomes was considered to be justified. We also note in our work that only published studies were included. However the use of Trim and Fill methods suggested that the impact of bias was unlikely to have a significant effect on the findings. Additionally, classification of types of medication review was based on the sometimes limited information available in the articles, and hence it is possible that the types of services may be mis-specified in some cases. However articles were independently reviewed and this effect is likely to be relatively minor. Nevertheless, care should be taken when interpreting the results of the subgroup analysis.
Conclusions
Fee-for-service pharmacist-led medication review services were shown to have a positive benefit on patient outcomes specifically on the attainment of clinical biomarkers. Furthermore services conducted as clinical medication reviews improved hospitalization, an important hard outcome for patients. Healthcare providers need to recognize the impact of different types of medication review on patients' outcomes. Further study specifically designed to compare the impact of different types of medication reviews is needed to quantify the importance of these findings.
Acknowledgments
Funding for this study was provided by the School of Pharmacy, University of Otago, New Zealand. EH was supported by Universiti Kebangsaan Malaysia and Ministry of Higher Education Malaysia. The funder and sponsor had no role in study design or data collection, analysis or interpretation.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1
Subject headings used to locate articles
Appendix S2
Summary of quality assessment criteria
Appendix S3
Summary of quality assessment for studies reporting primary outcomes
Appendix S4
Summary of subgroup analysis and publication bias assessment
Appendix S5
Summary of secondary outcomes reported in the studies included
References
- 1.Clyne W, Blenkinsopp A, Seal R. A Guide to Medication Review. 2008. Available at http://www.npc.nhs.uk/review_medicines/intro/resources/agtmr_web1.pdf (last accessed November 2011)
- 2.American Pharmacists Association and National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model. 2008. Available at http://www.pharmacist.com/sites/default/files/files/core_elements_of_an_mtm_practice.pdf (last accessed November 2011) [DOI] [PubMed]
- 3.Ontario Ministry of Health and Long-Term Care. MedsCheck. 2011. Availabe at http://www.health.gov.on.ca/en/pro/programs/drugs/medscheck/medscheck_original.aspx (last accessed September 2012)
- 4.Australia Government, Department of Human Services. Home Medicines Review (HMR) 2012. Available at http://www.medicareaustralia.gov.au/provider/pbs/fourth-agreement/hmr.jsp (last accessed August 2010)
- 5.Pharmacy Council of New Zealand. Medicines management: definition, levels, competence framework. 2006. Available at http://www.pharmacycouncil.org.nz/cms_show_download.php?id=124 (last accessed August 2011)
- 6.NHS Cumbria Medicine Management Team. Clinical medication review: a practice guide. 2011. Available at http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/MedicationReview-PracticeGuide2011.pdf (last accessed May 2012)
- 7.Lee E, Braund R, Tordoff J. Examining the first year of Medicines Use Review services provided by pharmacists in New Zealand, 2008. N Z Med J. 2009;122:26–35. [PubMed] [Google Scholar]
- 8.McCann L, Hughes CM, Adair CG. A self-reported work-sampling study in community pharmacy practice: a 2009 update. Pharm World Sci. 2010;32:536–543. doi: 10.1007/s11096-010-9405-x. [DOI] [PubMed] [Google Scholar]
- 9.Young S, Duffull SB. A learning-based approach for performing an in-depth literature search using MEDLINE. J Clin Pharm Ther. 2011;36:504–512. doi: 10.1111/j.1365-2710.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated March 2011]The Cochrane Collaboration, 2011. Available at http://www.cochrane-handbook.org (last accessed September 2012)
- 11.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: including non-randomzed studies. Cochrane Handbook for Systematic Reviews Interventions. Version 5.0.1 [updated September 2008]. 2008. Available at: http://www.cochrane-handbook.org (last accessed February 2013)
- 12.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 13.Koshman SL, Scot HS, Ross TT. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008;168:687–694. doi: 10.1001/archinte.168.7.687. [DOI] [PubMed] [Google Scholar]
- 14.Chisholm-Burns MA, Jeannie KL, Spivey CA, Slack M, Herrier RN, Hall-Lipsy E, Graff Zivin J, Abraham I, Palmer J, Martin JR, Kramer S, Wun T. US Pharmacists' Effect as Team Members on Patient Care Systematic Review and Meta-Analyses. Med Care. 2010;48:923–933. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- 15.Isetts BJ, Schondelmeyer SW, Artz MB, Lenarz LA, Heaton AH, Wadd WB, Brown LM, Cipolle RJ. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc. 2008;48:203–211. doi: 10.1331/JAPhA.2008.07108. [DOI] [PubMed] [Google Scholar]
- 16.Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, Shepstone L, Lipp A, Daly C, Howe A, Hall R, Harvey I. Effectiveness of visits from community pharmacists for patients with heart failure: heartMed randomised controlled trial. BMJ. 2007;334:1098–1101. doi: 10.1136/bmj.39164.568183.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado M, Bajcar J, Guzzo G, Einarson T. Sensitivity of patient outcomes to pharmacist interventions. Part II: systematic review and meta-analysis in hypertension management. Ann Pharmacother. 2007;41:1770–1781. doi: 10.1345/aph.1K311. [DOI] [PubMed] [Google Scholar]
- 18.Charrois TL, Zolezzi M, Koshman SL, Pearson G, Makowsky M, Durec T, Tsuyuki RT. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacotherapy. 2012;32:222–233. doi: 10.1002/j.1875-9114.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 19.Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2007;65:303–316. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting. J Am Pharm Assoc (Wash) 1996;NS36(Jul):443–451. doi: 10.1016/s1086-5802(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 21.Munroe WP, Kunz K, Dalmady-Israel C, Potter L, Schonfeld WH. Economic evaluation of pharmacist involvement in disease management in a community pharmacy setting. Clin Ther. 1997;19:113–123. doi: 10.1016/s0149-2918(97)80078-1. [DOI] [PubMed] [Google Scholar]
- 22.Begley S, Livingstone C, Hodges N, Williamson V. Impact of domiciliary pharmacy visits on medication management in an elderly population. Int J Pharm Prac. 1997;5:111–121. [Google Scholar]
- 23.Carter BL, Barnette DJ, Chrischilles E, Mazzotti GJ, Asali ZJ. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997;17:1274–1285. [PubMed] [Google Scholar]
- 24.Anonymous. Impact of pharmaceutical care delivered in the community pharmacy setting: results of a two year demonstration project. Iowa Pharm. 2000;55(Jan-Feb):18–24. [Google Scholar]
- 25.Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy-based program for patients with asthma. Pharmacotherapy. 2001;21(10 I):1196–1203. doi: 10.1592/phco.21.15.1196.33894. [DOI] [PubMed] [Google Scholar]
- 26.Herborg H, Soendergaard B, Froekjaer B, Fonnesbaek L, Jorgensen T, Hepler CD, Grainger-Rousseau T, Ersboell BK. Improving drug therapy for patients with asthma. Part 1. Patient outcomes. J Am Pharm Assoc (Wash) 2001;41(Jul-Aug):539–550. doi: 10.1016/s1086-5802(16)31278-5. [DOI] [PubMed] [Google Scholar]
- 27.Herborg H, Soendergaard B, Jorgensen T, Fonnesbaek L, Hepler CD, Holst H, Froekjaer B. Improving drug therapy for patients with asthma-part 2: use of antiasthma medications. J Am Pharm Assoc (Wash) 2001;41:551–559. doi: 10.1016/s1086-5802(16)31279-7. [DOI] [PubMed] [Google Scholar]
- 28.Schulz M, Verheyen F, Mühlig S, Ller JM, Hlbauer K, Knop-Schneickert E, Petermann F, Bergmann KC. Pharmaceutical care services for asthma patients: a controlled intervention study. J Clin Pharmacol. 2001;41:668–676. doi: 10.1177/00912700122010438. [DOI] [PubMed] [Google Scholar]
- 29.Finley PR, Rens HR, Pont JT, Gess SL, Louie C, Bull SA, Bero LA. Impact of a collaborative pharmacy practice model on the treatment of depression in primary care. Am J Health Syst Pharm. 2002;59:1518–1526. doi: 10.1093/ajhp/59.16.1518. [DOI] [PubMed] [Google Scholar]
- 30.Fischer LR, Defor TA, Cooper S, Scott LM, Boonstra DM, Eelkema MA, Goodman MJ. Pharmaceutical care and health care utilization in an HMO. Eff Clin Pract. 2002;5:49–57. [PubMed] [Google Scholar]
- 31.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60:1123–1129. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 32.Sturgess IK, McElnay JC, Hughes CM, Crealey G. Community pharmacy based provision of pharmaceutical care to older patients. Pharm World Sci. 2003;25:218–226. doi: 10.1023/a:1025860402256. [DOI] [PubMed] [Google Scholar]
- 33.Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, Goeree R, Cosby R, Trim K, Sebaldt R, Howard M, Hardcastle L, Poston J. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. Can Med Assoc J. 2003;169:17–22. [PMC free article] [PubMed] [Google Scholar]
- 34.Chabot I, Moisan J, Gregoire JP, Milot A. Pharmacist intervention program for control of hypertension. Ann Pharmacother. 2003;37:1186–1193. doi: 10.1345/aph.1C267. [DOI] [PubMed] [Google Scholar]
- 35.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoe AW, Leufkens HGM. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a Randomized Controlled Study. J Card Fail. 2003;9:404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 36.Chrischilles EA, Carter BL, Lund BC, Rubenstein LM, Chen-Hardee SS, Voelker MD, Park TR, Kuehl AK. Evaluation of the Iowa Medicaid pharmaceutical case management program. JAPhA. 2004;44:337–349. doi: 10.1331/154434504323063977. [DOI] [PubMed] [Google Scholar]
- 37.Krass I, Taylor SJ, Smith C, Armour CL. Impact on medication use and adherence of Australian pharmacists' diabetes care services. J Am Pharm Assoc. 2005;45:33–40. doi: 10.1331/1544345052843093. [DOI] [PubMed] [Google Scholar]
- 38.Paulos CP, Akesson Nygren CE, Celedon C, Carcamo CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Ann Pharmacother. 2005;39:939–943. doi: 10.1345/aph.1E347. [DOI] [PubMed] [Google Scholar]
- 39.Shane-McWhorter L, Oderda GM. Providing diabetes education and care to underserved patients in a collaborative practice at a Utah community health center. Pharmacotherapy. 2005;25:96–109. doi: 10.1592/phco.25.1.96.55623. [DOI] [PubMed] [Google Scholar]
- 40.Vrijens B, Belmans A, Matthys K, de Klerk E, Lesaffre E, Vrijens B, Belmans A, Matthys K, de Klerk E, Lesaffre E. Effect of intervention through a pharmaceutical care program on patient adherence with prescribed once-daily atorvastatin. Pharmacoepidemiol Drug Saf. 2006;15:115–121. doi: 10.1002/pds.1198. [DOI] [PubMed] [Google Scholar]
- 41.Christensen DB, Roth M, Trygstad T, Byrd J. Evaluation of a pilot medication therapy management project within the North Carolina State Health Plan. J Am Pharm Assoc. 2007;47:471–483. doi: 10.1331/JAPhA.2007.06111. [DOI] [PubMed] [Google Scholar]
- 42.Scott A, Tinelli M, Bond C. Costs of a community pharmacist-led medicines management service for patients with coronary heart disease in England – Healthcare system and patient perspectives. Pharmacoeconomics. 2007;25:397–411. doi: 10.2165/00019053-200725050-00004. [DOI] [PubMed] [Google Scholar]
- 43.Armour C, Bosnic-Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, Saini B, Smith L, Stewart K. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496–502. doi: 10.1136/thx.2006.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenaghan E, Holland R, Brooks A, Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care–the POLYMED randomised controlled trial. Age Ageing. 2007;36:292–297. doi: 10.1093/ageing/afm036. [DOI] [PubMed] [Google Scholar]
- 45.Community Pharmacy Medicines Management Project Evaluation Team. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract. 2007;24:189–200. doi: 10.1093/fampra/cml075. [DOI] [PubMed] [Google Scholar]
- 46.Saini B, Filipovska J, Bosnic-Anticevich S, Taylor S, Krass I, Armour C. An evaluation of a community pharmacy-based rural asthma management service. Aust J Rural Health. 2008;16:100–108. doi: 10.1111/j.1440-1584.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- 47.Mehuys E, Van Bortel L, De Bolle L, Van Tongelen I, Annemans L, Remon JP, Brusselle G. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir J. 2008;31:790–799. doi: 10.1183/09031936.00112007. [DOI] [PubMed] [Google Scholar]
- 48.Planas LG, Crosby KM, Mitchell KD, Farmer KC. Evaluation of a hypertension medication therapy management program in patients with diabetes. JAPhA. 2009;49:164–170. doi: 10.1331/JAPhA.2009.08164. [DOI] [PubMed] [Google Scholar]
- 49.Hugtenburg JG, Borgsteede SD, Beckeringh JJ. Medication review and patient counselling at discharge from the hospital by community pharmacists. Pharm World Sci. 2009;31:630–637. doi: 10.1007/s11096-009-9314-z. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch JD, Rosenquist A, Best BM, Miller TA, Gilmer TP, Hirsch JD, Rosenquist A, Best BM, Miller TA, Gilmer TP. Evaluation of the first year of a pilot program in community pharmacy: HIV/AIDS medication therapy management for Medi-Cal beneficiaries. J Manag Care Pharm. 2009;15:32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roughead EE, Barratt JD, Ramsay E, Pratt N, Ryan P, Peck R, Killer G, Gilbert AL. The effectiveness of collaborative medicine reviews in delaying time to next hospitalization for patients with heart failure in the practice setting: results of a cohort study. Circ Heart Fail. 2009;2:424–428. doi: 10.1161/CIRCHEARTFAILURE.109.861013. [DOI] [PubMed] [Google Scholar]
- 52.Hohmann C, Klotz JM, Radziwill R, Jacobs AH, Kissel T. Pharmaceutical care for patients with ischemic stroke: improving the patients quality of life. Pharm World Sci. 2009;31:550–558. doi: 10.1007/s11096-009-9315-y. [DOI] [PubMed] [Google Scholar]
- 53.Villeneuve J, Genest J, Blais L, Vanier MC, Lamarre D, Fredette M, Lussier MT, Perreault S, Hudon E, Berbiche D, Lalonde L. A cluster randomized controlled Trial to Evaluate an Ambulatory primary care Management program for patients with dyslipidemia: the TEAM study. CMAJ. 2010;182:447–455. doi: 10.1503/cmaj.090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.